Abstract
Aims
Spexin plays a role in regulating glucose metabolism. This study investigated the spexin levels in different glycemic status and its association with insulin secretion in humans.
Methods
A total of 462 subjects were recruited in this study, including 52 healthy subjects, 106 first-degree relatives (FDRs) of type 2 diabetes mellitus (T2DM), 115 impaired glucose regulation (IGR), 80 newly diagnosed T2DM, and 106 established T2DM. Serum spexin was measured using ELISA. The homeostasis model assessment of insulin resistance (HOMA2-IR) and β-cell function (HOMA2-β), and Stumvoll index estimating first- and second-phase insulin secretion were calculated.
Results
Spexin levels were higher in FDRs [235.53 pg/ml (185.28, 293.95)] and IGR [239.79 pg/ml (191.52, 301.69)], comparable in newly diagnosed T2DM [224.68 pg/ml (187.37, 279.74)], and lower in established T2DM [100.11 pg/ml (78.50, 137.34)], compared with healthy subjects [200.23 pg/ml (160.32, 275.65)]. Spexin levels were negatively correlated with fasting plasma glucose (FPG) (r = − 0.355, P < 0.001), hemoglobin A1C (HbA1c) (r = − 0.379, P < 0.001), and HOMA2-IR (r = − 0.225, P < 0.001), and positively correlated with HOMA2-β (r = 0.245, P < 0.001) after adjusting for age, sex, and BMI. Multivariate linear regression analysis showed that established T2DM and HOMA2-β were independently associated with serum spexin levels.
Conclusions
Serum spexin levels represented as a bell-shaped curve along the glycemic continuum and is closely related with insulin secretion in humans.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Spexin, also referred to as neuro-peptide Q, is a peptide hormone encoded by SPX gene [1, 2]. The protein sequence of spexin is highly conserved from fish to mammals [3]. Spexin has gained considerable attention for its role in regulating metabolism, energy homeostasis, and pancreatic β-cell function [4].
Spexin is involved in glucose regulation. In an obese mouse model with hyperglycemia, spexin treatment not only reduces body weight but also improves glucose tolerance with reduction of insulin resistance and hemoglobin A1c (HbA1c) levels [5], suggesting that spexin might regulate insulin secretion from β cells and insulin sensitivity of peripheral tissues. Subsequent studies demonstrate that spexin increases insulin-induced glucose uptake in skeletal muscle and liver in a diet-induced insulin resistance model [6, 7]. In rodents and humans, pancreatic islets have spexin expression at the transcript and protein level [8, 9]. Spexin reduces insulin secretion response to glucose in the cultured β-cell line, isolated pig islets, rats, and pigs [10, 11]. In addition, spexin increases cell proliferation in cultured β cells [10]. Consistent with the animal studies, numerous clinical studies explore the association of spexin with diabetes. Gu et al found, for the first time, that spexin levels decrease in type 2 diabetes mellitus (T2DM) and are correlated with fasting blood glucose (FBG), HbA1c, triglyceride (TG), and low-density lipoprotein cholesterol (LDL) [9]. Karaca et al. further found that spexin levels are lower in the lean type 1 diabetes mellitus (T1DM) and are not correlated with glycemic parameters, lipids, BMI, cortisol levels, and thyroid-stimulating hormone [12]. However, the correlation between spexin and diabetes might exist only in adults. Spexin is not significantly correlated with body composition, fitness, or blood biochemical measurements in adolescents [13]. Spexin levels are also influenced by the presence of gestational diabetes [14, 15]. Furthermore, exercise training increases spexin levels of T2DM with a concomitant improvement in metabolic profile [16, 17].
Reduced β-cell function and/or mass contributes to T2DM pathogenesis [18]. However, the relationship between serum spexin levels and β-cell function is unknown. Furthermore, both first-degree relatives (FDRs) of patients with T2DM and prediabetes subjects are at high risk of diabetes [19, 20]. Thus, the present study aims to investigate the circulating spexin levels in a group of patients consisting FDRs, prediabetes, newly diagnosed T2DM, and established T2DM, and the association of spexin with β-cell function.
Methods
Study subjects
A total of 462 subjects were recruited in this study, including 106 established T2DM, 80 newly diagnosed T2DM, 115 subjects with impaired glucose regulation (IGR, including both IFG and IGT), and 161 subjects with normal glucose tolerance (NGT, including 106 FDRs and 52 healthy controls). The minimal sample size was calculated according to the report from Peng et al. [6]. We supposed that the mean of spexin levels was significantly different between healthy subjects and one of other four groups. The minimal required sample size in each group was assessed according to the difference of spexin levels between the T2DM and NGT groups (mean difference = 2.13, SD1 = 1.54, SD2 = 1.07) in the reference. The criteria of type I error and type II error were set at α = 0.05/4 = 0.125 and β = 0.10, respectively. We found that at least 12 subjects need to be observed in each group.
The diagnoses of NGT, IGR, and T2DM were based on the WHO 1999 criteria as listed in Table1
All of 106 established T2DM cases were inpatients taking oral hypoglycemic drugs and receiving insulin therapy, who were not suitable for a 75-g oral glucose tolerance test (OGTT). The other 356 subjects without history of diabetes or oral hypotensive, hypolipidemic, anti-diabetic, or other medications known to affect glycolipid metabolism were performed an OGTT. The following exclusion criteria were used in this study: acute or chronic inflammatory disease, heart, liver or renal failure, cancer, or pregnancy. The study was approved by the human research ethics committee of the hospital, which conforms to the provision of the declaration of Helsinki (as revised in Fortaleza, Brazil, October 2013). All participants provided written informed consent before participating in this study.
Clinical measurements
Participants’ weight (in light clothing) and height (without shoes) were measured, and the body mass index (BMI) was calculated as weight in kilograms divided by the square of the height in meters. All blood samples were collected from the antecubital vein following 8–12 h of overnight fasting. An oral glucose tolerance test (OGTT) was conducted on 356 participants (excluding 106 established T2DM cases), and blood samples were obtained after 2 h. After clotting, the serum samples were separated from the blood specimens via centrifugation for 15 min at 1000 g and stored in aliquots at − 80 °C until spexin analysis. The FPG and 2-h postprandial glucose (2hPG) were measured using an automatic biochemical analyzer (DXC 800; Beckman coulter, Brea, CA, USA). The fasting insulin (FINS), fasting c-peptide, and 2-h postprandial insulin (2hINS) levels were determined using a Roche cobas e602 immunoassay analyzer and electrochemiluminescence immunoassay kit (Roche diagnostics, Indianapolis, IN, USA). The HbA1c was measured using high-pressure liquid chromatography (HLC-723G7; Tosoh, Tokyo, Japan).
The homeostasis model assessment of insulin resistance (HOMA2-IR) and β-cell function (HOMA2-β) was calculated from fasting plasma glucose and insulin (c-peptide in established T2DM subjects) concentrations by using the updated HOMA2 calculator (version 2.2.4; https://www.dtu.ox.ac.uk/). The Stumvoll first (1st) phase insulin secretion indices were calculated as 2032 + 4.681 × 6.965 × FINS (mU/L) − 135.0 × 2hPG (mmol/L) + 0.995 × 6.965 × 2hINS (mU/L) + 27.99 × body mass index (kg/m2) − 269.1 × FPG (mmol/L). The Stumvoll second (2nd) phase insulin secretion indices were calculated as 277 + 0.800 × 6.965 × FINS (mU/L) − 42.79 × 2hPG (mmol/L) + 0.321 × 6.965 × 2hINS (mU/L) + 5.338 × body mass index (kg/m2).
Serum spexin measurements
Serum spexin levels were measured using an ELISA kit (Catalog # EH4349, Fine biotech co., Wuhan, Hubei, China) with an intra- and inter-assay CV of < 8% and < 10%, respectively. This assay has high sensitivity and excellent specificity for detection of spexin. No significant cross-reactivity or interference between spexin and galanin, kisspeptin, insulin, and c-peptide (all 25 ng/ml) was observed.
Statistical analysis
The physical parameters and biochemistry indices were compared among the five groups. The distribution of continuous variables was assessed using the Kolmogorov–Smirnov test. Normally distributed data are shown as the mean ± standard deviation and non-normally distributed data are expressed as medians with the interquartile ranges. The right-skewed distributed data were log10-transformed before analysis. Comparisons among five groups of participants were performed by one-way ANOVA with fisher’s post hoc test, and then, multiple comparisons were performed using least-significant-difference t test or Dunnett t test to compare the differences between non-FDRs NGT with other four groups. To adjust covariate such as age, sex, and BMI at group comparisons, analysis of covariance was performed using the general linear model procedure. Pearson’s bivariate correlation and partial correlation analyses were used to explore the correlations between spexin and the clinical parameters. Multivariate linear regression analysis was used to examine the independent association of serum spexin and other parameters. The five groups of subjects which were transformed into dummy variables, as well as the variables that correlated significantly with spexin were selected to enter regression. (According to the collinearity diagnostic in liner regression, Supplementary Tables S1 and S2, the FPG was excluded because of effect of multicollinearity.) IBM SPSS (version 25.0) was used for all statistical analyses. GraphPad prism 7 was used for figures. A two-sided P value of < 0.05 was considered as significant.
Results
Clinical characteristics of the subjects
Table 2 shows the demographic, clinical, and metabolic characteristics of the study population stratified into five groups based on glycemic status. There was no difference in any characteristics between healthy and FDRs subjects. Age, sex, and BMI were comparable among NGT and IGR subjects. Established T2DM subjects were older than other groups (all P < 0.05). Both newly diagnosed and established T2DM subjects had higher BMI (both P < 0.05) compared with the healthy subjects. In addition, OGTT-2hPG, FINS, OGTT-2hINS, and HOMA2-IR index were higher in IGR subjects than those in healthy subjects (all P < 0.05). The newly diagnosed T2DM subjects had lower indices of HOMA2-β, and Stumvoll first- and second-phase insulin secretion, compared with healthy subjects (P < 0.05). Furthermore, established T2DM subjects had a decreased HOMA2-β index compared with healthy subjects (P < 0.05) and further increased HOMA2-IR index compared with newly diagnosed T2DM subjects (P < 0.05).
Serum spexin levels
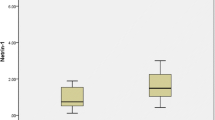
In a total of 462 subjects, serum levels of spexin ranged from 44.32 to 596.38 pg/ml (Fig. 1). There was a difference in spexin levels between male and female in all subjects (P < 0.001), and established T2DM subjects (P < 0.05) (supplementary Fig. S1). Bivariate correlation analysis found that spexin levels had a negative correlation with age (r = − 0.338, P < 0.001) in all subjects, and a positive correlation with BMI (r = 0.241, P < 0.01) in NGT subjects (Table 3). After adjustment for age and sex, serum spexin levels were positively correlated with BMI in all subjects (r = 0.131, P < 0.01) and NGT subjects (r = 0.223, P < 0.01) (Table 3).
Serum spexin concentrations [Median (min to max)] in subjects with NGT (n = 167) (including 52 healthy subjects and 109 FDRs), IGR (n = 115), newly diagnosed T2DM (n = 80), and established T2DM (n = 106). The analysis was performed using one-way ANOVA and covariance analyses. Further multiple comparisons were performed using least-significant-difference t test. The spexin levels were elevated in the FDR and IGR, and decreased in established T2DM, compared with the healthy subjects (**P < 0.001, unadjusted; $P < 0.05, $$P < 0.001, after age and sex adjustment; #P < 0.05, ##P < 0.05, after age, sex, and BMI adjustment)
Interestingly, serum spexin levels represented as a bell-shaped curve along the glycemic continuum (Fig. 1). Compared to healthy subjects [200.23 pg/ml (160.32, 275.65)], spexin levels increased in FDRs [235.53 pg/ml (185.28, 293.95)] and IGR [239.79 pg/ml (191.52, 301.69)] subjects, went to the similar level in newly diagnosed T2DM subjects [224.68 pg/ml (187.37, 279.74)], and then decreased significantly in established T2DM subjects [100.11 pg/ml (78.50, 137.34)] (P < 0.001). After age and sex adjustment, the spexin levels were still higher in subjects of FDRs (P < 0.05) and IGT (P < 0.05), and lower in established T2DM subjects (P < 0.001), compared to those of healthy subjects. After further adjustment for age, sex, and BMI, the spexin levels remained higher (P < 0.05) in IGR subjects, and lower in established T2DM subjects (P < 0.001), while it was not significantly higher in FDRs subjects, compared with those of healthy subjects.
Correlation between serum spexin levels and glucose-related variables
A bivariate correlation analysis was conducted between spexin levels and variables related to glucose metabolism in a total of 462 subjects (Table 3 and Fig. 2). The spexin levels were negatively correlated with FPG (r = − 0.444, P < 0.001), HbA1c (r = − 0.495, P < 0.001), and HOMA2-IR (r = − 0.232, P < 0.001) in all subjects. Conversely, the spexin levels were positively correlated with HOMA2-β (r = 0.343, P < 0.001). A partial correlation analysis showed that the spexin levels were still negatively correlated with FPG (r = − 0.355, P < 0.001), HbA1c (r = − 0.379, P < 0.001), and HOMA2-IR (r = − 0.225, P < 0.001), and positively correlated with HOMA2-β (r = 0.245, P < 0.001) after adjusting for age, sex, and BMI.
Correlation between serum spexin levels and glucose-related variables was analyzed using Pearson’s bivariate correlation. A Positive correlations of spexin levels (lg transformed) with HOMA2-β (lg transformed) in 462 subjects. B–C Positive correlation of spexin levels (lg transformed) with HOMA2-β (lg transformed) (B) HOMA2-IR (lg transformed) (C) in established diabetes subjects. D–F Positive correlation of spexin levels (lg transformed) with HOMA2-β (lg transformed) (D), Stumvoll first- E and second-phase F insulin secretion indices in NGT subjects
Furthermore, bivariate correlation analysis in each group was conducted. In NGT subjects, spexin levels were positively correlated with HOMA2-β, Stumvoll first- and second-phase insulin secretion indices. This correlation was still significant after adjusting for age and sex. In established T2DM subjects, spexin levels were positively correlated with fasting c-peptide, HOMA2-IR, and HOMA2-β. This correlation remained significant (P < 0.001) after age, sex, and BMI adjustments.
Independent association between serum spexin levels and indices of β-cell function
In correlation matrix analysis, we found a high correlation between FPG and HbA1c (Supplementary Table S1). A multivariate linear regression analysis was performed to determine which indices were independently associated with spexin levels (Table 3). The analysis involved glycemic status and the variables significantly correlated with serum spexin, including age, sex, BMI, FINS, HbA1c, HOMA2-IR, and HOMA2-β. The tolerance and variance inflation factors (VIF) of variables demonstrated that there was little multicollinearity in all variables (Supplementary Table S2). Established diabetes and HOMA2-β were found to be independently associated with serum spexin levels, while FDRs or IGR were not independently associated with elevated spexin levels after HOMA2-β adjustment.
Discussion
In this study, we described a profile of serum spexin levels along the glycemic continuum, from normoglycemia to T2DM. We demonstrated that serum spexin levels were higher in subjects of FDRs and IGR, while lower in subjects of established T2DM, compared with healthy subjects. Correlation analysis showed that serum spexin was correlated with variables related to glucose metabolism. Most importantly, serum spexin was independently associated with β-cell function and T2DM progression on fully adjusted analyses. Thus, serum spexin could provide insight into pathophysiology of diabetes, likely through its implications for β-cell function.
Obesity is a triggering factor for diabetes associated with insulin resistance [21]. A large body of evidence suggests a significant association between spexin levels and body weight status. Spexin-knockout zebrafish show higher food intake than wild-type controls and have altered expression of major appetite-regulating neuro-peptides [22]. Consistently, spexin injection induces anorexia in fish and reduced body weight gain in mice [22,23,24]. Clinical studies further show that circulating spexin levels are lower in obese than non-obese individuals in prepubertal children, adolescents, and adults [24,25,26,27]. Circulating spexin levels increase following weight loss [28]. Thus, in our study, we have fully considered the potential effects of body weight on these associations by adjusting the BMI during the correlation and regression analysis.
We found that spexin levels decreased in established T2DM which is in line with previous studies [9]. However, there is a disparity in the spexin levels in newly diagnosed T2DM [6]. We made a comparison and found that the characteristics of T2DM subjects in Gu’s study were more like those of the established T2DM in our study (shown in Table 1), as obtained from the values of HbA1c, FBG, and HOMA-IR. In our study, the subjects of the newly diagnosed diabetes were recognized from community screening and most of them had no obvious symptom of diabetes. So, one possibility is our newly diagnosed T2DM were freshly onset diabetes. In this regard, this study precisely described the spexin levels of different glycemic status toward T2DM. In addition, some subjects of newly diagnosed T2DM in our study had a family history of diabetes. We found that FDR had higher spexin levels than healthy subjects (shown in Fig. 1). Thus, another possibility is that the genetic background of our newly diagnosed T2DM might contribute to elevating spexin concentration of newly diagnosed diabetes to a level comparable to that of healthy subjects (Table 4).
The mRNA or protein expression levels of spexin in human [9], pigs [10], rats [8], mice [29], and goldfish [30] have been analyzed, and the results show a wild range of tissue distribution of spexin. In rodents and humans, spexin has been detected in tissues, including hypothalamus, hippocampus, cerebral cortex, stomach, small intestine, pancreas, liver, kidney, thyroid, ovary, testis, and adrenal gland [8, 9]. Subsequent studies are trying to clarify the tissue specific enrichment of spexin. Analyses based on integration of various omics technologies show that mRNA expression of spexin is highest in brain, pancreas (http://tiger.bsc.es), and adipose (https://www.proteinatlas.org) [31]. Immunohistochemistry analysis further shows that spexin has a colocalization with insulin in pancreas, suggesting that islet β cells produce spexin [10]. Thus, it can be speculated that the circulating levels of spexin are closely related with the expression of spexin in adipose and/or islet β cells. Actually, in obese human subjects, the circulating levels of spexin decrease and the mRNA levels of spexin are down-regulated in the omental and subcutaneous fat [32, 33]. In this study, we found that serum spexin was independently associated with T2DM. It is possible that the decreased circulating levels of spexin in established T2DM are due to the loss of β-cell mass in these subjects.
This study, for the first time, reported the clinical relevance of serum spexin with β-cell function. The indices of HOMA2 model and Stumvoll are used to measure β-cell function. HOMA is calculated using fasting blood concentrations of glucose and insulin to estimate the steady-state β-cell function. HOMA2 indices are more accurate representation of metabolic process than HOMA1 indices because calculation of HOMA2 indices has considered some physiological adjustments based on HOMA1 model [34]. Stumvoll index is assessed as an oral glucose tolerance test-based measure of β-cell function. Compared with hyperglycemic clamp method (the gold standard), Stumvoll index is a simpler, easier-to-use, and relatively accurate measurement of β-cell function in clinical and large epidemiological studies [35]. We found that spexin levels were independently and positively associated with HOMA-β. In NGT subjects, we also found a positive correlation between spexin levels and Stumvoll first- and second-phase insulin secretion.
There are several limitations of this study, including a relatively limited sample size. One limitation is that this study only detected the spexin levels under fasting condition. The spexin levels might be influenced by glucose ingestion during OGTT [9, 13, 36]. The association between spexin and blood glucose under different glycemic status should be measured in the future. Another limitation is the study that did not address the cause–effect relationship between spexin and β-cell dysfunction as well as diabetes progression. Further prospective studies are warranted to determine whether decreased serum spexin is causally related to obesity and diabetes or a concomitant phenomenon of these diseases.
In summary, we infer that spexin may be an important substance in the development of hyperglycemia and islet β-cell functional failure in patients with T2DM. Therefore, future studies will be critical for understanding the relationship between spexin and T2DM, and studies of spexin should focus on the effect of spexin on β-cell function.
References
Mirabeau O, Perlas E, Severini C et al (2007) Identification of novel peptide hormones in the human proteome by hidden Markov model screening. Genome Res 17:320–327
Wan B, Wang XR, Zhou YB, Zhang X, Huo K, Han ZG (2009) C12ORF39, a novel secreted protein with a typical amidation processing signal. Biosci Rep 30:1–10
Kim DK, Yun S, Son GH, et al (2014) Coevolution of the spexin/galanin/kisspeptin family: spexin activates galanin receptor type II and III. Endocrinology 155:1864–1873
Mills EG, Izzi-Engbeaya C, Abbara A, Comninos AN, Dhillo WS (2021) Functions of galanin, spexin and kisspeptin in metabolism, mood and behaviour. Nat Rev Endocrinol 17:97–113
Ge JF, Walewski JL, Anglade D, Berk PD (2016) Regulation of hepatocellular fatty acid uptake in mouse models of fatty liver disease with and without functional leptin signaling: roles of NfKB and SREBP-1C and the effects of spexin. Semin Liver Dis 36:360–372
Gu L, Ding X, Wang Y et al (2019) Spexin alleviates insulin resistance and inhibits hepatic gluconeogenesis via the FoxO1/PGC-1alpha pathway in high-fat-diet-induced rats and insulin resistant cells. Int J Biol Sci 15:2815–2829
Yu M, Wang M, Han S et al (2022) Spexin ameliorates skeletal muscle insulin resistance through activation of GAL2 receptor. Eur J Pharmacol 917:174731
Porzionato A, Rucinski M, Macchi V, Stecco C, Malendowicz LK, De Caro R (2010) Spexin expression in normal rat tissues. J Histochem Cytochem 58:825–837
Gu L, Ma Y, Gu M et al (2015) Spexin peptide is expressed in human endocrine and epithelial tissues and reduced after glucose load in type 2 diabetes. Peptides 71:232–239
Sassek M, Kolodziejski PA, Szczepankiewicz D, Pruszynska-Oszmalek E (2019) Spexin in the physiology of pancreatic islets-mutual interactions with insulin. Endocrine 63:513–519
Sassek M, Kolodziejski PA, Strowski MZ, Nogowski L, Nowak KW, Mackowiak P (2018) Spexin modulates functions of rat endocrine pancreatic cells. Pancreas 47:904–909
Karaca A, Bakar-Ates F, Ersoz-Gulcelik N (2018) Decreased spexin levels in patients with type 1 and type 2 diabetes. Med Princ Pract 27:549–554
Hodges SK, Teague AM, Dasari PS, Short KR (2018) Effect of obesity and type 2 diabetes, and glucose ingestion on circulating spexin concentration in adolescents. Pediatr Diabetes 19:212–216
Al-Daghri NM, Wani K, Yakout SM et al (2019) Favorable changes in fasting glucose in a 6-month self-monitored lifestyle modification programme inversely affects spexin levels in females with prediabetes. Sci Rep 9:9454
Akbas M, Koyuncu FM, Oludag Mete T, Taneli F, Ozdemir H, Yilmaz O (2019) Serum levels of spexin are increased in the third trimester pregnancy with gestational diabetes mellitus. Gynecol Endocrinol 35:1050–1053
Khadir A, Kavalakatt S, Madhu D et al (2020) Spexin as an indicator of beneficial effects of exercise in human obesity and diabetes. Sci Rep 10:10635
Mohammadi A, Bijeh N, Moazzami M, Kazem K, Rahimi N (2022) Effect of exercise training on spexin level, appetite, lipid accumulation product, visceral adiposity index, and body composition in adults with type 2 diabetes. Biol Res Nurs 24:152–162
Prentki M, Nolan CJ (2006) Islet beta cell failure in type 2 diabetes. J Clin Invest 116:1802–1812
Tabak AG, Herder C, Rathmann W, Brunner EJ, Kivimaki M (2012) Prediabetes: a high-risk state for diabetes development. Lancet 379:2279–2290
Poulsen P, Kyvik KO, Vaag A, Beck-Nielsen H (1999) Heritability of type II (non-insulin-dependent) diabetes mellitus and abnormal glucose tolerance–a population-based twin study. Diabetologia 42:139–145
Kahn SE, Hull RL, Utzschneider KM (2006) Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 444:840–846
Zheng B, Li S, Liu Y et al (2017) Spexin suppress food intake in zebrafish: evidence from gene knockout study. Sci Rep 7:14643
Jeong B, Kim KK, Lee TH et al (2022) Spexin regulates hypothalamic leptin action on feeding behavior. Biomol 12(2):236
Walewski JL, Ge F, Lobdell HT et al (2014) Spexin is a novel human peptide that reduces adipocyte uptake of long chain fatty acids and causes weight loss in rodents with diet-induced obesity. Obesity (Silver Spring) 22:1643–1652
Lin CY, Huang T, Zhao L et al (2018) Circulating spexin levels negatively correlate with age bmi, fasting glucose, and triglycerides in healthy adult women,. J Endocr Soc 2:409–419
Kolodziejski PA, Pruszynska-Oszmalek E, Korek E et al (2018) Serum levels of spexin and kisspeptin negatively correlate with obesity and insulin resistance in women. Physiol Res 67:45–56
Kumar S, Hossain J, Nader N, Aguirre R, Sriram S, Balagopal PB (2016) Decreased circulating levels of spexin in obese children. J Clin Endocrinol Metab 101:2931–2936
Kumar S, Hossain MJ, Javed A, Kullo IJ, Balagopal PB (2018) Relationship of circulating spexin with markers of cardiovascular disease: a pilot study in adolescents with obesity. Pediatr Obes 13:374–380
Sonmez K, Zaveri NT, Kerman IA et al (2009) Evolutionary sequence modeling for discovery of peptide hormones. PLoS Comput Biol 5:e1000258
Liu Y, Li S, Qi X et al (2013) A novel neuropeptide in suppressing luteinizing hormone release in goldfish, Carassius auratus. Mol Cell Endocrinol 374:65–72
Alonso L, Piron A, Moran I et al (2021) TIGER: The gene expression regulatory variation landscape of human pancreatic islets. Cell Rep 37:109807
Walewski JL, Ge F, Gagner M et al (2010) Adipocyte accumulation of long-chain fatty acids in obesity is multifactorial, resulting from increased fatty acid uptake and decreased activity of genes involved in fat utilization. Obes Surg 20:93–107
Bitarafan V, Esteghamati A, Azam K, Yosaee S, Djafarian K (2019) Comparing serum concentration of spexin among patients with metabolic syndrome, healthy overweight/obese, and normal-weight individuals. Med J Islam Repub Iran 33:93
Song YS, Hwang YC, Ahn HY, Park CY (2016) Comparison of the usefulness of the updated homeostasis model assessment (HOMA2) with the original HOMA1 in the prediction of type 2 diabetes mellitus in Koreans. Diabetes Metab J 40:318–325
Stumvoll M, Mitrakou A, Pimenta W et al (2000) Use of the oral glucose tolerance test to assess insulin release and insulin sensitivity. Diabetes Care 23:295–301
Wu H, Lin F, Chen H et al (2016) Ya-fish (Schizothorax prenanti) spexin: identification, tissue distribution and mRNA expression responses to periprandial and fasting. Fish Physiol Biochem 42:39–49
Funding
This study was funded by the national nature science foundation of China (Grant Numbers 81200622).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Managed by Massimo Federici.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dai, J., Ni, Y., Wu, D. et al. Circulating spexin levels are influenced by the glycemic status and correlated with pancreatic β-cell function in Chinese subjects. Acta Diabetol 60, 305–313 (2023). https://doi.org/10.1007/s00592-022-02010-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-022-02010-x