Abstract
Purpose
To investigate the association of omentin-1 and inflammatory factors in serum and visceral adipose tissue (VAT) of women with gestational diabetes mellitus (GDM) compared to normal pregnant (NP) subjects. Furthermore, to examine their correlation with maternal clinical characteristics.
Methods
We compared 116 GDM women to 115 NP women, at the time of cesarean section. Circulating omentin-1 and pro-inflammatory (IL-1β, IL-6, TNF-α), and anti-inflammatory cytokines (IL-1RA, IL-10) were examined. Moreover, their mRNA expression in VAT, along with inflammatory factors involved in the NF-κB pathway (TLR2, TLR4, NF-κB, IKκB), were examined.
Results
Circulating omentin-1 (p = 0.022) was lower and circulating IL-1-β, IL-1RA, as well as IL-10 (p = 0.005, p = 0.007, and p = 0.015, respectively), were higher in GDM compared to NP women. Omentin-1 correlated negatively with pre-pregnancy and gestational BMI, and HOMA-IR in all women, but was not associated with cytokines. TLR2, TLR4, IL-1β, IL-1RA, IL-6, IL-10 mRNA expression in VAT was lower in GDM compared with controls (p < 0.05 all). In multivariate analysis, BMI at delivery was significantly correlated to omentin-1 concentrations in all and NP subjects. In addition, omentin-1 expression was correlated to inflammatory gene expression in all, GDM and NP, women (p < 0.05 all).
Conclusion
Serum levels and VAT gene expression of omentin-1 are not independently linked to GDM; notwithstanding, GDM women have a VAT-altered inflammatory status. In addition, no systemic association between omentin-1 and inflammatory factors was found, whereas associations between their expression in all women were observed, indicating that expression of these adipokines is linked between them regardless of GDM.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gestational diabetes mellitus (GDM) is the most common medical complication of pregnancy, affecting from 1 to 30% of pregnant women worldwide. Maternal overweight and obesity are major GDM risk factors [1]. In recent years, various adipokines (adipocyte-secreted proteins) have been involved in the pathophysiology of GDM through their role in the regulation of insulin resistance, β-cell dysfunction, and bodyweight gain [2].
Omentin-1, also known as intelectin-1, is a recently-identified adipokine mainly expressed in visceral fat as well as vascular cells, intestine, and placenta [3]. Circulating omentin-1 levels are decreased in obesity and in patients with type 2 diabetes (T2DM) [4, 5]. Evidence has shown that omentin-1 stimulates glucose uptake via AMP-activated protein kinase/Akt in cultured adipocytes [3]. Furthermore, omentin-1 has anti-inflammatory properties, in cultured endothelial cells suppresses TNF-α-induced vascular inflammation and in smooth-muscle cells attenuates TNF-α-induced monocyte adhesion [6, 7].
Several cross-sectional studies have described decreased circulating concentrations of omentin-1 in women with GDM compared to controls [8,9,10,11,12]. Furthermore, a prospective study found that omentin-1 less than 38.4 ng/mL at 12–15 weeks of gestation was predictive of increased risk of GDM [13]. However, other studies have found no difference in omentin-1 circulating levels in women with GDM [14, 15], and recently, a few other studies have not shown GDM effect on omentin-1 gene expression in visceral adipose tissue (VAT) [8, 9].
GDM is characterized by elevated circulating free fatty acids and advanced glycation end products which activate toll-like receptors 2 and 4 (TLR2, TLR4) to promote the production of pro-inflammatory cytokines from macrophages and adipose tissue through the up-regulation of the nuclear transcription factor-κB (NF-κB) [16]. These cytokines can inhibit insulin action in the muscle and adipose tissue [17, 18]. Collectively, omentin-1 and inflammatory factors are associated with GDM [19, 20]; however, interactions among them in terms of their expression in VAT and how this correlates with their blood levels, and with related clinical parameters, have not yet been determined in a single setting.
Therefore, we aimed to investigate the association of circulating and mRNA expression in VAT of omentin-1 and pro-inflammatory (IL-1β, IL-6, TNF-α), and anti-inflammatory cytokines (IL-1RA, IL-10) along with inflammatory factors involved in the nuclear factor-κB (NF-κB) pathway (TLR2, TLR4, NF-κB, IKκB), in GDM compared to normal pregnant (NP) subjects. The secondary objective was to explore the correlation between omentin-1, inflammatory cytokines and clinical maternal characteristics.
Materials and methods
The cross-sectional study was approved by the Institutional Review Board of the Instituto Mexicano del Seguro Social (IMSS) in Mexico City (R-2018-785-026), and all participants gave written informed consent. Women who were scheduled for an elective Caesarean section at term (37–41 weeks of gestation) were recruited for participation in this study (indications for Caesarean section were breech presentation, previous Caesarean section, and/or when macrosomia was suspected by ultrasonography at 38 weeks of gestation) were consecutively recruited, attended in Hospital of Gynecology and Obstetrics 3, Medical Center La Raza, IMSS (Mexico City), and the Hospital of Gynecology and Obstetrics 221, IMSS (Toluca, State of Mexico). Women with pre-gestational diabetes or hypertension, and immunosuppressive, autoimmune, thyroid, renal, liver, infectious diseases or smoking and alcohol habits, as well as pregnancies complicated by fetal anomalies, were excluded from the study. This group of women has been previously analyzed for various other adipokines [21].
All women were screened for GDM at 24–28 weeks of gestation, and women with a negative screen were classified in the NP group. Women with GDM were diagnosed according to the International Association of Diabetes and Pregnancy Study Groups criteria (one or more abnormal glucose value during a 75 g-oral glucose tolerance test (OGTT), with fasting levels ≥ 5.1 mmol/L, 1 h ≥ 10 mmol/L or 2 h ≥ 8.5 mmol/L). treatment for GDM began with nutrition therapy (1600–1800 kcal/day, restricting carbohydrates to 35–40%), and moderate physical activity (30 min of moderate-intensity aerobic exercise at least 5 days a week), with assessments of glycemic control with fasting glucose and 2-h postprandial blood glucose at 2–4 week intervals. Women who did not achieve glycemic control with diet (fasting glucose levels > 5.27 mmol/L, and postprandial blood glucose values > 6.66 mmol/L at 2 h) began pharmacological therapy. Of the GDM cases, 33% (n = 38) were treated by diet and exercise only, and 14% (n = 16) received insulin, 41% (n = 48) metformin, or 12% (n = 14) a combination of both to reach glycemic goals.
A baseline questionnaire included information about current and previous pregnancies from medical records, demographic characteristics such as maternal age, past history of GDM and family history of T2DM in a first-degree relative. Maternal pre-pregnancy body mass index (BMI) was calculated from self-reported pre-pregnancy weight and height measured during the first visit of gestation. BMI was calculated using the subject´s weight in kilograms divided by the square of her height in meters, and was recalculated at the time of delivery. Maternal gestational weight gain (GWG) was obtained by the difference between the last recorded pre-delivery weight reported pre-pregnancy weight.
Biochemical analysis
On the day programmed for cesarean section, maternal fasting blood samples for biochemical analysis were obtained by venipuncture, along with the samples for routine laboratory tests. The samples were allowed to clot for at least 30 min before 15 min of centrifugation at 1000 g. Serum aliquots were frozen at − 70 °C until assayed. Levels of glucose, total cholesterol, high-density lipoprotein (HDL) cholesterol and triglycerides were measured in fresh samples on an ARCHITECT Plus c4000 Clinical Chemistry Analyzer (Abbot Diagnostics, Abbott Park, IL, USA). Levels of low-density lipoprotein (LDL) cholesterol were estimated with the use of the Friedewald formula. Omentin-1 determination was performed with an enzyme-linked immunoassay kit from BioVendor R&D Products (Brno, Czech Republic). IL-1β, IL-6, TNF-α, IL-1RA, IL-10, and insulin were measured through multiplex immunoassay using Magpix technology (Milliplex Map, Billerica, MA, USA). The calculated inter-assay and intra-assay coefficients of variation were all < 10%. Insulin resistance was calculated using the homeostasis model assessment for insulin resistance (HOMA-IR) method, where HOMA-IR = fasting insulin concentration [μU/mL] × fasting glucose concentration [mmol/L]/22.5 [22].
VAT collection
Omental adipose tissue was obtained from 50 NP women matched with 50 GDM patients according to age. Tissue was obtained within ten minutes of delivery and was washed in water treated with diethylpyrocarbonate to remove any blood, and then dissected into fragments that were placed in TRIzol® Reagent (In-vitrogen™, Carlsbad, CA, USA) and stored at − 70 °C until RNA extraction.
RNA extraction, cDNA synthesis and qPCR
Total RNA was isolated from VAT using Direct-zol™ RNA MiniPrep (Zymo Research Corp, CA, USA) according to the manufacturer´s protocol. RNA concentrations were quantified using a spectrophotometer (NanoDrop 2000, Thermo Fisher Scientific, DE, USA), and RNA quality was determined via the A260/A280 ratio. One μg of RNA was converted to cDNA using the SuperScript®III First-Strand kit (Invitrogen™, Carlsbad, CA, USA) according to the manufacture´s recommendations. The cDNA expression level was quantified by a real-time PCR using Taqman® Gene Expression Assays and Taqman® Universal PCR Master Mix (Applied Biosystems™, Foster City, CA, USA) according to the manufacturer´s protocol. Real-time PCR was performed with the StepOnePlus™ Real-Time PCR System (Applied Biosystems™, Foster City, CA, USA). The 2−ΔCT method of relative quantification was used to determine the fold change in the mRNA expressions with the GAPDH transcript as endogenous control. All the primers and probes were acquired from Applied Biosystems™: omentin-1 (Hs00914745_m1), IL-1β (Hs01555410_m1), IL-6 (Hs00985639_m1), TNF-α (Hs01113624_g1), IL-1RA (Hs00893626_m1), IL-10 (Hs00961622_m1), TLR2 (Hs02621280_s1), TLR4 (Hs00152939_m1), NF-κB (Hs00765730_m1), IKκB (Hs01559460_m1), and GAPDH (PN 4326317E).
Statistical analysis
Data distribution was assessed using Kolmogorov–Smirnov test. Results are presented as the medians with interquartile range (IQR). Differences between groups were analyzed by Mann–Whitney U test and correlations calculated using Spearman’s test. Correction for potential confounders was performed with analysis of covariance. Multiple regression analysis was used to determine which variables were independently associated with maternal levels and mRNA expression of omentin-1. We used IBM SPSS Statistics 23.0 (IBM SPSS Inc., Chicago, IL) for statistical analysis, and a p < 0.05 was considered statistically significant.
Results
One hundred sixteen women with GDM and 115 healthy NP women were enrolled. The clinical and metabolic maternal characteristics of the NP and GDM women are summarized in Table 1. Women diagnosed with gestational diabetes had a higher age, pre-pregnancy BMI, gestational BMI, parity, family history of T2DM, previous GDM, fasting glucose at delivery, and triglycerides compared with the NP individuals. In contrast, gestational weight gain, gestational age at delivery and HDL levels were significantly lower in GDM subjects than NP subjects.
Maternal circulating plasma levels
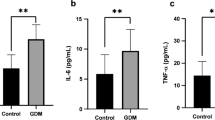
Circulating omentin-1 (p = 0.022) was lower and circulating IL-1β, IL-1RA, as well as IL-10 (p = 0.005, p = 0.007, and p = 0.015, respectively) were higher in GDM compared to NP women (Fig. 1). These significances did not continue after adjusting for age, pre-pregnancy BMI, BMI at term, and gestational age at delivery. No differences were found in circulating levels of IL-6 and TNF-α between GDM and non-GDM women (Table 2). We assessed the effect of pre-existing maternal obesity on omentin-1 and inflammatory cytokine levels in GDM participants. There was no difference in omentin-1, IL-1β, IL-6, IL-10, and TNF-α levels between obese and non-obese women. Circulating IL-1RA levels were significantly higher in women with obesity compared to non-obese women [8.1 (5.1–14.5 pg/mL) vs. 5.57 (3.1–8.7 pg/mL), respectively, p = 0.020]. In addition, no associations of maternal plasma omentin-1 levels with inflammatory factors were observed.
Box and whisker plots of circulating omentin-1, IL-1β, IL-1RA, and IL-10 in GDM compared to NP. GDM group showed decreased omentin-1 levels (a), and increased IL-1β (b), IL-1RA (c), as well as IL-10 (d) levels compared with controls. NP, n = 115; GDM, n = 116. *p < 0.05, **p < 0.01. NP normal pregnancy; GDM gestational diabetes mellitus; IL-1β interleukin 1β; IL-1RA IL-1 receptor antagonist; IL-10 interleukin 10
Omentin-1 and inflamatory markers gene expression
Median TLR2, TLR4, IL-1β, IL-1RA, IL-6, IL-10 expression levels in visceral adipose tissue were lower in GDM compared with controls (Fig. 2). There was no difference in VAT omentin-1, IKκB, NF-κB, TNF-α expression levels between GDM and controls (Table 2). As it has been suggested that an impaired balance between pro- and anti-inflammatory cytokines may play a role in the pathogenesis of GDM, we decided to investigate the pro-inflammatory cytokines/anti-inflammatory cytokines balance and we observed that VAT TNF-α/IL-10 expression ratio was higher in GDM compared with controls (p = 0.002). However, after controlling for gestational age, pre-pregnancy BMI and BMI at term, only IL-10 expression remained significantly lower in participants with GDM. Moreover, there was no significant difference in omentin-1 and cytokines expression and serum levels between women with GDM who were managed by dietary modification alone compared with women who were treated with insulin or metformin.
Inflammatory markers gene expression in visceral adipose tissue in GDM compared to NP. TLR2 (a), TLR4 (b), IL-1β (c), IL-6 (d), IL-1RA (e), and IL-10 (f) expression levels were lower in GDM compared with controls. NP, n = 50; GDM, n = 50. *p < 0.05, **p < 0.001. Each bar represents the mean ± SEM. NP normal pregnancy; GDM gestational diabetes mellitus; TLR2 toll-like receptor 2; TLR4 toll-like receptor 4; IL-1β interleukin 1β; IL-6 interleukin 6; IL-1RA IL-1 receptor antagonist; IL-10 interleukin 10
Omentin-1 and inflammatory cytokines VAT gene expression were not associated with maternal blood levels. Additionally, positive associations between omentin-1 and inflammatory markers expression in VAT in all, NP and GDM women, were observed (Table 3). The significance of the above correlations continued after adjustment for gestational age, pre-pregnancy BMI and BMI at term.
Correlations between omentin-1 and inflammatory markers serum levels as well as VAT gene expressions with maternal clinical parameters
In all women, serum omentin-1 levels correlated negatively with pre-pregnancy BMI, gestational BMI, and HOMA-IR (r = − 0.196, p = 0.003, r = − 0.289, p = 0.001, r = − 0.244, p = 0.048, respectively), and positively with HDL (r = 0.146, p = 0.035). IL-1β concentration was positively correlated with age (r = 0.175, p = 0.031), pre-pregnancy BMI (r = 0.201, p = 0.014), BMI at term (r = 0.165, p = 0.044), insulin (r = 0.344, p = 0.007), and HOMA-IR (r = 0.275, p = 0.042). The TNF-α level was positively correlated with BMI at term (r = 0.191, p = 0.020). IL-1RA had a positive correlation with BMI at term (r = 0.175, p = 0.033), and HOMA-IR (r = 0.326, p = 0.015). Furthermore, IL-10 was significantly negatively correlated with GWG (r = − 0.245, p = 0.002). In GDM subjects, serum IL-1β levels were positively correlated with insulin (r = 0.626, p = 0.022). IL1RA had positive correlations with BMI at term (r = 0.249, p = 0.034), and HOMA-IR (r = 0.553, p = 0.021). IL-10 levels were positively correlated with HOMA-IR (r = 0.660, p = 0.004), and were negatively associated with GWG (r = − 0.278, p = 0.016). In NP women, circulating omentin-1 correlated negatively with pre-pregnancy BMI and at term BMI (r = − 0.298, p = 0.001, r = − 0.379, p = 0.001, respectively), and correlated positively with triglycerides (r = 0.253, p = 0.006), and VLDL (r = 0.224, p = 0.016).
In all women, omentin-1 mRNA expression was positively related to the patient's age (r = 0.277, p = 0.005), and IL-6 expression was positively associated with GWG (r = 0.249, p = 0.013). In GDM subjects, omentin-1, TLR2, TLR4, TNF-α, and NF-κB expression were positively correlated with maternal age (r = 0.287, p = 0.044, r = 0.441, p = 0.001, r = 0.341, p = 0.015, r = 0.312, p = 0.028, r = 0.399, p = 0.004, respectively), and NF-κB expression was positively associated with pre-pregnancy BMI (r = 0.317, p = 0.028). In NP women, omentin-1 expression had a positive correlation with age (r = 0.320, p = 0.023). TLR2 expression was positively correlated with GWG (r = 0.391, p = 0.005). Finally, IL-1β and IL-10 expression had positive correlations with fasting plasma glucose levels (r = 0.290, p = 0.041, r = 0.300, p = 0.034).
Multiple linear regression analysis was performed to study the variables independently associated with maternal levels and expression of omentin-1 (Table 4). BMI at delivery was significantly correlated to omentin-1 concentrations in all and NP subjects. In addition, omentin-1 expression was significantly correlated to inflammatory markers mRNA expression.
Discussion
As far as we know, this is the first study that simultaneously determined adipose tissue expressions and serum levels of omentin-1 and inflammatory factors in GDM women at the time of delivery. Moreover, this study determined correlations between them and maternal parameters. We found that omentin-1 serum levels were lower in GDM compared with NP, and correlated negatively with BMI, and HOMA-IR in all women; however, there was no difference after adjustment for maternal factors such as age and BMI, and the result of the multiple regression analysis showed that BMI affects omentin-1 levels. In agreement with this, it has been shown that in conditions of increased adiposity, increased visceral adipocyte parameters including area, width, height, and perimeters are related to decreased serum levels of omentin-1 [23].
Case–control studies related to omentin-1 levels between GDM and NP women have had variable results. Barker et al. and others found that maternal omentin-1 was significantly lower in women with GDM compared with controls [8,9,10,11,12]. It is noteworthy that in the studies in which women were classified according to their BMI, omentin-1 was significantly decreased only in the non-obese subgroup of GDM women. On the other hand, Abell et al., in a longitudinal study, found that omentin-1 < 38.66 ng/mL at 12–15 weeks of gestation was associated with a fourfold increased risk of GDM [13]. However, Lewandowski et al., and Franz et al. did not find any difference in omentin-1 in women with GDM [14, 15]. The disagreement between different studies could be due to the definition of GDM, maternal BMI, study design, the different gestational ages at the time of the maternal blood collection, the methodological heterogeneity selected for the analysis, and the different treatments used for GDM. In this study, pharmacological treatment did not significantly affect adipokine levels.
Interestingly, in this study, omentin-1 mRNA synthesis was not significantly different in VAT of women with GDM compared with pregnant controls. Similarly, Barker et al. and Tsiotra et al. reported VAT omentin-1 mRNA expression being comparable between the NP and GDM groups [8, 9]. This is evidence that omentin-1 does not have a role in the modulation of adipocyte dysfunction in GDM. Of note, Barker et al. demonstrated that pre-existing maternal obesity was associated with lower expression and release of omentin-1 in VAT. Several studies have shown that serum levels of this adipokine reflect its expression in VAT, which decreases in parallel with the increase in VAT [23,24,25]. However, in this study, omentin-1 mRNA expression was not correlated with its serum levels, indicating that serum levels of omentin-1 might primarily be correlated to its secretion from other tissue, such as placental. It has recently been shown that placenta is the major source of omentin-1 levels, and its expression in this tissue is decreased in GDM compared to non-obese NP [8].
Omentin-1 is a recently-discovered adipokine originally identified as a soluble galactofuranose-binding lectin, and later identified from a human omental adipose tissue cDNA library [3]. Omentin-1 is abundantly expressed in the stromal vascular fraction of VAT, as well as vascular cells, intestine, and placenta. It has been shown that omentin-1 stimulates glucose uptake in cultured adipocytes in vitro in response to insulin, suggesting that may improve insulin sensitivity. Circulating omentin-1 levels are decreased in obese individuals, in patients with T2DM, with metabolic syndrome and in pregnancy [4, 5]. Additionally, it has been reported that omentin-1 has anti-inflammatory properties. In human endothelial cells inhibits TNF-α induced vascular inflammation, and in vascular smooth muscle cells it inhibits TNF-α-induced superoxide production, suggesting a protective role in vascular disease [6, 7]. In addition, in high fat diet-induced obese mice, omentin-1 attenuates adipose tissue inflammation, and in macrophages it attenuates its lipopolysaccharide-induced activation by inhibiting the TLR4/MyD88/NF-κB signaling [26, 27].
Increasing evidence suggests that GDM is a systemic pro-inflammatory state. It has been shown that pro-inflammatory cytokines such as IL-1β, TNF-α, and IL-6 increase in GDM conferring insulin resistance in liver, skeletal muscle, and vascular endothelium [16,17,18]. In this study, circulating IL-1β, IL-1RA, as well as IL-10, were higher in GDM compared to NP women. However, these significances did not continue after adjusting for maternal characteristics. We also observed that obese GDM women had higher IL-1RA levels than non-obese women, and that maternal BMI was in correlation with the serum IL-1β, IL-1RA and TNF-α levels, suggesting that one of the main sources of cytokine production is the adipose tissue, although it has also been documented that the feto-placental unit may also be an important source of TNF-α [2, 28]. Additionally, serum omentin-1 levels were not associated with circulating inflammatory factors.
Adipose tissue from GDM pregnancies displays an altered physiology; adipocytes display increased cell size, and adipose tissue displays reduced capillary density and also increased expression of markers of endothelial dysfunction and inflammatory cytokines when compared to NP [29]. However, it still remains to be determined whether the expression of these factors precedes the development of GDM or is rather secondary to the metabolic disturbances in GDM patients. In this study, we found that TLR2, TLR4, IL-1β, IL-1RA, IL-6, IL-10 mRNA expression in VAT was lower, and TNF-α/IL-10 ratio was higher, in GDM compared with controls. However, after controlling for maternal characteristics, only IL-10 expression remained significantly lower in participants with GDM, which further indicates altered anti-inflammatory status in patients with GDM. In agreement with this, several studies have shown decreased levels of IL-10 in GDM women [30].
Both age and BMI before pregnancy increase the risk of GDM. We showed that TLR2, TLR4, TNF-α, and NF-κB VAT expression were positively correlated with maternal age, and NF-κB expression was positively associated with pre-pregnancy BMI in GDM women. NF-κB is a primary regulator of inflammatory response. This nuclear factor exists in an inactive dimeric form in the cytoplasm bound by the inhibitory proteins IκBs. After activation by stimuli such as pathogens, T-cell activation signals, and pro-inflammatory cytokines, IκBs become phosphorylated by I-κB-Kinase β (IKκB), thus allowing the NF-κB to translocate to the nucleus and activate gene transcription of several genes, including pro-inflammatory cytokines, chemokines and adhesion molecules. Recent studies implicate a role of NF-κB and TLR in ageing, obesity and insulin resistance [31,32,33]. In GDM, several studies have reported increased expression of TLR4 mRNA and NF-κB mRNA in the placenta [34]. On the other hand, it has been shown that an elevated TLR2 expression in peripheral blood mononuclear cells of women with GDM [35], and high glucose and saturated fat increase TLR expression in these cells [33, 36].
In this study, we also reported associations between omentin-1 and inflammatory marker mRNA expression in VAT in all, NP, and GDM women, and the significance of correlations continued after adjustment for maternal characteristics, indicating that expression of these adipokines is linked between each other regardless of GDM. Interestingly, in NP, but not in GDM women, expression of omentin-1 was positively correlated with the expression of anti-inflammatory cytokine IL-1RA and was negatively correlated with pro-inflammatory cytokines IL1β, and IL-6, suggesting an anti-inflammatory property. One explanation of different associations between omentin-1 and cytokines expressions between GDM and non-GDM subjects might be because a hypertrophy of adipocytes is seen in individuals with GDM, which could contribute to a failure in expression levels of adipokines [29].
Our study has several limitations. First, the cross-sectional data analyses cannot make causal inferences regarding the relationships between omentin-1 and inflammatory factors in GDM. Second, there were differences in age and BMI between groups, although adjustments for these variables were made. Third, we did not measure the secretion of adipokines from adipose tissue obtained from studied women. It has been suggested that IL-1β gene expression on adipose tissue is independent of gene transcription. In addition, maternal adipokines were measured at the time of Caesarean section in only one population, there was a lack of HbA1c, and all the GDM patients received intervention once diagnosed, including diet, exercise and pharmacological treatment. The influence of these treatments on the cytokine levels is unclear and should be considered in future studies. Thus, large prospective cohort studies will be necessary to provide evidence concerning the association of VAT expression and release of the examined adipokines with GDM.
Conclusion
Serum levels and VAT gene expression of omentin-1 are not independently linked to GDM; notwithstanding, GDM women have a VAT-altered inflammatory status. In addition, no systemic association between omentin-1 and inflammatory factors was found, whereas associations between their expression in all women were observed, indicating that expression of these adipokines is linked between each other regardless of GDM.
Availability of data and material
The data used to support the findings of this study are available from the corresponding author by request.
Code availability
Not applicable for that section.
References
McIntyre HD, Catalano P, Zhang C, Desoye G, Mathiesen ER, Damm P (2019) Gestational diabetes mellitus. Nat Rev Dis Primers 5:47. https://doi.org/10.1038/s41572-019-0098-8
Fasshauer M, Blüher M, Stumvoll M (2014) Adipokines in gestational diabetes. Lancet Diabetes Endocrinol 2:488–499. https://doi.org/10.1016/S2213-8587(13)70176-1
Yang RZ, Lee MJ, Hu H, Pray J, Wu HB, Hansen BC, Shuldiner AR, Fried SK, McLenithan JC, Gong DW (2006) Identification of omentin as a novel depot-specific adipokine in human adipose tissue: possible role in modulating insulin action. Am J Physiol Endocrinol Metab 290:E1253-1261. https://doi.org/10.1152/ajpendo.00572.2004
Watanabe T, Watanabe KK, Takahashi Y, Kojima M, Watanabe R (2017) Adipose tissue-derived omentin-1 function and regulation. Compr Physiol 7:765–781. https://doi.org/10.1002/cphy.c160043
de Souza Batista CM, Yang RZ, Lee MJ, Glynn NM, Yu DZ, Pray J, Ndubuizu K, Patil S, Schwartz A, Kligman M, Fried SK, Gong DW, Shuldiner AR, Pollin TI, McLenithan JC (2007) Omentin plasma levels and gene expression are decreased in obesity. Diabetes 56:1655–1661. https://doi.org/10.2337/db06-1506
Yamawaki H, Kuramoto J, Kameshima S, Usui T, Okada M, Hara Y (2011) Omentin, a novel adipocytokine inhibits TNF-induced vascular inflammation in human endothelial cells. Biochem Biophys Res Commun 408:339–343. https://doi.org/10.1016/j.bbrc.2011.04.039
Kazama K, Usui T, Okada M, Hara Y, Yamawaki H (2012) Omentin plays an anti-inflammatory role through inhibition of TNF-α-induced superoxide production in vascular smooth muscle cells. Eur J Pharmacol 686:116–123. https://doi.org/10.1016/j.ejphar.2012.04.033
Barker G, Lim R, Georgiou HM, Lappas M (2012) Omentin-1 is decreased in maternal plasma, placenta and adipose tissue of women with pre-existing obesity. PLoS One 7:e42943. https://doi.org/10.1371/journal.pone.0042943
Tsiotra PC, Halvatsiotis P, Patsouras K, Maratou E, Salamalekis G, Raptis SA, Dimitriadis G, Boutati E (2018) Circulating adipokines and mRNA expression in adipose tissue and the placenta in women with gestational diabetes mellitus. Peptides 101:157–166. https://doi.org/10.1016/j.peptides.2018.01.005
Pan BL, Ma RM (2016) Correlation of serum omentin-1 and chemerin with gestational diabetes mellitus. Nan Fang Yi Ke Da Xue Xue Bao 36:1231–1236
Souvannavong-Vilivong X, Sitticharoon Ch, Klinjampa R, Keadkraichaiwat I, Sripong Ch, Chatree S, Sririwichitchai R, Lertbunnaphong T (2019) Placental expressions and serum levels of adiponectin, visfatin, and omentin in GDM. Acta Diabetol 56:1121–1131. https://doi.org/10.1007/s00592-019-01355-0
Mierzynski R, Dłuski D, Nowakowski L, Poniedziałek-Czajkowska E, Leszczyńska-Gorzelak B (2018) Adiponectin and omentin levels as predictive biomarkers of preterm birth in patients with gestational diabetes mellitus. Biomed Res Int 2018:7154216. https://doi.org/10.1155/2018/7154216
Abell SK, Shorakae S, Harrison CL, Hiam D, Moreno A, Stepto NK, De Courten B, Teede HJ (2017) The association between dysregulated adipocytokines in early pregnancy and development of gestational diabetes. Diabetes Metab Res Rev 33:e2926. https://doi.org/10.1002/dmrr.2926
Lewandowski K, Nadel I, Lewinski A, Bienkiewicz M, Tan B, Randeva HS, Cypryk K (2010) Positive correlation between serum omentin and thrombospondin-1 in gestational diabetes despite lack of correlation with insulin resistance indices. Ginekol Pol 81:907–912
Franz M, Polterauer M, Springer S, Kuessel L, Haslinger P, Worda C, Worda K (2018) Maternal and neonatal omentin-1 levels in gestational diabetes. Arch Gynecol Obstet 297:885–889. https://doi.org/10.1007/s00404-018-4652-5
Lappas M (2014) Activation of inflammasomes in adipose tissue of women with gestational diabetes. Mol Cell Endocrinol 382:74–83. https://doi.org/10.1016/j.mce.2013.09.011
Hotamisligil GS, Peraldi P, Budavari A, Ellis R, White MF, Spiegelman BM (1996) IRS-1- mediated inhibition of insulin receptor tyrosine kinase activity in TNF-alpha- and obesity-induced insulin resistance. Science 271:665–668. https://doi.org/10.1126/science.271.5249.665
Ballak DB, Stienstra R, Tack CJ, Dinarello CA, van Diepen JA (2015) IL-1 family members in the pathogenesis and treatment of metabolic disease: Focus on adipose tissue inflammation and insulin resistance. Cytokine 75:280–290. https://doi.org/10.1016/j.cyto.2015.05.005
Kirwan JP, Hauguel-De Mouzon S, Lepercq J, Challier JC, Huston PL, Friedman JE, Kalhan SC, Catalano PM (2002) TNF-alpha is a predictor of insulin resistance in human pregnancy. Diabetes 51:2207–2213. https://doi.org/10.2337/diabetes.51.7.2207
Atègbo JM, Grissa O, Yessoufou A, Hichami A, Dramane KL, Moutairou K, Miled A, Grissa A, Jerbi M, Tabka Z, Khan NA (2006) Modulation of adipokines and cytokines in gestational diabetes and macrosomia. J Clin Endocrinol Metab 91:4137–4143. https://doi.org/10.1210/jc.2006-0980
Saucedo R, Valencia J, Moreno-González LE, Peña-Cano MI, Aranda-Martínez A, García Y, Díaz-Velázquez MF, Hernández-Valencia M (2021) Maternal serum adipokines and inflammatory markers at late gestation and newborn weight in mothers with and without gestational diabetes mellitus. Ginekol Pol. https://doi.org/10.5603/GP.a2021.0083
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC (1985) Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28:412–419. https://doi.org/10.1007/BF00280883
Sitticharoon C, Nway NC, Chatree S, Churintaraphan M, Boonpuan P, Maikaew P (2014) Interactions between adiponectin, visfatin, and omentin in subcutaneous and visceral adipose tissues and serum, and correlations with clinical and peripheral metabolic factors. Peptides 62:164–175. https://doi.org/10.1016/j.peptides.2014.10.006
Auguet T, Quintero Y, Riesco D, Morancho B, Terra X, Crescenti A, Broch M, Aguilar C, Olona M, Porras JA, Hernandez M, Sabench F, del Castillo D, Richart C (2011) New adipokines vaspin and omentin. Circulating levels and gene expression in adipose tissue from morbidly obese women. BMC Med Genet 12:60. https://doi.org/10.1186/1471-2350-12-60
Tan BK, Adya R, Farhatullah S, Lewandowski KC, O’Hare P, Lehnert H, Randeva HS (2008) Omentin-1, a novel adipokine, is decreased in overweight insulin-resistant women with polycystic ovary syndrome: ex vivo and in vivo regulation of omentin-1 by insulin and glucose. Diabetes 57:801–808. https://doi.org/10.2337/db07-0990
Wang J, Gao Y, Lin F, Han K, Wang X (2020) Omentin-1 attenuates lipopolysaccharide (LPS)-induced U937 macrophages activation by inhibiting the TLR4/MyD88/NF-κB signaling. Arch Biochem Biophys 679:108187. https://doi.org/10.1016/j.abb.2019.108187
Zhou H, Zhang Z, Qian G, Zhou J (2020) Omentin-1 attenuates adipose tissue inflammation via restoration of TXNIP/NLRP3 signaling in high-fat diet-induced obese mice. Fundam Clin Pharmacol 34:721–735. https://doi.org/10.1111/fcp.12575
Lappas M, Mitton A, Permezel M (2010) In response to oxidative stress, the expression of inflammatory cytokines and antioxidant enzymes are impaired in placenta, but not adipose tissue, of women with gestational diabetes. J Endocrinol 204:75–84. https://doi.org/10.1677/JOE-09-0321
Rojas RR, Lifshitz LM, Bellve KD, Min SY, Pires J, Leung K, Boeras C, Sert A, Draper JT, Corvera S, Moore ST (2015) Human adipose tissue expansion in pregnancy is impaired in gestational diabetes mellitus. Diabetologia 58:2106–2114. https://doi.org/10.1007/s00125-015-3662-0
Kuzmicki M, Telejko B, Zonenberg A, Szamatowicz J, Kretowski A, Nikolajuk A, Laudanski P, Gorska M (2008) Circulating pro- and anti-inflammatory cytokines in Polish women with gestational diabetes. Horm Metab Res 40:556–560. https://doi.org/10.1055/s-2008-1073166
Kanigur Sultuybek G, Soydas T, Yenmis G (2019) NF-κB as the mediator of metformin’s effect on ageing and ageing-related diseases. Clin Exp Pharmacol Physiol 46:413–422. https://doi.org/10.1111/1440-1681.13073
He L, He M, Lv X, Pu D, Su P, Liu Z (2010) NF-κB binding activity and pro-inflammatory cytokines expression correlate with body mass index but not glycosylated hemoglobin in Chinese population. Diabetes Res Clin Pract 90:73–80. https://doi.org/10.1016/j.diabres.2010.06.016
Dasu MR, Devaraj S, Zhao L, Hwang DH, Jialal I (2008) High glucose induces toll-like receptor expression in human monocytes: mechanism of activation. Diabetes 57:3090–3098. https://doi.org/10.2337/db08-0564
Feng H, Su R, Song Y, Wang C, Lin L, Ma J, Yang H (2016) Positive correlation between enhanced expression of TLR4/MyD88/NF-κB with insulin resistance in placentae of gestational diabetes mellitus. PLoS One 11:e0157185. https://doi.org/10.1371/journal.pone.0157185
Kuzmicki M, Telejko B, Wawrusiewicz-Kurylonek N, Lipinska D, Pliszka J, Wilk J, Zielinska A, Skibicka J, Szamatowicz J, Kretowski A, Gorska M (2013) The expression of genes involved in NF-κB activation in peripheral blood mononuclear cells of patients with gestational diabetes. Eur J Endocrinol 168:419–427. https://doi.org/10.1530/EJE-12-0654
Ghanim H, Abuaysheh S, Sia CL, Korzeniewski K, Chaudhuri A, Fernandez-Real JM, Dandona P (2009) Increase in plasma endotoxin concentrations and the expression of toll-like receptors and suppressor of cytokine signaling-3 in mononuclear cells after a high-fat, high-carbohydrate meal implications for insulin resistance. Diabetes Care 32:2281–2287. https://doi.org/10.2337/dc09-0979
Funding
This study was supported by scientific grants from Instituto Mexicano del Seguro Social (FIS/IMSS/PROT/G18/1826). RS, JVO, EMA, and RGD hold a fellowship from the National System of Investigators (Consejo Nacional de Ciencia y Tecnología).
Author information
Authors and Affiliations
Contributions
MIPC, JVO, and RS planned and designed the study. MIPC, and JVO performed laboratory biomarker analysis. EMA, MFDV, RGD, and RS drafted the manuscript. MIPC, and RS performed the data analysis. All authors provided significant intellectual contribution in interpreting data and critical review of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Ethics approval
The study protocol was approved by the Institutional Review Board of the Instituto Mexicano del Seguro Social (IMSS) in Mexico City (R-2018-785-026).
Consent to participate
All participants gave written informed consent.
Consent for publication
All authors approved the final version of the manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Peña-Cano, M.I., Valencia-Ortega, J., Morales-Ávila, E. et al. Omentin-1 and its relationship with inflammatory factors in maternal plasma and visceral adipose tissue of women with gestational diabetes mellitus. J Endocrinol Invest 45, 453–462 (2022). https://doi.org/10.1007/s40618-021-01671-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-021-01671-9