Abstract
Objective
This study aimed to investigate the concentration of Wingless-type (Wnt)-inducible signaling pathway protein-1 (WISP1) in the serum, and its expression in the abdominal subcutaneous adipose tissue (SAT), and placenta of women with gestational diabetes mellitus (GDM).
Methods
A total of 69 patients with GDM and 71 pregnant women with normal glucose tolerance (NGT, control) were recruited. Carbohydrate metabolism, alanine aminotransferase (ALT), lipid profiles, thyroid function, interleukin-6 (IL-6), and WISP1 levels were assessed. Fasting sera were collected between 25 and 30 weeks of gestation. Tissues of placenta and abdominal SAT samples were obtained from 24 women who had undergone cesarean section and were divided into a GDM group and a control group. Reverse transcription polymerase chain reaction (RT-PCR) and western blot were used to detect the WISP1 expression.
Results
The serum WISP1 concentrations were higher in the GDM group than in the control group (p < 0.01) and positively associated with body mass index (BMI), fasting glucose, fasting insulin, HOMA-insulin resistance (HOMA-IR), and IL-6 levels. BMI, fasting glucose, and HOMA-IR independently and positively predicted WISP1 levels. Further, WISP1 mRNA and protein expression were higher in tissues from the placenta and abdominal SAT from the GDM group (p < 0.01).
Conclusions
WISP1 may be an important adipokine in modulating carbohydrate metabolism in women with GDM.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gestational diabetes mellitus (GDM) is a common condition that occurs in up to 16% of pregnant women in high-risk populations [1]. Hyperglycemia during pregnancy can result in adverse pregnancy outcomes in both fetus and the mother. Macrosomia, premature rupture of membranes, and preeclampsia are more common in patients with GDM [2]. A good mental health status as well as regular exercises and a good diet will help the diabetic mother to achieve good glycemic control [3,4,5]. In addition, women with previously diagnosed GDM are approximately 30% more likely to develop type 2 diabetes within 10 years [6]. It is worth noting that the incidence of GDM has increased rapidly over the past decade as the global rate of obesity has increased [7].
The pathophysiology of GDM is not fully understood currently. Accumulating evidence indicates that the maternal adipose tissue plays an important role in pathogenesis of GDM [8]. Maternal obesity is considered to be one of the important variable risk factors. Recently, the role of inflammation as a regulator of metabolic disorder in obesity has received increasing attention [9]. Adipose tissue not only stores energy, but also modulates the endocrine function by secreting molecules known as adipokines or adipocytokines. Dysfunction of adipose tissue can cause low grade chronic inflammation and insulin resistance [10]. The risk of GDM is higher in overweight and obese women, and this may be partly related to adipokines released from the adipose tissue [11].
The placenta is not only an additional source of systemic cytokines during pregnancy, but also a target of cytokine action [12]. Studies have confirmed that chronic inflammation in the placenta tissue during pregnancy with obesity and GDM play an important role in determining the fetal environment [13, 14]. Moreover, maternal obesity and GDM have been related to changes in maternal–fetal transport of nutrients [15].
Wingless-type (Wnt)-inducible signaling pathway protein-1(WISP1) was recently identified as a novel adipokine. The expression of WISP1 correlates with index of insulin resistance and inflammation. In addition, treatment of macrophages with WISP1 may lead to pro-inflammatory responses [16]. Barchetta et al. [17] indicated that WISP1 expression increased in obese subjects and was linked with higher levels of C-reactive protein and interleukin (IL)-8 in adipocytes. Wang et al. [18] found that the level of serum WISP1 was correlated to increased adiponectin, leptin, and IL-18 expression, as well as with markers of insulin resistance. They confirmed that higher WISP1 expression was linked with increased adipocyte inflammation. In another study, Jung et al. [19] found that treatment with WISP1 significantly induced inflammation in hepatocytes and in the skeletal muscle cells of mice. These studies suggest that WISP1 may play an active part in the pathogenesis of chronic inflammation-related diseases such as obesity.
WISP1 mRNA expression has also been detected in the placenta and pancreas [20]. However, there is a lack of data regarding expression of WISP1 in adipose tissue and the placenta during pregnancy, particularly under conditions such as GDM. In this study, we measured the levels of WISP1 in serum and changes of WISP1 expression in the abdominal subcutaneous adipose tissue (SAT) and placenta of patients with GDM and pregnant women with NGT.
Material and methods
Study design and subjects
The subjects were divided into a GDM group and a normal glucose tolerance (NGT, control) group. Inclusion criteria for pregnant women include the following: (1) women who had singleton pregnancies; (2) women who had no current regular medications; and (3) women without history of glucose intolerance. Exclusion criteria are patients with adverse medical conditions (pregnancy-induced hypertension, preeclampsia, premature membrane rupture). GDM was diagnosed according to the criteria of the International Association of Diabetic Pregnancy Study Group (IADPSG). Oral glucose tolerance test (OGTT) was conducted using 75 g glucose loading at 24–28 weeks, and diagnosis of GDM was made by either a fasting venous glucose level of ≥ 5.1 mmol/L, or 1 h ≥ 10.0 mmol/L, or 2 h ≥ 8.5 mmol/L [21].
Study 1 population: effect of GDM on circulating maternal WISP1 levels
A total of 140 serum samples were collected from Chinese pregnant women with regular follow-up between 25 and 30 weeks of gestation visiting the outpatient department of Shengjing Hospital of China Medical University. Body mass index (BMI) was assessed at the day of inclusion. Whole blood samples were immediately centrifuged at 1,000 rpm for 5 min, and then serum samples were collected and stored at − 80 ℃ for further analysis.
Fasting plasma glucose (FPG) was detected by an automated glucose oxidase method using Olympus AU5800 (Tokyo, Japan). Fasting serum insulin (FIN) levels were determined using chemiluminescence assays (Huamei Biotech, Wuhan, China). Hemoglobin A1c (HbA1c) level was examined by high performance liquid chromatography (HPLC) using D-10 Hemoglobin Testing System (Bio-Rad, Shanghai, China). Blood triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), and alanine aminotransferase (ALT) levels were determined by routine methods with an Olympus AU 5400 Automatic Analyzer (Tokyo, Japan). Thyroid function was detected by chemiluminescence immunoassay with commercially available diagnostic kits (Mingde Biotech, Wuhan, China). Insulin resistance was expressed by the HOMA-insulin resistance (HOMA-IR) index (HOMA-IR = FPG (mmol/L) × FIN (μU/mL) / 22.5). The concentrations of serum WISP1 and IL-6 were evaluated with the enzyme-linked immunosorbent assay (ELISA) method (Cloud-Clone Corp, Wuhan, China) according to the manufacturer’s instructions.
Study 2 population: effect of GDM on adipose tissue and placental WISP1 gene expression
All participants in this study signed written informed consent. Pregnant women were selected for cesarean section in Shengjing Hospital of China Medical University, including 12 cases in GDM group and 12 cases in NGT group, respectively. All pregnant women in Study 2 have clinical indications to perform cesarean section. The maternal side of the placenta tissue and abdominal SAT (~ 1 cm3) were collected from the pregnant woman shortly after delivery.
RNA extraction and quantitative RT-PCR (qRT-PCR)
Total RNA was isolated from the placental tissue and abdominal SAT with Trizol reagent (Ambion, TX, USA). Then, 1 µg of total RNA was converted to cDNA using a cDNA synthesis kit (Transgen, Beijing, China). The cDNA was diluted tenfold and 2 µL was taken for RT-PCR using an Exicycler 96 (Bioneer, South Korea) and 100 nM of primers. The samples were incubated in the Exicycler 96 for 40 PCR cycles according to the manufacturer’s instructions. The primer sequences were shown in Table 1.
Western blot
Total protein of placental tissue and abdominal SAT was extracted with radioimmunoprecipitation assay lysis buffer. Protein concentration was determined using the bicinchoninic acid (BCA) protein assay (Beyotime, Shanghai, China). Protein samples were separated by 10% sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis and transferred onto polyvinylidene fluoride (PVDF) membranes. After 1 h of blocking with 5% non-fat milk at 2 5 ℃, the membranes were incubated with anti-WISP1 polyclonal antibody (1:1000; Boster, Wuhan, China) overnight at 4 ℃. The membranes were then incubated with horseradish peroxidase-conjugated secondary antibodies (1:5000; Wanleibio, Shenyang, China) for 2 h at 25 ℃. The immune complexes were observed with enhanced chemiluminescence reagent kit. Band strength was quantified using Gel-Pro-Analyzer software, and data were expressed as a ratio of the WISP1 and GAPDH integral optic density (IOD) values.
Statistical analysis
Data are presented as the mean ± standard deviation (SD) and Student’s t tests were used to analyze two sample comparisons. Univariate and multivariate regression analyses were utilized to identify independent variables of WISP1. The level of significance was considered at p < 0.05. All statistical analyses were performed using SPSS 13.0 program (SPSS, Chicago, IL, USA).
Results
Clinical and demographic characteristics of the subjects in Study 1
A total of 140 subjects were enrolled, including 69 patients in the GDM group and 71 in the control group. The clinical and biochemical characteristics of the two groups are shown in Table 2. Compared to the control group, GDM women had higher fasting glucose levels (5.45 ± 1.37 vs. 4.82 ± 1.41, p = 0.008), fasting insulin levels (13.89 ± 8.07 vs. 10.23 ± 7.64, p = 0.007), HbA1c (5.32 ± 0.84% vs. 4.97 ± 0.73%, p = 0.009), HOMA-IR (3.63 ± 1.34 vs. 2.42 ± 0.97, p < 0.001), and IL-6 (2.25 ± 0.51 vs. 1.92 ± 0.45, p < 0.001) values. However, there was no difference with respect to age, BMI, ALT, FT3, FT4, sTSH, TG, LDL-C, or HDL-C levels between the two groups.
Serum WISP1 concentration was higher in the GDM group
As shown in Fig. 1, the serum WISP1 concentrations were higher in the GDM group compared with that in the control group (2.29 ± 0.35 vs. 1.40 ± 0.30, respectively, p < 0.01).
Correlation between serum WISP1 levels and clinical and demographic characteristics
By using bivariate correlation analyses, we found that WISP1 was positively correlated with BMI (r = 0.332, p < 0.001), fasting glucose (r = 0.306, p < 0.001), fasting insulin (r = 0.246, p = 0.003), HOMA-IR (r = 0.296, p < 0.001), and IL-6 (r = 0.182, p < 0.031). In contrast, circulating WISP1 levels were not significantly correlated with age, HbA1c, FT3, FT4, sTSH, ALT, TG, HDL-C, or LDL-C levels (Table 3). Multivariate regression analysis demonstrated that BMI (β = 0.142, p < 0.001), fasting glucose (β = 0.216, p = 0.003), and HOMA-IR (β = 0.125, p = 0.005) independently and positively predicted serum WISP1 levels (Table 3).
Demographics of the subjects at cesarean section in Study 2
The demographics of the Study 2 population are shown in Table 4: there were no statistical differences between the control and GDM groups (p > 0.05).
WISP1 expression was upregulated in the placenta and SAT of GDM group
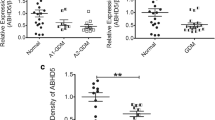
As shown in Fig. 2, compared to the control group, the WISP1 mRNA expression was significantly upregulated in the maternal placenta and abdominal SAT of the GDM group (p < 0.01). Similarly, the WISP1 protein expression was significantly upregulated in the maternal placenta (0.532 ± 0.162 vs. 0.323 ± 0.128, p < 0.01) and abdominal SAT (0.398 ± 0.032 vs. 0.201 ± 0.053, p < 0.01) of the GDM group than that in the control group (Fig. 3).
Relative WISP1 mRNA levels in the placentae and SAT of the GDM and control groups. SAT: subcutaneous adipose tissue. A and B Quantification of the differences in the WISP1 mRNA levels in the placenta and SAT between the GDM and control groups (n = 12 for each of the placenta and SAT sample per group). GAPDH served as an internal control. *p < 0.01, compared with the control group
Western blot analysis of the WISP1 protein levels in the placentae and SAT of the GDM and control groups. SAT: subcutaneous adipose tissue. A and B Quantification of the differences in the WISP1 protein levels in the placenta and SAT between the GDM and control groups (n = 12 for each of the placenta and SAT sample per group). GAPDH served as an internal control. *p < 0.01, compared with the control group
Discussion
In this study, we analyzed WISP1 levels in a population of women with GDM and a group of age and gestational week-matched NGT pregnant controls. Our results revealed that circulating WISP1 level was higher in GDM patients and correlated with fasting glucose and insulin levels, indicating that WISP1 is positively correlated with glucose intolerance.
WISP1 is thought to contribute to inflammatory events in metabolic diseases and insulin resistance through different pathways [22]. Barchetta et al. [17] found that WISP1 level was increased in obese individuals and was directly related to adiposity. A recent study on GDM in China showed that WISP1 level was positively correlated with fasting blood glucose, ALT, and systolic blood pressure and was negatively correlated with HDL-C [23]. Another study on GDM in the USA showed that WISP1 was positively correlated with BMI, fasting blood glucose and fasting insulin, HOMA-IR, and TG [24]. Our findings are consistent with these studies with respect to the association of WISP1 and fasting glucose and fasting insulin levels. The discrepancies between our study and previous researches may be related to differences in the sample size, study population, and GDM severity.
WISP1 is a recently discovered adipokine that is secreted by adipocytes and stimulates cytokine responses in macrophages. The release of WISP1 was significantly increased during adipocyte differentiation; thus, fat cells are probably a main source of WISP1 released into the blood circulation [16]. Our study supports this hypothesis as we found that SAT from women with GDM displayed significantly higher WISP1 expression and release. The mechanisms underlying increased circulating WISP1 levels in women with GDM may be related to unknown factors, while the relationship between WISP1 and deterioration of glucose metabolism and insulin sensitivity remains to be elucidated. It is plausible that the increased WISP1 levels with adipose tissue inflammation may induce adipose tissue remodeling and aberrant fibrogenesis, which, in turn, may be responsible for the loss of adipose tissue function, insulin resistance, and downstream consequences [25, 26].
Our data are also consistent with the notion that WISP1 may interfere with insulin signaling in insulin target tissues. For instance, WISP1 can inhibit insulin-mediated protein kinase B (Akt) phosphorylation, which regulates multiple important aspects of glucose metabolism [27, 28]. Contrary to this, during pancreatic regeneration, WISP1 is one of several overexpressed genes, suggesting that WISP1 may exert reparative effects during GDM [29]. Moreover, during oxidative stress WISP1 can upregulate phosphoinositide 3-kinase (PI3-K) and Akt to protect against DNA damage [30]. These studies suggest that WISP1 may play dual roles in modulating glucose homeostasis, and further studies are needed to elucidate its underlying pathophysiological mechanisms.
Our study found that WISP1 expression also significantly increased in the placentas of the GDM group compared to that in the control group. As an endocrine organ, the placenta can secrete many cytokines through adipocytes, which are well known to be essential for maintaining a normal pregnancy [31]. The placenta plays an important role in the mediation of chronic inflammation response in women with GDM. For instance, in the adaptive response to obesity during pregnancy, the function and structure of the placenta may be changed, and some adipocytokines are also co-secreted by the placenta [32]. Numerous researches have indicated an increase in inflammatory mediators in the placentas of women with obesity-related GDM [33, 34]. Some changes found in the placenta when maternal obesity may be adaptations to limit the fetal exposure to inflammation and oxidative stress [35]; however, inflammatory mediators may also work in utero, causing the fetal adipose tissue, skeletal muscle, and liver to develop insulin resistance in later life [36]. Recently, in a pregnant rat model with a predisposition to obesity, maternal obesity was shown to decrease placental efficiency and cause significant placental lipid accumulation by aberrantly activating placental Wnt signaling, indicating that the placentas of obese rats were less effective at supporting fetal development compared to those of lean rats. Wnt signaling can also contribute to obesity-associated metabolic disorder by increasing placental inflammation [37]. As a downstream target of Wnt signaling, WISP1 has been shown to impact multiple other signal transduction pathways to affect cellular injury and cellular proliferation [38].
To our knowledge, this is the first study to compare the expression of WISP1 in the abdominal SAT and placental tissue in individuals with GDM and controls. There are two major limitations of this study. Firstly, since this is a descriptive study, further studies are needed to fully elucidate the pathophysiological processes underlying our observations and their possible clinical implications. Secondly, the sample size in this study was limited; therefore, similar researches with a larger cohort are needed to confirm our observations.
Conclusion
In conclusion, this study presents novel data showing increased plasma WISP1 levels and increased WISP1 expression in the abdominal SAT and placenta of women with GDM. Our current findings may support the hypothesis that WISP1 plays a role in the pathogenesis of GDM. Although the physiological and pathological significance of these findings remains unclear, they may explain a mechanism by which insulin resistance occurs in pregnant women with GDM.
Data availability
The datasets generated and analyzed during the present study are available from the corresponding author on reasonable request.
References
Association AD. Classification and diagnosis of diabetes. Diabetes Care. 2016;39(Suppl 1):S13-22.
Poulakos P, Mintziori G, Tsirou E, Taousani E, Savvaki D, Harizopoulou V, et al. Comments on gestational diabetes mellitus: from pathophysiology to clinical practice. Hormones (Athens, Greece). 2015;14(3):335–44.
Sook LW, Sablihan NI, Ismail S, Devaraj NK, Mooi CS. Factors associated with the level of physical activities among non-academic staffs in the faculty of medicine and health sciences of a public university in Selangor. Malaysia Mal J Med Health Sci. 2019;15(2):47–55.
Lee KW, Ching SM, Hoo FK, Ramachandran V, Chong SC, Tusimin M, et al. Neonatal outcomes and its association among gestational diabetes mellitus with and without depression, anxiety and stress symptoms in Malaysia: a cross-sectional study. Midwifery. 2020;81:102586.
Devaraj NK, Mohamed M, Hussein N. Prevalence, factors influencing and knowledge about adherence to lipid-lowering therapy among hyperlipidemia patients. Med J Malaysia. 2017;72(3):157–64.
Kintiraki E, Goulis DG, Mameletzi S, Kasmas S, Athanasiadis A, Assimakopoulos E, et al. Large- and small-for-gestational-age neonates born by women with gestational diabetes mellitus diagnosed by the new IADPSG criteria: a case-control study of 289 patients and 1108 controls. Exp Clin Endocrinol Diabetes. 2013;121(5):262–5.
Stuebe AM, Landon MB, Lai Y, Spong CY, Carpenter MW, Ramin SM, et al. Maternal BMI, glucose tolerance, and adverse pregnancy outcomes. Am J Obstet Gynecol. 2012;207(1):62.e1-7.
Tsiotra PC, Halvatsiotis P, Patsouras K, Maratou E, Salamalekis G, Raptis SA, et al. Circulating adipokines and mRNA expression in adipose tissue and the placenta in women with gestational diabetes mellitus. Peptides. 2018;101:157–66.
Ben-Haroush A, Yogev Y, Hod M. Epidemiology of gestational diabetes mellitus and its association with type 2 diabetes. Diabet Med. 2004;21(2):103–13.
Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11(2):85–97.
Jung UJ, Choi MS. Obesity and its metabolic complications: the role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int J Mol Sci. 2014;15(4):6184–223.
Hauguel-de Mouzon S, Guerre-Millo M. The placenta cytokine network and inflammatory signals. Placenta. 2006;27(8):794–8.
Aye IL, Lager S, Ramirez VI, Gaccioli F, Dudley DJ, Jansson T, et al. Increasing maternal body mass index is associated with systemic inflammation in the mother and the activation of distinct placental inflammatory pathways. Biol Reprod. 2014;90(6):129.
Kleiblova P, Dostalova I, Bartlova M, Lacinova Z, Ticha I, Krejci V, et al. Expression of adipokines and estrogen receptors in adipose tissue and placenta of patients with gestational diabetes mellitus. Mol Cell Endocrinol. 2010;314(1):150–6.
Jansson N, Rosario FJ, Gaccioli F, Lager S, Jones HN, Roos S, et al. Activation of placental mTOR signaling and amino acid transporters in obese women giving birth to large babies. J Clin Endocrinol Metab. 2013;98(1):105–13.
Murahovschi V, Pivovarova O, Ilkavets I, Dmitrieva RM, Docke S, Keyhani-Nejad F, et al. WISP1 is a novel adipokine linked to inflammation in obesity. Diabetes. 2015;64(3):856–66.
Barchetta I, Cimini FA, Capoccia D, De Gioannis R, Porzia A, Mainiero F, et al. WISP1 is a marker of systemic and adipose tissue inflammation in dysmetabolic subjects with or without type 2 diabetes. J Endocr Soc. 2017;1(6):660–70.
Wang AR, Yan XQ, Zhang C, Du CQ, Long WJ, Zhan D, et al. Characterization of Wnt1-inducible signaling pathway protein-1 in obese children and adolescents. Curr Med Sci. 2018;38(5):868–74.
Jung TW, Kang C, Goh J, Chae SI, Kim HC, Lee TJ, et al. WISP1 promotes non-alcoholic fatty liver disease and skeletal muscle insulin resistance via TLR4/JNK signaling. J Cell Physiol. 2018;233(8):6077–87.
Maiese K. WISP1: Clinical insights for a proliferative and restorative member of the CCN family. Curr Neurovasc Res. 2014;11(4):378–89.
Association AD. Standards of medical care in diabetes–2011. Diabetes Care. 2011;34(Suppl 1):S11-61.
Palsgaard J, Emanuelli B, Winnay JN, Sumara G, Karsenty G, Kahn CR. Cross-talk between insulin and Wnt signaling in preadipocytes: role of Wnt co-receptor low density lipoprotein receptor-related protein-5 (LRP5). J Biol Chem. 2012;287(15):12016–26.
Liu L, Hu J, Yang L, Wang N, Liu Y, Wei X. Association of WISP1/CCN4 with Risk of overweight and gestational diabetes mellitus in Chinese pregnant women. Dis Markers. 2020;2020:4934206.
Sahin Ersoy G, Altun Ensari T, Subas S, Giray B, Simsek EE, Cevik O. WISP1 is a novel adipokine linked to metabolic parameters in gestational diabetes mellitus. J Matern Fetal Neonatal Med. 2017;30(8):942–6.
Morrison MC, Kleemann R. Role of macrophage migration inhibitory factor in obesity, insulin resistance, type 2 diabetes, and associated hepatic co-morbidities: a comprehensive review of human and rodent studies. Front Immunol. 2015;6:308.
Pellegrinelli V, Carobbio S, Vidal-Puig A. Adipose tissue plasticity: how fat depots respond differently to pathophysiological cues. Diabetologia. 2016;59(6):1075–88.
Venkatesan B, Prabhu SD, Venkatachalam K, Mummidi S, Valente AJ, Clark RA, et al. WNT1-inducible signaling pathway protein-1 activates diverse cell survival pathways and blocks doxorubicin-induced cardiomyocyte death. Cell Signal. 2010;22(5):809–20.
Wang S, Chong ZZ, Shang YC, Maiese K. Wnt1 inducible signaling pathway protein 1 (WISP1) blocks neurodegeneration through phosphoinositide 3 kinase/Akt1 and apoptotic mitochondrial signaling involving Bad, Bax, Bim, and Bcl-xL. Curr Neurovasc Res. 2012;9(1):20–31.
Lim HW, Lee JE, Shin SJ, Lee YE, Oh SH, Park JY, et al. Identification of differentially expressed mRNA during pancreas regeneration of rat by mRNA differential display. Biochem Biophys Res Commun. 2002;299(5):806–12.
Wang S, Chong ZZ, Shang YC, Maiese K. WISP1 neuroprotection requires FoxO3a post-translational modulation with autoregulatory control of SIRT1. Curr Neurovasc Res. 2013;10(1):54–69.
Campos DB, Palin MF, Bordignon V, Murphy BD. The “beneficial” adipokines in reproduction and fertility. Int J Obes (Lond). 2008;32(2):223–31.
Challier JC, Basu S, Bintein T, Minium J, Hotmire K, Catalano PM, et al. Obesity in pregnancy stimulates macrophage accumulation and inflammation in the placenta. Placenta. 2008;29(3):274–81.
Kuzmicki M, Telejko B, Wawrusiewicz-Kurylonek N, Citko A, Lipinska D, Pliszka J, et al. The expression of suppressor of cytokine signaling 1 and 3 in fat and placental tissue from women with gestational diabetes. Gynecol Endocrinol. 2012;28(11):841–4.
Lepercq J, Cauzac M, Lahlou N, Timsit J, Girard J, Auwerx J, et al. Overexpression of placental leptin in diabetic pregnancy: a critical role for insulin. Diabetes. 1998;47(5):847–50.
Pantham P, Aye IL, Powell TL. Inflammation in maternal obesity and gestational diabetes mellitus. Placenta. 2015;36(7):709–15.
Segovia SA, Vickers MH, Gray C, Reynolds CM. Maternal obesity, inflammation, and developmental programming. Biomed Res Int. 2014;2014:418975.
Strakovsky RS, Pan YX. A decrease in DKK1, a WNT inhibitor, contributes to placental lipid accumulation in an obesity-prone rat model. Biol Reprod. 2012;86(3):81.
Maiese K, Chong ZZ, Shang YC, Wang S. Targeting disease through novel pathways of apoptosis and autophagy. Expert Opin Ther Targets. 2012;16(12):1203–14.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
This study was approved by the Human Ethics Committee of the Shengjing Hospital of China Medical University. It was designed in accordance with the principle of the Helsinki Declaration.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, Lc., Xu, St. & Li, L. WISP1 is increased in the maternal serum, adipose tissue, and placenta of women with gestational diabetes mellitus. Int J Diabetes Dev Ctries 42, 269–275 (2022). https://doi.org/10.1007/s13410-021-00972-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13410-021-00972-2