Abstract
Background
In recent clinical practice, an increasing number of elderly patients suffering from head and neck squamous cell carcinoma (HNSCC) of unknown pathophysiology is observed. The majority of HNSCC patients can roughly be divided into three subcategories. First, a small group of young patients who present with variants of genomic aberrations and inheritable diseases like Fanconi anaemia. Second, an increasing population of HPV-related HNSCCs that are regarded as genomic stable tumours with a more favourable prognosis. Though HPV-related tumours used to be more common among younger males, a notable rise in the elderly population is observed. The third subcategory, that of HPV-negative tumours, has been shown to be more heterogeneous with involvement of a variety of oncogenic pathways related to lifestyle factors like smoking and alcohol consumption, often seen in middle-aged males. Some of these pathways could be related to age, such as TP53 alterations, EGFR activation, apoptotic pathway alterations and field cancerization.
Conclusions
In this narrative review, we provide an overview of established and newly discovered age-specific pathophysiological mechanisms underlying HNSCC. We propose a fourth subcategory of patients with a suspected different pathophysiology: elderly (HPV-negative) HNSCC patients without a history of tobacco and alcohol consumption. In this subcategory, carcinogenesis seems to be a multi-step process based on genomic instability, immunosenescence, cell cycle disruption and telomere shortening. To conclude, we discuss suggestions for future research to fill the knowledge gap about age-dependent HNSCC carcinogenesis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Head and neck cancer is the sixth most common type of cancer worldwide, with approximately 600,000 new diagnoses and 250,000 deaths annually [1, 2]. Most head and neck cancers comprise head and neck squamous cell carcinomas (HNSCCs), derived from the mucosal epithelium in the oral cavity, pharynx or larynx. HNSCCs commonly require aggressive multimodality treatment, consisting of surgery, radiotherapy and systemic treatment. Importantly, the prognosis of HNSCC not only depends on tumour stage, treatment modality and patient-specific factors, but also on the underlying pathophysiology, which varies among patients of different ages. When considering the younger population of HNSCC patients, inherited diseases such as Fanconi anaemia (FA), Dyskeratosis Congenita (DC) and Bloom’s syndrome are acknowledged risk factors for developing HNSCC [3]. Familial oral squamous cell carcinoma (OSCC) and nasopharyngeal carcinoma are also more common in younger patients [4]. Furthermore, Human Papilloma Virus (HPV)-related tumours are predominately observed in young males [5]. This group of HNSCCs appears to be a distinct entity with a stable genome and relatively better treatment response [6,7,8,9]. The patterns noted above differ from the middle-aged patients with HNSCC. This latter category typically encompasses male patients with traditional risk factors such as excessive tobacco and alcohol consumption [10]. Tumours in this group of patients are mostly HPV-negative, and the pathophysiology of the tumours is assumed to be based on a complex multi-step process, rather than on a single molecular event/pathway [11]. Finally, ageing has contributed to an increased incidence of HPV-negative HNSCCs in the elderly population, resulting in 25 to 30% of patients being over the age of 70 [12, 13]. In this relatively new but expanding group of patients, the aetiology and pathophysiology remain largely unknown. In other tumour types, ageing has been found to be associated with genomic instability, telomere attrition, epigenetic changes, proteostasis, nutrient sensing and metabolism, as well as cellular senescence and stem cell function [14]. Based on the age-related distribution of risk factors as described above, the absence of well-known aetiological factors for HNSCC (i.e., tobacco and alcohol consumption) in elderly patients suggests the involvement of different oncogenic pathway(s). This could subsequently influence treatment response and prognosis [15]. Unravelling potential differences in oncogenic pathways in this specific subcategory of elderly patients with HNSCC could be helpful to facilitate treatment decisions and possibly generate age-specific treatment strategies. As yet, however, the literature is scarce on this issue.
The aim of this review was to describe the distinct age-specific characteristics of HNSCC. We will provide an overview of the literature on potential age-specific oncogenic pathways in the four suggested subcategories of HNSCC patients, being (i) inherited HNSCC, (ii) HPV-related HNSCC, (iii) HPV-negative HNSCC in young and middle-aged patients and (iv) elderly patients not exposed to tobacco and/or alcohol. Distinction of the latter group of elderly HNSCC patients is a novelty and may have clinical consequences, leading to different treatment policies for this group, which will be discussed in the final chapter. Given the broad scope and aim of this review, an open literature search rather than a systematic literature search was performed in Pubmed/Medline. The description of our search strategy is summarized in Appendix 1.
2 Inherited HNSCC
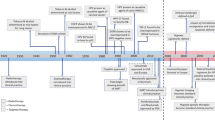
Inherited cancers occur due to hereditary genetic mutations, and account for 5–10% of all cancer cases [4]. These cancer types often affect younger patients [4]. An overview of the most well-known inherited head and neck cancer syndromes is listed below and shown in Fig. 1.
Inherited head and neck cancer syndromes. Autosomal dominant, autosomal recessive and X-linked inheritance patterns have been described for inherited HNSCC, affecting mostly young patients. In familial OCSCC a series of mutations in tumour suppresser genes has been described [4, 16]. In Li Fraumeni syndrome, germline mutations in TP53 have been found to be responsible for an increased cancer risk [28]. Germline mutations in Fanconi anemia deregulate cellular DNA repair pathways, resulting in HNSCC [18]. In Dyskeratosis Congenita, mutations in telomere maintenance genes cause telomere shortening leading to an increased cancer risk [4, 23]. A high rate of loss-of-heterozygosity resulting in chromosomal instability is responsible for an increased cancer risk in patients with Bloom’s syndrome [24, 26]. The increased risk for familial nasopharyngeal carcinoma is thought to be multifactorial, including genetic, ethnic and environmental factors, as well as Epstein-Barr virus infection [22]
Familial Oral Squamous Cell Carcinoma (OSCC) is an autosomal dominant disorder [4]. Impairment in regulator proteins such as overexpression of MDM2 [16] and deletion of the CDKN2 locus and loss of ADP-Ribosylation Factor 1 (ARF) affecting p53 degradation and p53 response to DNA damage are found in familial HNSCC [4]. Germline TP16 mutations, associated with increased p16/CDKN2A protein levels are particularly linked to OSCCs [16].
Fanconi anaemia (FA) results from chromosomal instability due to biallelic mutations in one of the 17 known FA genes (FANCA to FANCS), inherited in an autosomal recessive or X-linked recessive pattern [17]. Germline mutations in genes of cellular DNA repair pathways are assumed to facilitate accumulation of mutations during HNSCC development [18]. Mutations in these FA genes may also contribute to the development of HNSCC in general [18]. Several studies have investigated the role of sporadic genetic variants of FA in the development of HNSCC in non-Fanconi patients, i.e., downregulation [19], loss of heterozygosity [18] and increased mutational loads [20] of multiple FA genes. Increased mutation of FANCD2, FANCE, and lower expression of Glutathione S-Transferase P1 (GSTP1), FANCA and FANCG proteins has been reported to be particularly common among younger patients [20, 21].
The aetiology of Familial Nasopharyngeal Carcinoma (fNPC) is thought to be multifactorial, being based on variations in ethnicity, genetics, environmental factors and infection with Epstein-Barr virus (EBV) [22]. fNPC has a pronounced geographic distribution [4]. Thus far, several susceptibility loci or genes and polymorphisms in immune-related cytokines and surface proteins on immune cells have been found to be associated with fNP, but the results are not concordant and, therefore, preclude firm conclusions [22].
Dyskeratosis Congenita follows various inheritance patterns: X-linked, autosomal dominant or autosomal recessive [4]. In Dyskeratosis Congenita-related cancer, mutations in 10 genes have been described (including DKC1, TERC, TERT, TINF2, NOP10, NHP2, TCAB1, C16orf57, RTEL1) [4, 23]. Mutations in nine of these genes affect telomere maintenance, leading to excessively short telomeres and, consequently, cancer development [23].
Bloom's syndrome is an autosomal recessive disorder characterized by early predisposition to multiple cancers, including HNSCC [24, 25]. Loss-of-function mutations of the BLM gene cause chromosome instability. A resulting fourfold higher rate of mutations and a 50-fold higher rate of loss of heterozygosity are likely to be responsible for the increased cancer risk [24, 26].
Li Fraumeni syndrome is an autosomal dominant disorder [27]. Individuals with this syndrome often harbour germline mutations in the p53 tumour suppressor gene [28], resulting in a 50% increased risk of developing cancer by the age of 30 and a 90% increased risk by the age of 70 [28, 29]. Also missense mutations located primarily in exons 4–9, harbouring hot spot codons 205–248, have been associated with Li Fraumeni syndrome [16].
Based on these observations, it appears that inherited HNSCC syndromes share pathways involved in (dis)functional DNA damage repair systems and surveillance of genetic stability. The affected patients are essentially of younger age [30]. It remains unclear why they have a predilection for squamous cell carcinoma, but it is worth noting that the affected genes are similar to those seen in non-inherited HNSCC, and include p53, p16 and FANCA-M [3].
3 Age-related pathways in HPV-related HNSCC
The prevalence of HPV-related HNSCC, mostly oropharyngeal squamous cell carcinoma (OPSCC), is described as between 36% and 46% [31, 32]. Previous studies showed that most patients with HPV-related OPSCC are males between 45 and 60 years of age [5, 33], have fewer comorbidities, report less tobacco exposure and higher numbers of sexual partners compared to traditional HNSCC patients [5, 34,35,36,37]. However, a recent study showed that the incidence of (HPV-related) oropharyngeal cancer is also increasing in the older population [38]. HPV-related tumours have fewer genomic aberrations than HPV-negative tumours [9], suggesting that these tumours have a relatively stable genome compared to HPV-negative tumours. Infection with HPV into the host cellular genome and expression of the E6 and E7 oncoproteins result in degradation of p53 and functional inactivation of the Retinoblastoma (Rb) protein [39] (Fig. 2). E7-driven inactivation of Rb leads to p16 overexpression, as Rb normally represses p16 transcription [6]. The p16 protein decelerates cell cycle progression from the G1 phase to the S phase by binding to cyclin dependent kinase (CDK)4 or 6, thereby preventing the formation of a catalytically active cyclin D–CDK4/CDK6 complex to release E2F through phosphorylation of the Rb protein. This liberates E2F1 from its bound state in the cytoplasm and allows it to enter the nucleus. Once in the nucleus, E2F1 promotes the transcription of target genes that are essential for transition from the G1 to S phase [40,41,42]. Hereby, cells are released from their growth inhibitory effects, resulting in abnormal cell cycling and growth.
Role of ageing in HPV-driven HNSCC. HPV-driven HNSCCs are located at the oropharyngeal subsite (tonsils, base of the tongue), mostly in male patients < 45 years of age with oral sexual contacts. In this category, induction of carcinogenesis through up-regulation of E6 and E7 has been reported, leading to cell cycle arrest failure and cell cycle disruption, respectively [39,40,41,42]. In addition, some genetic subtypes have been formulated in younger patients that may increase the risk to develop HPV-driven OSCC (blue dotted squares) [43,44,45,46]. Disrupted apoptosis through Noxa/MCL and PUMA has been suggested as a possible pathway for carcinogenesis in younger HPV+ patients (blue dotted squares) [47,48,49]. In addition, an increasing prevalence of HPV-driven HNSCC has been observed in elderly patients over thee age of 70 (red dotted square). Although the pathophysiology underlying this latter category is as yet unclear, it has been suggested that other generational norms in sexual behaviour and co-infection with other viruses such as EBV could play a role [5, 50, 51]
When it comes to ageing, certain genetic changes also increase the risk for developing HPV-related tumours, particularly in younger patients (Fig. 2). HPV-related tumours exhibit specific deletions of the chromosomal regions 14q32 and 9q, which contain tumour necrosis factor receptor associated factor 3 (TRAF3), focal amplification of E2F1 and, in contrast to HPV-negative HNSCC, lack of deletions in the 9q21.3 region containing the CDNK2A gene [43, 44]. Also, amplification of the chromosome 3q26-28 region containing tumour protein 63 (TP63), sex determining region Y-box 2 (SOX2) and Phosphatidylinositol-4,5-Bisphosphate 3-Kinase Catalytic Subunit Alpha (PIK3CA) have been observed in HPV-related tumours [39]. PIK3CA, the gene that encodes the p110 alpha subunit of Phosphoinositide-3-kinase (PI3K), is the most frequently altered oncogene in HNSCC overall, with a possible enrichment in HPV-related tumours [45]. The apolipoprotein B mRNA-editing enzyme catalytic subunit (APOBEC) has emerged as a potential mutagenic factor in HPV-related tumours, resulting from high cytosine deaminase activity [44]. Besides this, mutations in Fibroblast Growth Factor Receptor (FGFR) 2 and 3 have also been identified in HPV-related tumours [46], as well as rare FGFR3-Transforming Acidic Coiled-coil-Containing 3 (TACC3) fusions [43]. Mutation rate differences were not found to be associated with HPV status [39, 44].
In younger patients, another possible pathway in HPV-related tumours is that of disrupted apoptosis and/or uncontrolled cellular growth (Fig. 2). Apoptosis (programmed cell death) can be activated via two routes, i.e., by intracellular mitochondrial signalling (intrinsic pathway) or receptor mediated signalling (extrinsic pathway) [47]. Genetic variants of Phorbol 12-myristate 13-acetate induced protein 1 (PMAIP1 gene, also known as Noxa), myeloid cell leukaemia 1 (MCL1) [48] and p53 upregulated modulator of apoptosis protein (PUMA) [49] seem to be involved in the apoptotic cascade of HPV-related HNSCC. Noxa is a pro-apoptotic protein, while MCL1 is an anti-apoptotic protein; both are regulated by p53. Based on these mechanisms, the balance between Noxa and MCL1 is influencing the intrinsic apoptotic pathway. Puma, another pro-apoptotic protein, influences the intrinsic apoptotic pathway via E6‐mediated p53 degradation [49]. Both intrinsic apoptotic pathways seem to be related to younger patients [48, 49].
Over the past years the age of diagnosis for HPV-related oropharyngeal squamous cell carcinoma (OPSCC) has increased rapidly, with a simultaneous rise in the proportion of HPV-related OPSCCs among all age groups – especially in the elderly population [50]. Elderly patients with HPV-related OPSCC have an inferior survival rate compared to younger HPV-related OPSCC patients [50]. In a study of Rettig et al. [50], the authors further specified the characteristics of the elderly cohort with HPV-related OPSCC and noted significantly higher comorbidity scores in elderly patients compared to younger patients. Moreover, elderly patients tended to present with higher T-stages and less likely to be treated by surgery. Furthermore, the 70+ population received palliative treatment more often than the sub-cohort of patients under 50 years. All these factors may contribute to inferior survival outcomes in the elderly. Another explanation may be co-infection with EBV or other viruses that are associated with the effect of increasing cultural acceptability of certain sexual behaviours [5, 50]. Viral co-infection with EBV in particular could enhance invasive phenotypes of HPV-related OPSCC by a delay in epithelial differentiation and the establishment of EBV latency [51]. However, results on viral co-infection and HPV-related tumours remain conflicting, since some researchers claim that this is mainly the case in OSCC rather than in OPSCC [52], while others could not find subsite-related correlations nor age-related differences [53].
Overall, HPV-related HNSCCs show a different biological behaviour with a more favourable prognosis compared to HPV-negative tumours [6]. The survival advantage of HPV-positivity is, however, attenuated in older age groups [50].
4 Age-related pathways in HPV-negative HNSCC
According to the Surveillance, Epidemiology, and End Results (SEER) Cancer Statistics data, the peak incidence of head and neck cancer is between 55–65 years [10]. Tobacco and alcohol abuse are the most well-known aetiological factors with a synergistic effect in the development of HNSCC [54], particularly in this ‘middle aged’ patient category [15]. These types of head and neck tumours are remarkably heterogeneous, as highlighted by various RNA and DNA profiling studies [55]. Several potential pathophysiological pathways have been described for this third sub-category of HNSCC patients, and are listed below and summarized in Fig. 3.
Age-related pathways in HPV-negative HNSCC. Tobacco and alcohol consumption have been linked with various pathways leading to HNSCC. Mutations in TP53, associated with tobacco and alcohol consumption, are responsible for cell cycle de-regulation and apoptosis. A high p53 expression is more frequently observed in young patients with HNSCC and tongue SCC [70, 71]. Also higher expression of EGFR, causing (in)activation of various pathways that influence cell proliferation, apoptosis, metastasis and angiogenesis, has been found in younger patients [78, 79]. Loss of heterozygosity at chromosomes 3p, 9p and 17p and TP53-mutated clonal units represent early oncogenic changes in the mucosa, described as field cancerization, which is related to middle-aged patients [59]. Conflicting results concerning age and GST polymorphisms, involved in the cellular detoxification pathway, have been reported [73, 74, 76]. For angiogenesis, young and elderly OSCC patients showed similar results [83, 141]. MSI is characterized by expansion or contraction of short tandem repeats, and indicates genomic instability. In normal human somatic cells, MSI increases linearly with age. It has also been associated with head and neck tumours, although the age-specific results in HNSCC vary between studies [86, 90, 91]. Epigenetic alterations represent heritable changes that affect gene expression without changing the DNA sequence. Hypermethylation is more commonly seen in tumours of younger female patients, particularly at the anterior tongue [95]. The most frequently mutated genes in young patients with tongue SCC have been reported to be TP53, CDKN2A, NOTCH1, CASP8, FAT1, PIK3CA and MLL2 [98]
The process of field cancerization can be explained by a mechanism in which multiple cell groups undergo neoplastic transformation due to stress resulting from regional carcinogenic activity (i.e., smoking and/or excessive alcohol usage). Presumably, a critical genetic alteration in a single cell attains a growth advantage over its neighbouring cells. At some point after transformation, cells harbouring these early genetic alterations migrate to colonize contiguous tracts of mucosa, accumulate other alterations, acquire additional growth advantages, and ultimately transform into aggressive subclones [56, 57]. At the molecular level in dysplasia, loss of heterozygosity at chromosomes 3p, 9p and 17p reflect these early carcinogenic steps (Fig. 3) [15]. In addition, p53-mutated clonal units represent early oncogenic changes in the mucosa [15, 56]. A significant increase in both numerical and structural chromosome abnormalities has been found to be associated with an increased age [58, 59]. The size of precancerous fields has also been found to be larger in older patients compared to younger patients [60]. Consequently, older patients (> 50 years) appear to have an increased recurrence risk after surgical removal of head and neck tumours compared to younger patients [60].
Tobacco and alcohol consumption are primarily associated with alterations in the tumour suppressor gene TP53 [61,62,63], reported to be affected in 53–80% of HNSCCs [11, 15, 64]. During the cell cycle, p53 induces p21, a CDK inhibitor that arrests the cell cycle [65]. The presence of a TP53 mutation has been found to be a negative pro gnostic factor for HNSCC in various studies [11, 15, 64, 66, 67]. Over the last decades, the relation between TP53 mutations and age has extensively been studied [67,68,69,70,71]. Since populations and methodologies vary widely among these studies it is difficult to compare the results. In most studies no relation with age was found [67, 70,71,72], but two studies found an increase in TP53 mutations in younger HNSCC patients compared to older patients [68, 69].
Glutathione S-transferase (GST) is an important enzyme in the detoxification of cells from carcinogenic substrates, such as tobacco components, that can cause DNA damage. Besides the strong correlation with smoking, a GST polymorphism has also been found to be a potential risk factor for HNSCC [73] and, more specifically, for LSCC in young adults [74]. In patients with a GSTM1 and GSTT1 null genotype, the entire gene is absent, resulting in complete loss of functional activity of the respective enzymes [75]. The GSTT1 null genotype seems to be related to a higher age of onset in patients with OSCC [76].
Amplification of Epidermal Growth Factor Receptor (EGFR) plays an important role in the development of HPV-negative HNSCC and may act as a driver [43]. As a result, specific signalling functions of EGFR may be disturbed, causing (in)activation of different pathways that affect cell proliferation, apoptosis, invasion, angiogenesis and metastasis. The exact contribution of the EGFR pathway in HNSCC is still elusive. Numerous studies have investigated the prognostic value of EGFR expression in head and neck cancer, concluding that elevated EGFR levels are associated with a reduced survival [77]. Also, higher EGFR expression seems to be relate d to younger age [78, 79].
Enhanced angiogenesis is suggested to be involved in tumour growth, advanced clinical stage, metastasis, and to be associated with a worse prognosis [80, 81]. The angiogenesis profile does not seem to differ betwe en young and elderly HNSCC patients [82,83,84], or to be correlated with age at all in HNSCC patients [80]. Distinct mutational clusters in regions that regulate angiogenesis have been identified in very old patients (aged 81 to 87) compared to young HNSCC patients (aged 19 to 40) [85]. No differences among gene mutation patterns were seen, rather an accumulation of mutations in the elderly group. The role of the tumour microenvironment (TME) harbouring stimulating neovascularization factors has been discussed widely over the last decades. To our knowledge, the impact of ageing on specific parts of the TME involved in angiogenesis has not yet been described, and this gap in the literature is mentioned in some studies (e.g. ref. 84).
Loss of heterozygosity (LOH) has also been associated with the development of HNSCC. Most studies on this topic focus on correlations between LOH and prognosis, which is described for regions on chromosome arms 3p, 8p, 9p, 14q, 17p and 18q [86, 87]. No relation between LOH and age in HNSCC has been found so far [88, 89]. Also, no consensus has yet been reached on the relation between microsatellite instability (MSI) and age, i.e., the results are contradictory [86, 90, 91]. MSI is characterized by expansion or contraction in the length of short tandem repeats and, just like LOH, it indicates genomic instability. In normal human somatic cells, MSI increases linearly with age, but it has also been associated with head and neck tumours [92].
Epigenetic alterations such as DNA methylation, histone modification, chromatin remodelling and non-coding RNA effects have been associated with HNSCC [93], which suggests that they may play a role in driving HNSCC development [94]. As yet, contradictions exist regarding the relation between p16 methylation a nd age in HNSCC [45, 95].
The incidence of oral tongue squamous cell carcinoma (OTSCC) in young patients without exposure to conventional risk factors is increasing worldwide [96]. The most frequently mutated genes in OTSCCs in young patients are TP53, CDKN2A, NOTCH1, CASP8, FAT1, PIK3CA and MLL2 [69, 97], comparable to those affected in elderly patients [98]. Recently, two novel driver genes, ATXN1 and CDC42EP, have been added to this list of frequently mutated genes in OTSCC [97]. Early‐onset OTSCC patients seem to have fewer (non-silent) mutations than older OTSCC patients, when adjusted for tobacco use [59, 97].
To conclude, it seems that in HPV-negative tumours cells can accommodate various genetic alterations that lead to the same clinical disease state. Several pathways can be distinguished and, therefore, this type of HNSCC appears to be heterogeneous. TP53, EGFR and apoptotic pathway alterations seem to be age-dependent and to more frequently affect younger patients. The mutational burden seems to be lower in younger OTSCC patients, while field cancerization seems more pronounced in the ‘classic’ middle-aged patient category. For both angiogenesis and genomic instability pathways, no relation with age has been found. Conflicting results regarding age in relation with the GST-pathway and epigenetic alterations have been reported. Further exploration of these pathways could be important for the development of molecular targeted therapies, regardless patient age.
5 Pathophysiology of HNSCC in elderly patients
In patients over the age of 70, the most affe cted tumour sites comprise the larynx, oropharynx and oral cavity [99]. In contrast to the middle-aged population —whic h is either ex posed to extensive tobacco and alcohol usage or HPV-infection – the underlying cause and tumour biology amongst the elderly re main largely unknown. This knowledge gap may result in inferior treatment outcomes in the elderly. Currently, elderly patients receive comparable treatment to younger patients, including radiation therapy and/or surgery. However, based on a meta-analysis by Pignon et al. [100], it has become clear that patients over the age of 70 do not receive adjuvant chemotherapy due to the absence of survival benefit. A better insight into the pathophysiology of HNSCC could form a basis for clinical studies on novel, age-specific treatment strategies in the elderly. Unfortunately, studies regarding this topic are scarce.
It is well-known that the risk of developing cancer increases with age: by the time an individual passes the age of 70, the chance of developing any type of invasive cancer has risen to 33%, compared to approximately 6% of individuals below the age of 60 [101]. This suggests an overlap in molecular biology of ageing with carcinogenesis. Overlapping mechanisms between ageing and carcinogenesis of various tumours have been described regarding telomere shortening, genomic instability, epigenetic alterations, loss of proteostasis, mitochondrial dysfunction, cellular senescence and stem cell exhaustion [14, 102]. However, only very few basic and translational studies focusing on the pathophysiology of HNSCC in elderly patients as a separate group have been reported. These are discussed below and summarized in Fig. 4.
Possible pathophysiological mechanisms of HNSCC in elderly patients. Overlapping mechanisms between ageing and carcinogenesis have been described regarding telomere shortening, genomic instability, epigenetic alterations, loss of proteostasis, mitochondrial dysfunction, cellular senescence and stem cell exhaustion [14, 102]. However, only four mechanisms have been described in HNSCC in elderly patients as a separate group. The first, genomic instability, seems more frequent in elderly patients causing impaired cell mobility and increased tumour invasion and angiogenesis [85]. Second, elderly patients developing HNSCC are thought to have deteriorated or weaker immune responses (immunosenescence) compared to younger patients with an active immune system. As suggested by Schuler et al., young subjects may have more CD8+ T cells expressing mainly CCR7 and CD73, while old subjects may have less CD8+ T cells expressing more PD-1. Young patients have an active immune system with a strong tumour-induced immune suppression with many Tregs, while old patients have a senile immune system with a weak immune suppression and less Tregs [128]. Third, ageing is believed to cause inactivation of the FAT1 tumour suppressor which, subsequently, leads to activation of the Wnt signalling pathway causing cell cycle disruption [8, 125]. Many studies have investigated the role of telomere shortening and telomerase reverse transcriptase (TERT) in HNSCC. Both smoking and HPV infection have been shown to induce TERT activity. Remarkably, this has not yet been proven for ageing in HNSCC, while telomere shortening is believed to be one of the driving factors of ageing [107, 109, 120]
5.1 Telomere shortening
Shortening of telomere length is a well-known phenomenon associated with ageing [103]. The lifetime of a cell is ultimately limited by the length of its telomeres, which shorten after every cell division until all telomeres become critically short and the cell becomes senescent. Senescence prevents infinite cell divisions with a concurrent accumulation of mutations, thereby also reducing the risk of cancer development. This process is counteracted by telomerase reverse transcriptase (TERT), an enzyme whose activity can result in prolonged or even unlimited cell divisions [104].
Carcinogenesis in HNSCC has also been associated with a shortened telomere length and subsequent TERT overexpression [103, 105, 106]. Aberrant telomere shortening has been observed in pre-cancerous lesions of HNSCC and has been suggested to be a marker for field cancerization [107,108,109]. Increased expression of TERT has been found to be highly specific for malignant LSCC [110], but has also been reported in other HNSCC sub-sites [106]. Considering the molecular pathways underlying TERT overexpression, one can distinguish HPV-related OPSCC from non-HPV related HNSCC. In non-HPV related HNSCC, overexpression is often the result of mutations in the TERT gene promoter [111,112,113]. This phenomenon has been linked to life-style factors such as smoking, and results in a poor overall survival of LSCC patients [114,115,116]. In HPV-related OPSCC, the high-risk oncoproteins E6 and E7 are thought to be responsible for inactivation of Rb, p53 and subsequent increased expression of TERT. Nevertheless, in vitro studies with TERT inhibitors revealed alternative lengthening of telomeres in keratinocytes [117], suggesting that telomere homeostasis is maintained by various pathways [118].
Although ageing leads to telomere shortening, TERT overexpression in HNSCC strongly correlates with tumour aggressiveness (poor differentiation grade, increased risk for metastasis and poor response to treatment), rather than with patient age [107, 119, 120]. In one study, age and telomere length were investigated in HNSCC, but no correlation was found in neither cancer tissue nor in surrounding mucosa [107]. The authors speculated that patients with short telomeres found in mucosa surrounding the tumour had a higher risk of mucosal failure based on a study performed on colorectal carcinoma in which telomere length in non-cancerous cells was inversely correlated with age [121].
5.2 Patterns of genetic variants
In HNSCC a comprehensive genetic analysis on mutational load and mutational patterns during ageing has been performed [85]. In this study, somatic single nucleotide polymorphisms (SNP’s) in 203 selected HPV-negative and TP53-negative patients whose genetic data were available via The Cancer Genome Atlas (TCGA) were investigated. In concordance with other studies on ageing, the investigators found that mutation frequency rather than mutation spectrum differed between young and old patients with HNSCC. By analysing genetic clusters of ‘very old’ patients (defined as 81–87 years old, n = 11), four pathways were found to be enriched compared to those of young patients (defined as 19–40 years old, n = 11), being the ‘axon-guidance’ pathway, the ‘extracellular matrix receptor interaction’ pathway, the ‘focal adhesion’ pathway and the ‘notch-signalling’ pathway. Although this was one of the few studies to compare genetic mutations between young and old HNSCC patients, the sample size was very limited. Also, the tumour sub-sites varied, the majority being oral cavity and larynx. Despite these limitations, the study points to possible pathways involved in HNSCC development in the ageing population and suggests further investigations on this topic.
Chromosomal aneuploidy, or the phenomenon of aberrant chromosome numbers in cells, is not only associated with syndromic disorders, but is also a common feature in tumour cells. The biological severity generally correlates with the size of the chromosome anomaly and related gene copy number changes [122]. Furthermore, the International Workshop on Genotoxicity Testing (IWGT) workgroup report stated that the frequency of aneuploidy increases with age, but is not associated with smoking or gender. This is in concordance with another study that investigated ageing and aneuploidy in 220 OSCC patients, in which in non-smokers the association of patient age with DNA aneuploid OPMDs/OSCCs was significantly higher compared to those with DNA diploid OPMDs/OSCCs [123]. The mechanism of aneuploidy further underlines the importance of genomic instability involved both in carcinogenesis and ageing, but whether this applies to other sub-sites of HNSCC and possible treatment regimens remains a subject for further investigation.
5.3 Disruption of apoptosis and cell cycle progression
Protocadherin FAT1 (FAT1) is a tumour suppressor gene located on chromosome 4q35.2 and is mutated in 23% of HNSCC cases and lost or deleted in 8% of them [11]. Inactivation of this gene promotes carcinogenesis by inducing the WNT signalling pathway [124]. WNT signalling plays a key role in cell orientation and cell fate and thereby in stem cell maintenance. In two studies, patient age was generally higher among individuals with a FAT1 mutation [69, 125]. One study showed a lower mutation frequency of FAT1 among younger patients (in this study defined as < 45 years) compared to older patients (≥ 45 years) with SCC of the oral tongue [69]. Interestingly, despite the mutation frequency of TP53 usually being associated with cigarette smoking, this study found that the mutation rate was higher in young patients who were all non-smokers. In another study, the authors suggest that FAT1 could be a potential prognostic marker in HNSCC patients based on an association between lower expression of FAT1 and improved survival in HPV-negative HNSCCs [125]. This study showed a higher mean age of individuals with the FAT1 mutation. Therefore, the actual effect of FAT1 expression is speculative and seems to play a more important role in elderly patients than in younger patients.
5.4 Immune cell signalling
Immunosenescence, defined as gradual deterioration of the immune system associated with age, is described as an important factor contributing to carcinogenesis in elderly patients [126]. Tumour infiltrating lymphocytes (TILs) and tumour-associated macrophages (TAMs) involved in the innate immune system are key components of the tumour microenvironment and drive tumour progression [127]. Changes in the total number of circulating innate immune cells or in the relative percentage of different subpopulations have been reported in elderly patients. With the introduction of new immunotherapeutic agents in the treatment of head and neck cancer (e.g. Nivolumab, Pembrolizumab), immunosenescence mechanisms may have direct clinical implications. In a recently published study, differences in T-cell subgroups and their expression profile with increasing age were investigated in healthy subjects consisting of young (40–69 years; n = 17) and elderly (70–90 years; n = 20) HNSCC patients. The authors found a lower concentration of TILs in peripheral blood samples of the elderly patients, suggesting that in elderly tumour patients the immune system is impaired and the tumour-induced immune escape is less pronounced [128]. This concept is illustrated in Fig. 4, which contains an adapted version of the figure reported by Schuler et al.
Programmed death-ligand 1 (PD-L1) is becoming increasingly important as a biomarker and therapeutic target in HNSCC [129]. PD-1 is variably expressed by head and neck tumour cells, and immunotherapies that block inhibitory immune cell signalling have demonstrated clinical efficacy in advanced head and neck cancers [130, 131]. During carcinogenesis, anti-tumour activity by the immune system is suppressed by upregulation of PD-L1 on tumour cells, which binds to PD-1 on T-cells. Conflicting results on PD-L1 levels in HNSCC and age have been reported. In one study, higher PD-L1 expression on tumour cells in HNSCC patients ≤ 45 years has been described [37]. An association was noted between a) higher PD-L1 levels, b) higher numbers of Inducible T-cell Co-Stimulator (ICOS)‐positive TILs and c) a higher ratio of FOXP3+ Tregs and ICOS+ TILs relative to effector CD8+ T cells and younger patients [37]. In contrast, in a large meta-analysis on the prognostic and clinicopathological significance of PD-L1 overexpression in OSCC, no significant association was found between PD-L1 overexpression and age (> 56, > 60, > 65 years) [132]. Conversely, two other studies found an association between elevated levels of PD-L1 in HNSCC tissues [133] and peripheral blood lymphocytes [128] and older patients. PD-L1 expression was observed in 80% of patients and was significantly associated with old age (≥ 65 years) [133]. In the second study, peripheral blood lymphocytes were obtained from HNSCC patients (n = 33, 47–90 years) and healthy volunteers (n = 48, 21–84 years). A higher PD-L1 expression was found to be associated with increased age [128]. In both studies, PD-L1 positivity [37] and high PD-L1 expression (≥ 50%) [133] seemed to be prognostic factors for a poor survival. Another study found that young female OSCC patients < 45 years with increased membranous PD-L1 positivity showed a decreased risk of recurrence and an improved survival [134].
In summary, PD-L1 expression seems to play a more important role in patient prognosis than age. However, results on immunosenescence are conflicting and consensus on the effects of immune checkpoint molecules on the prognosis of HNSCC has not yet been reached. Detailed knowledge on age-related alterations of the immune system is necessary in order to offer an adequate treatment option for this growing group of HNSCC patients and must be further investigated.
5.5 Impact of ageing on the biology of tumours other than HNSCC; DNA methylation and epigenetic clocks
Many overlapping mechanisms involving ageing and carcinogenesis have not been studied in HNSCC. A particular mechanism of interest is DNA methylation. In DNA methylation, methyl groups are covalently linked to cytosines. When such methylation occurs in a gene promoter, it can result in repression of gene transcription. In carcinogenesis, DNA methylation has been associated with inactivation of tumour suppressor genes [135]. Interestingly, DNA-methylation as a marker for “epigenetic aging” has been shown to correlate well with biological aging [136]. The first “epigenetic clock” theory originates from Horvath et al. [137] and has inspired others to identify methylation biomarkers and drifts in blood that can predict aging [138]. Based on application of these methods, investigators were able to predict a biological age acceleration in colorectal carcinoma that was associated with disease onset and/or death [139]. Other subsites showing epigenetic drift include hematopoietic stem cells and skin cells [138]. Unfortunately, as mentioned previously, DNA methylation has so far only been investigated in younger patients with HNSCC leading to contradictory results.
Based on the mechanisms described above, we conclude that in elderly HNSCC patients accumulation of mutations is the most relevant driving force [85]. Carcinogenesis seems to represent a multi-step process rather than a single hit mutation in this specific group of patients. Further research, especially in the field of immunosenescence, holds promise for the identification of potential prognostic biomarkers and the development of novel therapeutic strategies.
6 Conclusions, recommendations for future research and possible clinical applications
The aim of this review was to provide an overview of potential age-specific molecular pathways in HNSCC. By doing so, we introduced a possible new biological entity: non-intoxication driven and non-HPV related HNSCC of the elderly. Age-related pathophysiology in inherited HNSCC, HPV-related HNSCC and HPV-negative HNSCC has been outlined. The number of studies investigating the molecular background of HNSCC in general is overwhelming. Our search for age-related carcinogenesis in particular was, however, hampered by the fact that most studies do not focus on ageing as a central question, resulting in poorly defined age categories with divergent cut-off values, and a subsequent lack of specific comparisons among age categories. In addition, many results on age-specific molecular mechanisms seem to be ambiguous.
Due to the lack of molecular studies in elderly patients in particular, identification of possible pathways required deduction of findings in studies from other tumour types. These studies suggest a higher degree of accumulating genetic mutations acquired with ageing, which was also cautiously confirmed in HNSCC [85]. Furthermore, immunosenescence is intensely studied, but consensus on this subject has not yet been reached, while studies on mechanisms like loss of proteostasis or differences in epigenetics and cellular senescence in HNSCC still need to be initiated.
Because of the lack of studies and the heterogeneous character of HNSCC in elderly patients, it seems impossible to point out one specific targeted therapy for this patient category. An attractive strategy to identify molecular pathways and potential therapeutic targets, however, is the use of multi-omics profiling technologies. Multi-omics profiling or integrative omics is a comprehesive molecular approach in which multiple molecular features, such as the genome, proteome, phosphoproteome, transcriptome, epigenome, metabolome and microbiome, are measured as comprehensive as the analytical technology allows. In contrast to single-omic analyses, multi-omic analysis integrates molecular data layers in multiple steps based on molecular information (genomics to proteomics and metabolomics) in order to find a causal relationship between molecular alterations, for instance at pre- and post-translational levels. Therefore, a multi-omic approach is thought to provide a more complete tumour biology picture.
A recent study by Huang et al. used this principle to identify subgroups of HNSCC patients that could respond to targeted therapy [140]. Based on multi-omic data in 110 patients with HPV-negative tumours, the investigators could distinguish three clusters of (epi)genetic, proteomic and phospho-proteomic profiles that could potentially respond to treatment with CDK4/6 inhibitors, anti-EGFR antibodies or immunotherapy. Each cluster contains its own set of markers and it would be interesting to use these markers in the analysis of HPV-negative elderly patients to see in which molecular cluster they fit, and to estimate whether they could benefit from one of the suggested targeted therapies. Once future multi-omic analysis techniques become less costly and available for clinical practice, individualized multi-omics may become the diagnostic approach for HNSCC patients in all age-categories, not only the elderly, to provide tailor-made treatment options.
Data availability
Not Applicable as the manuscript is a review containing no original data.
References
F. Bray, J. Ferlay, I. Soerjomataram, R. L. Siegel, L. A. Torre, A. Jemal, Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J. Clin. 68, 394–424 (2018). https://doi.org/10.3322/caac.21492.
J. Ferlay, M. Colombet, I. Soerjomataram, C. Mathers, D.M. Parkin, M. Piñeros, A. Znaor, F. Bray, Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 144, 1941–1953 (2019). https://doi.org/10.1002/ijc.31937
H.S. Van Monsjou, V.B. Wreesmann, M.W.M. Van Den Brekel, A.J.M. Balm, Head and neck squamous cell carcinoma in young patients. Oral Oncol. 49, 1097–1102 (2013). https://doi.org/10.1016/j.oraloncology.2013.09.001
R. Venugopal, R. M. Bavle, P. Konda, S. Muniswamappa, S. Makarla, Familial cancers of head and neck region. J. Clin. Diagn. Res. 11, ZE01–ZE06 (2017). https://doi.org/10.7860/JCDR/2017/25920.9967.
A.K. Chaturvedi, E.A. Engels, W.F. Anderson, M.L. Gillison, Incidence trends for human papillomavirus-related and -unrelated oral squamous cell carcinomas in the United States. J. Clin. Oncol. 26, 612–619 (2008). https://doi.org/10.1200/JCO.2007.14.1713
F. Dayyani, C.J. Etzel, M. Liu, C.H. Ho, S.M. Lippman, A.S. Tsao, Meta-analysis of the impact of human papillomavirus (HPV) on cancer risk and overall survival in head and neck squamous cell carcinomas (HNSCC). Head Neck Oncol. 2, 15 (2010). https://doi.org/10.1186/1758-3284-2-15
K.K. Ang, J. Harris, R. Wheeler, R. Weber, D.I. Rosenthal, P.F. Nguyen-Tân, W.H. Westra, C.H. Chung, R.C. Jordan, C. Lu, H. Kim, R. Axelrod, C.C. Silverman, K.P. Redmond, M.L. Gillison, Human papillomavirus and survival of patients with oropharyngeal cancer. N. Engl. J. Med. 363, 24–35 (2010). https://doi.org/10.1056/nejmoa0912217
B. O’Sullivan, S.H. Huang, J. Su, A.S. Garden, E.M. Sturgis, K. Dahlstrom, N. Lee, N. Riaz, X. Pei, S.A. Koyfman, D. Adelstein, B.B. Burkey, J. Friborg, C.A. Kristensen, A.B. Gothelf, F. Hoebers, B. Kremer, E.J. Speel, D.W. Bowles, D. Raben, S.D. Karam, E. Yu, W. Xu, Development and validation of a staging system for HPV-related oropharyngeal cancer by the international collaboration on oropharyngeal cancer network for staging (ICON-S): a multicentre cohort study. Lancet Oncol. 17, 440–451 (2016). https://doi.org/10.1016/S1470-2045(15)00560-4
A. Villepelet, S. Hugonin, S. Atallah, B. Job, B. Baujat, J. Lacau St Guily, R. Lacave, Effects of tobacco abuse on major chromosomal instability in human papilloma virus 16-positive oropharyngeal squamous cell carcinoma. Int. J. Oncol. 55, 527–535 (2019). https://doi.org/10.3892/ijo.2019.4826.
N. Howlader, A. M. Noone, M. Krapcho, J. Garshell, D. Miller, S. F. Altekruse, C. L. Kosary, M. Yu, J. Ruhl, Z. Tatalovich, A. Mariotto, D. R. Lewis, H. S. Chen, E. J. Feuer, K. A. Cronin, SEER Cancer Statistics Review, 1975–2011, National Cancer Institute. Based on November 2013 SEER data submission, posted to the SEER web site, April 2014. Seercancergov/Csr. (2014). Accessed October 2020.
C.R. Leemans, P.J.F. Snijders, R.H. Brakenhoff, The molecular landscape of head and neck cancer. Nat. Rev. Cancer 18, 269–282 (2018). https://doi.org/10.1038/nrc.2018.11
S. Hartmann, J.R. Grandis, Treatment of head and neck cancer in the elderly. Expert Opin. Pharmacother. 17, 1903–1921 (2016). https://doi.org/10.1080/14656566.2016.1220540
T.T.A. Peters, B.A.C. Van Dijk, J.L.N. Roodenburg, B.F.A.M. Van Der Laan, G.B. Halmos, Relation between age, comorbidity, and complications in patients undergoing major surgery for head and neck cancer. Ann. Surg. Oncol. 21, 963–970 (2014). https://doi.org/10.1245/s10434-013-3375-x
J.R. Aunan, W.C. Cho, K. Søreide, The biology of aging and cancer: a brief overview of shared and divergent molecular hallmarks. Aging Dis. 8, 628–642 (2017). https://doi.org/10.14336/AD.2017.0103
C.R. Leemans, B.J.M. Braakhuis, R.H. Brakenhoff, The molecular biology of head and neck cancer. Nat. Rev. Cancer 11, 9–22 (2011). https://doi.org/10.1038/nrc2982
S.S. Prime, N.S. Thakker, M. Pring, P.G. Guest, I.C. Paterson, A review of inherited cancer syndromes and their relevance to oral squamous cell carcinoma. Oral Oncol. 37, 1–16 (2001). https://doi.org/10.1016/S1368-8375(00)00055-5
A.T. Wang, A. Smogorzewska, SnapShot: fanconi anemia and associated proteins. Cell 160, 354-354.e1 (2015). https://doi.org/10.1016/j.cell.2014.12.031
C. Turke, S. Horn, C. Petto, D. Labudde, G. Lauer, G. Wittenburg, Loss of heterozygosity in FANCG, FANCF and BRIP1 from head and neck squamous cell carcinoma of the oral cavity. Int. J. Oncol. 50, 2207–2220 (2017). https://doi.org/10.3892/ijo.2017.3974
V.B. Wreesmann, C. Estilo, D.W. Eisele, B. Singh, S.J. Wang, Downregulation of fanconi anemia genes in sporadic head and neck squamous cell carcinoma. ORL J. Otorhinolaryngol. Relat. Spec. 69, 218–225 (2007). https://doi.org/10.1159/000101542
S.C. Chandrasekharappa, S.B. Chinn, F.X. Donovan, N.I. Chowdhury, A. Kamat, A.A. Adeyemo, J.W. Thomas, M. Vemulapalli, C.S. Hussey, H.H. Reid, J.C. Mullikin, Q. Wei, E.M. Sturgis, Assessing the spectrum of germline variation in fanconi anemia genes among patients with head and neck carcinoma before age 50. Cancer 123, 3943–3954 (2017). https://doi.org/10.1002/cncr.30802
S. Tremblay, P.P. Dos Reis, G. Bradley, N.N. Galloni, B. Perez-Ordonez, J. Freeman, D. Brown, R. Gilbert, P. Gullane, J. Irish, S. Kamel-Reid, Young patients with oral squamous cell carcinoma: study of the involvement of GSTP1 and deregulation of the fanconi anemia genes. Arch. Otolaryngol. Head Neck Surg. 132, 958–966 (2006). https://doi.org/10.1001/archotol.132.9.958
J.X. Bei, W.H. Jia, Y.X. Zeng, Familial and large-scale case-control studies identify genes associated with nasopharyngeal carcinoma. Semin. Cancer Biol. 22, 96–106 (2012). https://doi.org/10.1016/j.semcancer.2012.01.012
M. S. Fernández García, J. Teruya-Feldstein, The diagnosis and treatment of dyskeratosis congenita: A review. J. Blood Med. 5, 157–167 (2014). https://doi.org/10.2147/JBM.S47437.
C. Cunniff, J.A. Bassetti, N.A. Ellis, Bloom’s syndrome: clinical spectrum, molecular pathogenesis, and cancer predisposition. Mol. Syndromol. 8, 4–23 (2017). https://doi.org/10.1159/000452082
A.S. Berkower, H.F. Biller, Head and neck cancer associated with bloom’s syndrome. Laryngoscope 98, 746–748 (1988). https://doi.org/10.1288/00005537-198807000-00012
A.M.R. Taylor, C. Rothblum-Oviatt, N.A. Ellis, I.D. Hickson, S. Meyer, T.O. Crawford, A. Smogorzewska, B. Pietrucha, C. Weemaes, G.S. Stewart, Chromosome instability syndromes. Nat. Rev. Dis. Primers. 5, 64 (2019). https://doi.org/10.1038/s41572-019-0113-0
P.L. Friedlander, Genomic instability in head and neck cancer patients. Head Neck 23, 683–691 (2001). https://doi.org/10.1002/hed.1096
O. Michaeli, D. Malkin, Li-Fraumeni Syndrome. Encyclopedia of cancer. 356–368 (2018). https://doi.org/10.1016/B978-0-12-801238-3.96122-1.
M. Akashi, H.P. Koeffler, Li-Fraumeni syndrome and the role of the p53 tumor suppressor gene in cancer susceptibility. Clin. Obstet. Gynecol. 41, 172–199 (1998). https://doi.org/10.1097/00003081-199803000-00024
T.N. Toporcov, A. Znaor, Z.F. Zhang, G.P. Yu, D.M. Winn, Q. Wei, M. Vilensky, T. Vaughan, P. Thomson, R. Talamini, N. Szeszenia-Dabrowska, E.M. Sturgis, E. Smith, O. Shangina, S.M. Schwartz, S. Schantz, P. Rudnai, L. Richiardi, H. Ramroth, M.P. Purdue, A.F. Olshan, J. Eluf-Neto, J. Muscat, R.A. Moyses, H. Morgenstern, A. Menezes, M. McClean, K. Matsuo, D. Mates, T.V. Macfarlane, J. Lissowska, F. Levi, P. Lazarus, C. La Vecchia, P. Lagiou, S. Koifman, K. Kjaerheim, K. Kelsey, I. Holcatova, R. Herrero, C. Healy, R.B. Hayes, S. Franceschi, L. Fernandez, E. Fabianova, A.W. Daudt, O.A. Curioni, L.D. Maso, M.P. Curado, D.I. Conway, C. Chen, X. Castellsague, C. Canova, G. Cadoni, P. Brennan, S. Boccia, J.L.F. Antunes, W. Ahrens, A. Agudo, P. Boffetta, M. Hashibe, Y.C.A. Lee, V.W. Filho, Risk Factors for Head and Neck Cancer in Young Adults: A Pooled Analysis in the INHANCE Consortium. Int. J. Epidemiol. 44, 169–185 (2015). https://doi.org/10.1093/ije/dyu255
A.R. Kreimer, G.M. Clifford, P. Boyle, S. Franceschi, Human papillomavirus types in head and neck squamous cell carcinomas worldwide: a systemic review. Cancer Epidemiol. Biomark. Prev. 14, 467–475 (2005). https://doi.org/10.1158/1055-9965.EPI-04-0551
C. Ndiaye, M. Mena, L. Alemany, M. Arbyn, X. Castellsagué, L. Laporte, F.X. Bosch, S. de Sanjosé, H. Trottier, HPV DNA, E6/E7 MRNA, and P16INK4a detection in head and neck cancers: a systematic review and meta-analysis. Lancet Oncol. 15, 1319–1331 (2014). https://doi.org/10.1016/S1470-2045(14)70471-1
P. S. Hammerman, D. Neil Hayes, J. R. Grandis, Therapeutic insights from genomic studies of head and neck squamous cell carcinomas. Cancer Discov. 5, 239–244 (2015). https://doi.org/10.1158/2159-8290.CD-14-1205.
D. Rischin, R. J. Young, R. Fisher, S. B. Fox, Q. T. Le, L. J. Peters, B. Solomon, J. Choi, B. O’Sullivan, L. M. Kenny, G. A. McArthur, Prognostic significance of P16INK4Aand human papillomavirus in patients with oropharyngeal cancer treated on TROG 02.02 Phase III Trial. J. Clin. Oncol. 28, 4142–4148 (2010). https://doi.org/10.1200/JCO.2010.29.2904.
M.L. Gillison, Current topics in the epidemiology of oral cavity and oropharyngeal cancers. Head Neck 29, 779–792 (2007). https://doi.org/10.1002/hed.20573
G. D’Souza, A.R. Kreimer, R. Viscidi, M. Pawlita, C. Fakhry, W.M. Koch, W.H. Westra, M.L. Gillison, Case-control study of human papillomavirus and oropharyngeal cancer. N. Engl. J. Med. 356, 1944–1956 (2007). https://doi.org/10.1056/nejmoa065497
H.J. Ryu, E.K. Kim, B.C. Cho, S.O. Yoon, Characterization of head and neck squamous cell carcinoma arising in young patients: particular focus on molecular alteration and tumor immunity. Head Neck 41, 198–207 (2019). https://doi.org/10.1002/hed.25507
Z.S. Zumsteg, G. Cook-Wiens, E. Yoshida, S.L. Shiao, N.Y. Lee, A. Mita, C. Jeon, M.T. Goodman, A.S. Ho, Incidence of oropharyngeal cancer among elderly patients in the United States. JAMA Oncol. 2, 1617–1623 (2016). https://doi.org/10.1001/jamaoncol.2016.1804
B. Solomon, R.J. Young, D. Rischin, Head and neck squamous cell carcinoma: genomics and emerging biomarkers for immunomodulatory cancer treatments. Semin. Cancer Biol. 52, 228–240 (2018). https://doi.org/10.1016/j.semcancer.2018.01.008
S. Stone, P. Jiang, P. Dayananth, S.V. Tavtigian, H. Katcher, D. Parry, G. Peters, A. Kamb, Complex structure and regulation of the P16 (MTS1) locus. Cancer Res. 55, 2988–2994 (1995)
G.I. Shapiro, C.D. Edwards, L. Kobzik, J. Godleski, W. Richards, D.J. Sugarbaker, B.J. Rollins, Reciprocal Rb inactivation and P16INK4 expression in primary lung cancers and cell lines. Cancer Res. 55, 505–509 (1995)
A. Merlo, J.G. Herman, L. Mao, D.J. Lee, E. Gabrielson, P.C. Burger, S.B. Baylin, D. Sidransky, 5’ CpG island méthylation is associated with transcriptional silencing of the tumour suppressor P16/CDKN2/MTS1 in human cancers. Nat. Med. 1, 686–692 (1995). https://doi.org/10.1038/nm0795-686
M. S. Lawrence, C. Sougnez, L. Lichtenstein, K. Cibulskis, E. Lander, S. B. Gabriel, G. Getz, A. Ally, M. Balasundaram, I. Birol, R. Bowlby, D. Brooks, Y. S. N. Butterfield, R. Carlsen, D. Cheng, A. Chu, N. Dhalla, R. Guin, R. A. Holt, S. J. M. Jones, D. Lee, H. I. Li, M. A. Marra, M. Mayo, R. A. Moore, A. J. Mungall, A. G. Robertson, J. E. Schein, P. Sipahimalani, A. Tam, N. Thiessen, T. Wong, A. Protopopov, N. Santoso, S. Lee, M. Parfenov, J. Zhang, H. S. Mahadeshwar, J. Tang, X. Ren, S. Seth, P. Haseley, D. Zeng, L. Yang, A. W. Xu, X. Song, A. Pantazi, C. A. Bristow, A. Hadjipanayis, J. Seidman, L. Chin, P. J. Park, R. Kucherlapati, R. Akbani, T. Casasent, W. Liu, Y. Lu, G. Mills, T. Motter, J. Weinstein, L. Diao, J. Wang, Y. Hong Fan, J. Liu, K. Wang, J. T. Auman, S. Balu, T. Bodenheimer, E. Buda, D. N. Hayes, K. A. Hoadley, A. P. Hoyle, S. R. Jefferys, C. D. Jones, P. K. Kimes, Y. Liu, J. S. Marron, S. Meng, P. A. Mieczkowski, L. E. Mose, J. S. Parker, C. M. Perou, J. F. Prins, J. Roach, Y. Shi, J. V. Simons, D. Singh, M. G. Soloway, D. Tan, U. Veluvolu, V. Walter, S. Waring, M. D. Wilkerson, J. Wu, N. Zhao, A. D. Cherniack, P. S. Hammerman, A. D. Tward, C. S. Pedamallu, G. Saksena, J. Jung, A. I. Ojesina, S. L. Carter, T. I. Zack, S. E. Schumacher, R. Beroukhim, S. S. Freeman, M. Meyerson, J. Cho, M. S. Noble, D. DiCara, H. Zhang, D. I. Heiman, N. Gehlenborg, D. Voet, P. Lin, S. Frazer, P. Stojanov, Y. Liu, L. Zou, J. Kim, D. Muzny, H. V. Doddapaneni, C. Kovar, J. Reid, D. Morton, Y. Han, W. Hale, H. Chao, K. Chang, J. A. Drummond, R. A. Gibbs, N. Kakkar, D. Wheeler, L. Xi, G. Ciriello, M. Ladanyi, W. Lee, R. Ramirez, C. Sander, R. Shen, R. Sinha, N. Weinhold, B. S. Taylor, B. A. Aksoy, G. Dresdner, J. Gao, B. Gross, A. Jacobsen, B. Reva, N. Schultz, S. O. Sumer, Y. Sun, T. A. Chan, L. G. Morris, J. Stuart, S. Benz, S. Ng, C. Benz, C. Yau, S. B. Baylin, L. Cope, L. Danilova, J. G. Herman, M. Bootwalla, D. T. Maglinte, P. W. Laird, T. Triche, D. J. Weisenberger, D. J. Van Den Berg, N. Agrawal, J. Bishop, P. C. Boutros, J. P. Bruce, L. A. Byers, J. Califano, T. E. Carey, Z. Chen, H. Cheng, S. I. Chiosea, E. Cohen, B. Diergaarde, A. M. Egloff, A. K. El-Naggar, R. L. Ferris, M. J. Frederick, J. R. Grandis, Y. Guo, R. I. Haddad, T. Harris, A. B. Y. Hui, J. J. Lee, S. M. Lippman, F. F. Liu, J. B. McHugh, J. Myers, P. K. S. Ng, B. Perez-Ordonez, C. R. Pickering, M. Prystowsky, M. Romkes, A. D. Saleh, M. A. Sartor, R. Seethala, T. Y. Seiwert, H. Si, C. Van Waes, D. M. Waggott, M. Wiznerowicz, W. G. Yarbrough, J. Zhang, Z. Zuo, K. Burnett, D. Crain, J. Gardner, K. Lau, D. Mallery, S. Morris, J. Paulauskis, R. Penny, C. Shelton, T. Shelton, M. Sherman, P. Yena, A. D. Black, J. Bowen, J. Frick, J. M. Gastier-Foster, H. A. Harper, K. Leraas, T. M. Lichtenberg, N. C. Ramirez, L. Wise, E. Zmuda, J. Baboud, M. A. Jensen, A. B. Kahn, T. D. Pihl, D. A. Pot, D. Srinivasan, J. S. Walton, Y. Wan, R. A. Burton, T. Davidsen, J. A. Demchok, G. Eley, M. L. Ferguson, K. R. Mills Shaw, B. A. Ozenberger, M. Sheth, H. J. Sofia, R. Tarnuzzer, Z. Wang, L. Yang, J. C. Zenklusen, C. Saller, K. Tarvin, C. Chen, R. Bollag, P. Weinberger, W. Golusiński, P. Golusiński, M. Ibbs, K. Korski, A. Mackiewicz, W. Suchorska, B. Szybiak, E. Curley, C. Beard, C. Mitchell, G. Sandusky, J. Ahn, Z. Khan, J. Irish, J. Waldron, W. N. William, S. Egea, C. Gomez-Fernandez, L. Herbert, C. R. Bradford, D. B. Chepeha, A. S. Haddad, T. R. Jones, C. M. Komarck, M. Malakh, J. S. Moyer, A. Nguyen, L. A. Peterson, M. E. Prince, L. S. Rozek, E. G. Taylor, H. M. Walline, G. T. Wolf, L. Boice, B. S. Chera, W. K. Funkhouser, M. L. Gulley, T. G. Hackman, M. C. Hayward, M. Huang, W. K. Rathmell, A. H. Salazar, W. W. Shockley, C. G. Shores, L. Thorne, M. C. Weissler, S. Wrenn, A. M. Zanation, B. T. Brown, M. Pham, Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature. 517, 576–582 (2015). https://doi.org/10.1038/nature14129.
D.N. Hayes, C. Van Waes, T.Y. Seiwert, Genetic landscape of human papillomavirus-associated head and neck cancer and comparison to tobacco-related tumors. J. Clin. Oncol. 33, 3227–3234 (2015). https://doi.org/10.1200/JCO.2015.62.1086
L.C. Farias, C.A.D.C. Fraga, M.V.M. De Oliveira, T.F. Silva, L. Marques-Silva, P.R. Moreira, A.M.B. De-Paula, R.S. Gomez, A.L.S. Guimarães, Effect of age on the association between P16CDKN2A methylation and DNMT3B polymorphism in head and neck carcinoma and patient survival. Int. J. Oncol. 37, 167–176 (2010). https://doi.org/10.3892/ijo-00000664
T.Y. Seiwert, Z. Zuo, M.K. Keck, A. Khattri, C.S. Pedamallu, T. Stricker, C. Brown, T.J. Pugh, P. Stojanov, J. Cho, M.S. Lawrence, G. Getz, J. Brägelmann, R. DeBoer, R.R. Weichselbaum, A. Langerman, L. Portugal, E. Blair, K. Stenson, M.W. Lingen, E.E.W. Cohen, E.E. Vokes, K.P. White, P.S. Hammerman, Integrative and comparative genomic analysis of HPV-positive and HPV-negative head and neck squamous cell carcinomas. Clin. Cancer Res. 21, 632–641 (2015). https://doi.org/10.1158/1078-0432.CCR-13-3310
S. Fulda, K.M. Debatin, Extrinsic versus intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene 25, 4798–4811 (2006). https://doi.org/10.1038/sj.onc.1209608
Z. Zhou, E.M. Sturgis, Z. Liu, L.E. Wang, Q. Wei, G. Li, Genetic variants of NOXA and MCL1 modify the risk of HPV16-associated squamous cell carcinoma of the head and neck. BMC Cancer 12, 159 (2012). https://doi.org/10.1186/1471-2407-12-159
Z. Zhou, E.M. Sturgis, Z. Liu, L.E. Wang, Q. Wei, G. Li, Genetic variants of a BH3-Only pro-apoptotic gene, puma, and risk of HPV16-associated squamous cell carcinoma of the head and neck. Mol. Carcinog. 51, E54-64 (2012). https://doi.org/10.1002/mc.21838
E.M. Rettig, M. Zaidi, F. Faraji, D.W. Eisele, M. El Asmar, N. Fung, G. D’Souza, C. Fakhry, Oropharyngeal cancer is no longer a disease of younger patients and the prognostic advantage of human papillomavirus is attenuated among older patients: analysis of the national cancer database. Oral Oncol. 83, 147–153 (2018). https://doi.org/10.1016/j.oraloncology.2018.06.013
J.T. Guidry, R.S. Scott, The interaction between human papillomavirus and other viruses. Virus Res. 231, 139–147 (2017). https://doi.org/10.1016/j.virusres.2016.11.002
F. Broccolo, G. Ciccarese, A. Rossi, L. Anselmi, F. Drago, A. Toniolo, Human papillomavirus (HPV) and epstein-barr virus (EBV) in keratinizing versus non- keratinizing squamous cell carcinoma of the oropharynx. Infect. Agents Cancer 13, 32 (2018). https://doi.org/10.1186/s13027-018-0205-6
B. Drop, M. Strycharz-Dudziak, E. Kliszczewska, M. Polz-Dacewicz, Coinfection with Epstein–Barr Virus (EBV), Human Papilloma Virus (HPV) and Polyoma Bk Virus (BKPyV) in laryngeal, oropharyngeal and oral cavity cancer. Int. J. Mol. Sci. 18, (2017). https://doi.org/10.3390/ijms18122752.
D. H. E. Maasland, P. A. van den Brandt, B. Kremer, R. A. (. Goldbohm, L. J. Schouten, Alcohol consumption, cigarette smoking and the risk of subtypes of head-neck cancer: results from the Netherlands cohort study. BMC Cancer 14, 187 (2014). https://doi.org/10.1186/1471-2407-14-187.
N. Riaz, L.G. Morris, W. Lee, T.A. Chan, Unraveling the molecular genetics of head and neck cancer through genome-wide approaches. Genes and Diseases 1, 75–86 (2014). https://doi.org/10.1016/j.gendis.2014.07.002
J. Califano, W.H. Westra, G. Meininger, R. Corio, W.M. Koch, D. Sidransky, Genetic progression and clonal relationship of recurrent premalignant head and neck lesions. Clin. Cancer Res. 6, 347–352 (2000)
S.I. Pai, W.H. Westra, Molecular pathology of head and neck cancer: implications for diagnosis, prognosis, and treatment. Annu. Rev. Pathol. 4, 49–70 (2009). https://doi.org/10.1146/annurev.pathol.4.110807.092158
C. Jin, Y. Jin, J. Wennerberg, J. Åkervall, B. Grenthe, N. Mandahl, S. Heim, F. Mitelman, F. Mertens, Clonal chromosome aberrations accumulate with age in upper aerodigestive tract mucosa. Mut. Res. - Fundamental and Molecular Mechanisms of Mutagenesis 374, 63–72 (1997). https://doi.org/10.1016/S0027-5107(96)00219-9
E.M. O’Regan, M.E. Toner, P.C. Smyth, S.P. Finn, C. Timon, S. Cahill, R. Flavin, J.J. O’Leary, O. Sheils, Distinct array comparative genomic hybridization profiles in oral squamous cell carcinoma occurring in young patients. Head Neck 28, 330–338 (2006). https://doi.org/10.1002/hed.20354
M.D. Ryser, W.T. Lee, N.E. Readyz, K.Z. Leder, J. Foo, Related head and neck cancer : A multi-scale modeling approach. Cancer Res. 76, 7078–7088 (2016). https://doi.org/10.1158/0008-5472.CAN-16-1054.Quantifying
J.K. Field, V. Zoumpourlis, D.A. Spandidos, A.S. Jones, P53 expression and mutations in squamous cell carcinoma of the head and neck: expression correlates with the patients’ use of tobacco and alcohol. Cancer Detect. Prev. 18, 197–208 (1994)
J.A. Brennan, J.O. Boyle, W.M. Koch, S.N. Goodman, R.H. Hruban, Y.J. Eby, M.J. Couch, A.A. Forastiere, D. Sidransky, Association between cigarette smoking and mutation of the p53 gene in squamous-cell carcinoma of the head and neck. N. Engl. J. Med. 332, 712–717 (1995). https://doi.org/10.1056/nejm199503163321104
J.K. Field, Z.P. Pavelic, D.A. Spandidos, P.J. Stambrook, A.S. Jones, J.L. Gluckman, The role of the P53 tumor suppressor gene in squamous cell carcinoma of the head and neck. Arch. Otolaryngol. - Head and Neck Surgery 119, 1118–1122 (1993). https://doi.org/10.1001/archotol.1993.01880220064009
M.L. Poeta, J. Manola, M.A. Goldwasser, A. Forastiere, N. Benoit, J.A. Califano, J.A. Ridge, J. Goodwin, D. Kenady, J. Saunders, W. Westra, D. Sidransky, W.M. Koch, TP53 mutations and survival in squamous-cell carcinoma of the head and neck. N. Engl. J. Med. 357, 2552–2561 (2007). https://doi.org/10.1056/nejmoa073770
A.J. Levine, M. Oren, The first 30 years of P53: growing ever more complex. Nat. Rev. Cancer 9, 749–758 (2009). https://doi.org/10.1038/nrc2723
D. Bergamaschi, M. Gasco, L. Hiller, A. Sullivan, N. Syed, G. Trigiante, I. Yulug, M. Merlano, G. Numico, A. Comino, M. Attard, O. Reelfs, B. Gusterson, A.K. Bell, V. Heath, M. Tavassoli, P.J. Farrell, P. Smith, X. Lu, T. Crook, P53 Polymorphism influences response in cancer chemotherapy via modulation of P73-DEPENDENT apoptosis. Cancer Cell 3, 387–402 (2003). https://doi.org/10.1016/S1535-6108(03)00079-5
W.M. Koch, J.A. Brennan, M. Zahurak, S.N. Goodman, W.H. Westra, D. Schwab, G.H. Yoo, D.J. Lee, A.A. Forastiere, D. Sidransky, P53 mutation and locoregional treatment failure in head and neck squamous cell carcinoma. J. Natl. Cancer Inst. 88, 1580–1586 (1996). https://doi.org/10.1093/jnci/88.21.1580
J. Gugić, P. Strojan, Squamous cell carcinoma of the head and neck in the elderly. Rep. Pract. Oncol. Radiother. 18, 16–25 (2013). https://doi.org/10.1016/j.rpor.2012.07.014
C.R. Pickering, J. Zhang, D.M. Neskey, M. Zhao, S.A. Jasser, J. Wang, A. Ward, C.J. Tsai, M.V.O. Alves, J.H. Zhou, J. Drummond, A.K. El-Naggar, R. Gibbs, J.N. Weinstein, D.A. Wheeler, J. Wang, M.J. Frederick, J.N. Myers, Squamous cell carcinoma of the oral tongue in young non-smokers is genomically similar to tumors in older smokers. Clin. Cancer Res. 20, 3842–3848 (2014). https://doi.org/10.1158/1078-0432.CCR-14-0565
A.M.B. De Paula, L.R. Souza, L.C. Farias, G.T.B. Corrêa, C.A.C. Fraga, N.B. Eleutério, A.C.O. Silveira, F.B.G. Santos, D.S. Haikal, A.L.S. Guimarães, R.S. Gomez, Analysis of 724 cases of primary head and neck squamous cell carcinoma (HNSCC) with a focus on young patients and p53 immunolocalization. Oral Oncol. 45, 777–782 (2009). https://doi.org/10.1016/j.oraloncology.2008.11.015
R.S.R. Adduri, V. Kotapalli, N.A. Gupta, S. Gowrishankar, M. Srinivasulu, M.M. Ali, S. Rao, S.G. Uppin, U.K. Nayak, S. Dhagam, M.V. Chigurupati, M.D. Bashyam, P53 nuclear stabilization is associated with fhit loss and younger age of onset in squamous cell carcinoma of oral tongue. BMC Clin. Pathol. 14, 37 (2014). https://doi.org/10.1186/1472-6890-14-37
J. A. Regezi, N. P. Dekker, A. McMillan, V. Ramirez-Amador, A. Meneses-Garcia, L. M. Ruiz-Godoy Rivera, E. Chrysomali, I. O. L. Ng, P53, P21, Rb, and MDM2 proteins in tongue carcinoma from patients < 35 versus > 75 Years. Oral Oncol. 35, 379–383 (1999). https://doi.org/10.1016/S1368-8375(98)00126-2.
W. Gawȩcki, M. Kostrzewska-Poczekaj, M. Gajȩcka, P. Milecki, K. Szyfter, W. Szyfter, The role of genetic factor in etiopathogenesis of squamous cell carcinoma of the head and neck in young adults. Eur. Arch. Otorhinolaryngol. 264, 1459–1465 (2007). https://doi.org/10.1007/s00405-007-0386-x
M. Gajecka, M. Rydzanicz, R. Jaskula-Sztul, M. Kujawski, W. Szyfter, K. Szyfter, CYP1A1, CYP2D6, CYP2E1, NAT2, GSTM1 and GSTT1 polymorphisms or their combinations are associated with the increased risk of the laryngeal squamous cell carcinoma. Mut. Res. - Fundamental and Molecular Mechanisms of Mutagenesis. 574, 112–123 (2005). https://doi.org/10.1016/j.mrfmmm.2005.01.027
M. Ünal, L. Tamer, N. Aras Ateş, Y. Akbaş, Y. S. Pata, Y. Vayisoǧlu, B. Ercan, K. Görür, U. Atik, Glutathione S-Transferase M1, T1, and P1 gene polymorphism in laryngeal squamous cell carcinoma. Am. J. Otolaryngol. - Head and Neck Medicine and Surgery 25, 318–322 (2004). https://doi.org/10.1016/j.amjoto.2004.04.003.
C.J. Liu, C.S. Chang, M.T. Lui, C.W. Dang, Y.H. Shih, K.W. Chang, Association of GST genotypes with age of onset and lymph node metastasis in oral squamous cell carcinoma. J. Oral Pathol. Med. 34, 473–477 (2005). https://doi.org/10.1111/j.1600-0714.2005.00345.x
X. Zhu, F. Zhang, W. Zhang, J. He, Y. Zhao, X. Chen, Prognostic role of epidermal growth factor receptor in head and neck cancer: a meta-analysis. J. Surg. Oncol. 108, 387–397 (2013). https://doi.org/10.1002/jso.23406
V. Costa, L.P. Kowalski, C.M. Coutinho-Camillo, M.D. Begnami, V.F. Calsavara, J.I. Neves, E. Kaminagakura, EGFR amplification and expression in oral squamous cell carcinoma in young adults. Int. J. Oral Maxillofac. Surg. 47, 817–823 (2018). https://doi.org/10.1016/j.ijom.2018.01.002
M. Miranda Galvis, A. R. Santos-Silva, J. Freitas Jardim, F. Paiva Fonseca, M. A. Lopes, O. P. de Almeida, C. A. Lópes Pinto, E. Kaminagakura, I. Sawazaki-Calone, P. M. Speight, L. P. Kowalski, Different patterns of expression of cell cycle control and local invasion-related proteins in oral squamous cell carcinoma affecting young patients. J. Oral Pathol. Med. 47, 32–39 (2018). https://doi.org/10.1111/jop.12601.
G. Ascani, P. Balercia, M. Messi, L. Lupi, G. Goteri, A. Filosa, D. Stramazzotti, T. Pieramici, C. Rubini, Angiogenesis in oral squamous cell carcinoma. Acta otorhinolaryngologica Italica : organo ufficiale della Società italiana di otorinolaringologia e chirurgia cervico-facciale 25, 13–17 (2005)
S.M. Shivamallappa, N.T. Venkatraman, B. Shreedhar, L. Mohanty, S. Shenoy, Role of angiogenesis in oral squamous cell carcinoma development and metastasis: an immunohistochemical study. Int. J. Oral Sci. 3, 216–224 (2011). https://doi.org/10.4248/IJOS11077
T.G. Benevenuto, C.F.W. Nonaka, L.P. Pinto, L.B. De Souza, Immunohistochemical comparative analysis of cell proliferation and angiogenic index in squamous cell carcinomas of the tongue between young and older patients. Appl. Immunohistochem. Mol. Morphol. 20, 291–297 (2012). https://doi.org/10.1097/PAI.0b013e31823277f6
L.R. Teixeira, L.Y. Almeida, R.N. Silva, A.T.M. Mesquita, C.B.N. Colturato, H.A. Silveira, A. Duarte, A. Ribeiro-Silva, J.E. León, Young and elderly oral squamous cell carcinoma patients present similar angiogenic profile and predominance of m2 macrophages: comparative immunohistochemical study. Head Neck 41, 4111–4120 (2019). https://doi.org/10.1002/hed.25954
M. Khalili, N. Mahdavi, R. Beheshti, F. Baghai Naini, Immunohistochemical evaluation of angiogenesis and cell proliferation in tongue squamous cell carcinoma. J. Dentistry 12, 846–52 (2015).
S. Meucci, U. Keilholz, I. Tinhofer, O.A. Ebner, Mutational load and mutational patterns in relation to age in head and neck cancer. Oncotarget 7, 69188–69199 (2016). https://doi.org/10.18632/oncotarget.11312
S. Koy, J. Plaschke, H. Luksch, K. Friedrich, E. Kuhlisch, U. Eckelt, R. Martinez, Microsatellite instability and loss of heterozygosity in squamous cell carcinoma of the head and neck. Head Neck 30, 1105–1113 (2008). https://doi.org/10.1002/hed.20857
S.W. Coon, A.T. Savera, R.J. Zarbo, M.S. Benninger, G.A. Chase, B.A. Rybicki, D.L. Van Dyke, Prognostic implications of loss of heterozygosity at 8p21 and 9p21 in head and neck squamous cell carcinoma. Int. J. Cancer 111, 206–212 (2004). https://doi.org/10.1002/ijc.20254
W.M. Koch, M. Lango, D. Sewell, M. Zahurak, D. Sidransky, Head and neck cancer in nonsmokers: a distinct clinical and molecular entity. Laryngoscope 109, 1544–1551 (1999). https://doi.org/10.1097/00005537-199910000-00002
Y.T. Jin, J. Myers, S.T. Tsai, H. Goepfert, J.G. Batsakis, A.K. El-Naggar, Genetic alterations in oral squamous cell carcinoma of young adults. Oral Oncol. 35, 251–256 (1999). https://doi.org/10.1016/S1368-8375(98)00112-2
Y. Wang, J. Irish, C. MacMillan, D. Brown, Y. Xuan, C. Boyington, P. Gullane, S. Kamel-Reid, High frequency of microsatellite instability in young patients with head-and-neck squamous-cell carcinoma: lack of involvement of the mismatch repair genes HMLH1 and HMSH2. Int. J. Cancer 93, 353–360 (2001). https://doi.org/10.1002/ijc.1337
Y. Wang, X. Liu, Y. Li, Target genes of microsatellite sequences in head and neck squamous cell carcinoma: mononucleotide repeats are not detected. Gene 506, 195–201 (2012). https://doi.org/10.1016/j.gene.2012.06.056
M.I. Coolbaugh-Murphy, J. Xu, L.S. Ramagli, B.W. Brown, M.J. Siciliano, Microsatellite instability (MSI) increases with age in normal somatic cells. Mech. Ageing Dev. 126, 1051–1059 (2005). https://doi.org/10.1016/j.mad.2005.06.005
R.M. Castilho, C.H. Squarize, L.O. Almeida, Epigenetic modifications and head and neck cancer: implications for tumor progression and resistance to therapy. Int. J. Mol. Sci. 18, 1506 (2017). https://doi.org/10.3390/ijms18071506
D.E. Johnson, B. Burtness, C.R. Leemans, V.W.Y. Lui, J.E. Bauman, J.R. Grandis, Head and neck squamous cell carcinoma. Nat. Rev. Dis. Primers. 6, 92 (2020). https://doi.org/10.1038/s41572-020-00224-3
E.M. O’Regan, M.E. Toner, S.P. Finn, C.Y. Fan, M. Ring, B. Hagmar, C. Timon, P. Smyth, S. Cahill, R. Flavin, O.M. Sheils, J.J. O’Leary, P16INK4A genetic and epigenetic profiles differ in relation to age and site in head and neck squamous cell carcinomas. Hum. Pathol. 39, 452–458 (2008). https://doi.org/10.1016/j.humpath.2007.08.004
J.H. Ng, N.G. Iyer, M.H. Tan, G. Edgren, Changing epidemiology of oral squamous cell carcinoma of the tongue: a global study. Head Neck 39, 297–304 (2017). https://doi.org/10.1002/hed.24589
B. R. Campbell, Z. Chen, D. L. Faden, N. Agrawal, R. J. Li, G. J. Hanna, N. G. Iyer, A. Boot, S. G. Rozen, A. L. Vettore, B. Panda, N. M. Krishnan, C. R. Pickering, J. N. Myers, X. Guo, K. A. Lang Kuhs, The mutational landscape of early- and typical-onset oral tongue squamous cell carcinoma. Cancer 127, 544–553 (2021). https://doi.org/10.1002/cncr.33309.
A. Paderno, R. Morello, C. Piazza, Tongue carcinoma in young adults: a review of the literature (2018), pp. 175–180
A. Coca-Pelaz, G. B. Halmos, P. Strojan, R. de Bree, P. Bossi, C. R. Bradford, A. Rinaldo, V. Vander Poorten, A. Sanabria, R. P. Takes, A. Ferlito, The role of age in treatment-related adverse events in patients with head and neck cancer: a systematic review. Head and Neck. 41, 2410–2429 (2019). doi: https://doi.org/10.1002/hed.25696.
J.P. Pignon, A. le Maître, E. Maillard, J. Bourhis, Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients. Radiother. Oncol. 92, 4–14 (2009). https://doi.org/10.1016/j.radonc.2009.04.014
R. L. Siegel, K. D. Miller, A. Jemal, Cancer Statistics, 2017. CA: Cancer J. Clin. 67, 7–30 (2017). https://doi.org/10.3322/caac.21387.
C. López-Otín, M.A. Blasco, L. Partridge, M. Serrano, G. Kroemer, The hallmarks of aging. Cell 153, 1194 (2013). https://doi.org/10.1016/j.cell.2013.05.039
B. Bernardes de Jesus, M.A. Blasco, Telomerase at the intersection of cancer and aging. Trends Genet. 29, 513–520 (2013). https://doi.org/10.1016/j.tig.2013.06.007
E.H. Blackburn, E.S. Epel, J. Lin, Human telomere biology: a contributory and interactive factor in aging, disease risks, and protection. Science 350, 1193–1198 (2015). https://doi.org/10.1126/science.aab3389
I.M. Wentzensen, L. Mirabello, R.M. Pfeiffer, S.A. Savage, The association of telomere length and cancer: a meta-analysis. Cancer Epidemiol. Biomark. Prev. 20, 1238–1250 (2011). https://doi.org/10.1158/1055-9965.EPI-11-0005
P. Boscolo-Rizzo, M.C. Da Mosto, E. Rampazzo, S. Giunco, A. Del Mistro, A. Menegaldo, L. Baboci, M. Mantovani, G. Tirelli, A. De Rossi, Telomeres and telomerase in head and neck squamous cell carcinoma: from pathogenesis to clinical implications. Cancer Metastasis Rev. 35, 457–474 (2016). https://doi.org/10.1007/s10555-016-9633-1
P. Boscolo-Rizzo, E. Rampazzo, E. Perissinotto, M.A. Piano, S. Giunco, L. Baboci, G. Spinato, R. Spinato, G. Tirelli, M.C. Da Mosto, A. Del Mistro, A. De Rossi, Telomere shortening in mucosa surrounding the tumor: biosensor of field cancerization and prognostic marker of mucosal failure in head and neck squamous cell carcinoma. Oral Oncol. 51, 500–507 (2015). https://doi.org/10.1016/j.oraloncology.2015.02.100
J. Aida, T. Izumo, N. Shimomura, K. ichi Nakamura, N. Ishikawa, M. Matsuura, S. S. Poon, M. Fujiwara, M. Sawabe, T. Arai, K. Takubo, Telomere lengths in the oral epithelia with and without carcinoma. Eur. J. Cancer 46, 430–438 (2010). https://doi.org/10.1016/j.ejca.2009.10.018.
J. Aida, T. Kobayashi, T. Saku, M. Yamaguchi, N. Shimomura, K.I. Nakamura, N. Ishikawa, S. Maruyama, J. Cheng, S.S.S. Poon, M. Sawabe, T. Arai, K. Takubo, Short Telomeres in an oral precancerous lesion: Q-FISH analysis of leukoplakia. J. Oral Pathol. Med. 41, 372–378 (2012). https://doi.org/10.1111/j.1600-0714.2011.01120.x
Y. Liu, X. li Dong, C. Tian, H. gang Liu, Human Telomerase RNA Component (HTERC) gene amplification detected by fish in precancerous lesions and carcinoma of the larynx. Diagn. Pathol. 7, 34 (2012). https://doi.org/10.1186/1746-1596-7-34.
K.P. Chang, C.I. Wang, C.R. Pickering, Y. Huang, C.N. Tsai, N.M. Tsang, H.K. Kao, M.H. Cheng, J.N. Myers, Prevalence of promoter mutations in the TERT gene in oral cavity squamous cell carcinoma. Head Neck 39, 1131–1137 (2017). https://doi.org/10.1002/hed.24728
W. Barczak, W.M. Suchorska, A. Sobecka, K. Bednarowicz, P. Machczynski, P. Golusinski, B. Rubis, M.M. Masternak, W. Golusinski, HTERT C250T promoter mutation and telomere length as a molecular markers of cancer progression in patients with head and neck cancer. Mol. Med. Rep. 16, 441–446 (2017). https://doi.org/10.3892/mmr.2017.6590
D.T. Bau, S.M. Lippman, E. Xu, Y. Gong, J.J. Lee, X. Wu, J. Gu, Short telomere lengths in peripheral blood leukocytes are associated with an increased risk of oral premalignant lesion and oral squamous cell carcinoma. Cancer 119, 4277–4283 (2013). https://doi.org/10.1002/cncr.28367
Y. Qu, S. Dang, K. Wu, Y. Shao, Q. Yang, M. Ji, B. Shi, P. Hou, TERT promoter mutations predict worse survival in laryngeal cancer patients. Int. J. Cancer 135, 1008–1010 (2014). https://doi.org/10.1002/ijc.28728
J. Lin, E. Epel, E. Blackburn, Telomeres and lifestyle factors: roles in cellular aging. Mut. Res. - Fundamental and Molecular Mechanisms of Mutagenesis. 730, 85–89 (2012). https://doi.org/10.1016/j.mrfmmm.2011.08.003
J. Raschenberger, B. Kollerits, J. Ritchie, B. Lane, P.A. Kalra, E. Ritz, F. Kronenberg, Association of relative telomere length with progression of chronic kidney disease in two cohorts: effect modification by smoking and diabetes. Sci. Rep. 5, 11887 (2015). https://doi.org/10.1038/srep11887
O.G. Opitz, Y. Suliman, W.C. Hahn, H. Harada, H.E. Blum, A.K. Rustgi, Cyclin D1 overexpression and P53 inactivation immortalize primary oral keratinocytes by a telomerase-independent mechanism. J. Clin. Investig. 108, 725–732 (2001). https://doi.org/10.1172/jci11909
W. Chen, B.K. Xiao, J.P. Liu, S.M. Chen, Z.Z. Tao, Alternative lengthening of telomeres in HTERT-inhibited laryngeal cancer cells. Cancer Sci. 101, 1769–1776 (2010). https://doi.org/10.1111/j.1349-7006.2010.01611.x
V. Vitelli, P. Falvo, S. G. Nergadze, M. Santagostino, L. Khoriauli, P. Pellanda, G. Bertino, A. Occhini, M. Benazzo, P. Morbini, M. Paulli, C. Porta, E. Giulotto, Telomeric Repeat-CONTAINING RNAs (TERRA) Decrease in squamous cell carcinoma of the head and neck is associated withworsened clinical outcome. Int. J. Mol. Sci. 19, 274 (2018). https://doi.org/10.3390/ijms19010274.
H.H. Chen, C.H. Yu, J.T. Wang, B.Y. Liu, Y.P. Wang, A. Sun, T.C. Tsai, C.P. Chiang, Expression of human telomerase reverse transcriptase (HTERT) protein is significantly associated with the progression, recurrence and prognosis of oral squamous cell carcinoma in Taiwan. Oral Oncol. 43, 122–129 (2007). https://doi.org/10.1016/j.oraloncology.2006.01.011
E. Rampazzo, R. Bertorelle, L. Serra, L. Terrin, C. Candiotto, S. Pucciarelli, P. Del Bianco, D. Nitti, A. De Rossi, Relationship between telomere shortening, genetic instability, and site of tumour origin in colorectal cancers. Br. J. Cancer 102, 1300–1305 (2010). https://doi.org/10.1038/sj.bjc.6605644
D. Tweats, D. A. Eastmond, A. M. Lynch, A. Elhajouji, R. Froetschl, M. Kirsch-Volders, F. Marchetti, K. Masumura, F. Pacchierotti, M. Schuler, Role of Aneuploidy in the Carcinogenic Process: Part 3 of the Report of the 2017 IWGT workgroup on assessing the risk of aneugens for carcinogenesis and hereditary diseases. Mut. Res. - Genetic Toxicology and Environmental Mutagenesis 847, 403032 (2019). https://doi.org/10.1016/j.mrgentox.2019.03.005.
P. Castagnola, S. Gandolfo, D. Malacarne, C. Aiello, R. Marino, G. Zoppoli, A. Ballestrero, W. Giaretti, M. Pentenero, DNA aneuploidy relationship with patient age and tobacco smoke in OPMDs/OSCCs. PLoS ONE 12, e0184425 (2017). https://doi.org/10.1371/journal.pone.0184425
L.G.T. Morris, A.M. Kaufman, Y. Gong, D. Ramaswami, L.A. Walsh, Ş Turcan, S. Eng, K. Kannan, Y. Zou, L. Peng, V.E. Banuchi, P. Paty, Z. Zeng, E. Vakiani, D. Solit, B. Singh, I. Ganly, L. Liau, T.C. Cloughesy, P.S. Mischel, I.K. Mellinghoff, T.A. Chan, Recurrent somatic mutation of FAT1 in multiple human cancers leads to aberrant Wnt activation. Nat. Genet. 45, 253–261 (2013). https://doi.org/10.1038/ng.2538
K.T. Kim, B.S. Kim, J.H. Kim, Association between FAT1 mutation and overall survival in patients with human papillomavirus-negative head and neck squamous cell carcinoma. Head Neck 38, E2021–E2029 (2016). https://doi.org/10.1002/hed.24372
B. Bottazzi, E. Riboli, A. Mantovani, Aging, inflammation and cancer. Semin. Immunol. 40, 74–82 (2018). https://doi.org/10.1016/j.smim.2018.10.011
E. Bonavita, S. Gentile, M. Rubino, V. Maina, R. Papait, P. Kunderfranco, C. Greco, F. Feruglio, M. Molgora, I. Laface, S. Tartari, A. Doni, F. Pasqualini, E. Barbati, G. Basso, M.R. Galdiero, M. Nebuloni, M. Roncalli, P. Colombo, L. Laghi, J.D. Lambris, S. Jaillon, C. Garlanda, A. Mantovani, PTX3 is an extrinsic oncosuppressor regulating complement-dependent inflammation in cancer. Cell 160, 700–714 (2015). https://doi.org/10.1016/j.cell.2015.01.004
S.S. Jeske, P.J. Schuler, J. Doescher, M.N. Theodoraki, S. Laban, C. Brunner, T.K. Hoffmann, M.C. Wigand, Age-related changes in t lymphocytes of patients with head and neck squamous cell carcinoma. Immunity and Ageing 17, 3 (2020). https://doi.org/10.1186/s12979-020-0174-7
S. Alfieri, S. Cavalieri, L. Licitra, Immunotherapy for recurrent/metastatic head and neck cancer. Curr. Opin. Otolaryngol. Head Neck Surg. 26, 152–156 (2018). https://doi.org/10.1097/MOO.0000000000000448
R. L. Ferris, G. Blumenschein, J. Fayette, J. Guigay, A. D. Colevas, L. Licitra, K. Harrington, S. Kasper, E. E. Vokes, C. Even, F. Worden, N. F. Saba, L. C. Iglesias Docampo, R. Haddad, T. Rordorf, N. Kiyota, M. Tahara, M. Monga, M. Lynch, W. J. Geese, J. Kopit, J. W. Shaw, M. L. Gillison, Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N. Eng. J. Med. 375, 1856–1867 (2016). https://doi.org/10.1056/nejmoa1602252.
T.Y. Seiwert, B. Burtness, R. Mehra, J. Weiss, R. Berger, J.P. Eder, K. Heath, T. McClanahan, J. Lunceford, C. Gause, J.D. Cheng, L.Q. Chow, Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): an open-label, multicentre, phase 1b trial. Lancet Oncol. 17, 956–965 (2016). https://doi.org/10.1016/S1470-2045(16)30066-3
D. Lenouvel, M.Á. González-Moles, I. Ruiz-Ávila, L. Gonzalez-Ruiz, I. Gonzalez-Ruiz, P. Ramos-García, Prognostic and clinicopathological significance of PD-L1 overexpression in oral squamous cell carcinoma: a systematic review and comprehensive meta-analysis. Oral Oncol. 106, 104722 (2020). https://doi.org/10.1016/j.oraloncology.2020.104722
N. Ngamphaiboon, T. Chureemas, T. Siripoon, L. Arsa, N. Trachu, C. Jiarpinitnun, P. Pattaranutaporn, E. Sirachainan, N. Larbcharoensub, Characteristics and impact of programmed death-ligand 1 expression, CD8+ tumor-infiltrating lymphocytes, and P16 status in head and neck squamous cell carcinoma. Med. Oncol. 36, 1–10 (2019). https://doi.org/10.1007/s12032-018-1241-1
G.J. Hanna, S.B. Woo, Y.Y. Li, J.A. Barletta, P.S. Hammerman, J.H. Lorch, Tumor PD-L1 expression is associated with improved survival and lower recurrence risk in young women with oral cavity squamous cell carcinoma. Int. J. Oral Maxillofac. Surg. 47, 568–577 (2018). https://doi.org/10.1016/j.ijom.2017.09.006
P.A. Jones, S.B. Baylin, The fundamental role of epigenetic events in cancer. Nat. Rev. Genet. 3, 415–428 (2002). https://doi.org/10.1038/nrg816
B.H. Chen, R.E. Marioni, E. Colicino, M.J. Peters, C.K. Ward-Caviness, P.C. Tsai, N.S. Roetker, A.C. Just, E.W. Demerath, W. Guan, J. Bressler, M. Fornage, S. Studenski, A.R. Vandiver, A.Z. Moore, T. Tanaka, D.P. Kiel, L. Liang, P. Vokonas, J. Schwartz, K.L. Lunetta, J.M. Murabito, S. Bandinelli, D.G. Hernandez, D. Melzer, M. Nalls, L.C. Pilling, T.R. Price, A.B. Singleton, C. Gieger, R. Holle, A. Kretschmer, F. Kronenberg, S. Kunze, J. Linseisen, C. Meisinger, W. Rathmann, M. Waldenberger, P.M. Visscher, S. Shah, N.R. Wray, A.F. McRae, O.H. Franco, A. Hofman, A.G. Uitterlinden, D. Absher, T. Assimes, M.E. Levine, A.T. Lu, P.S. Tsao, L. Hou, J.A.E. Manson, C.L. Carty, A.Z. LaCroix, A.P. Reiner, T.D. Spector, A.P. Feinberg, D. Levy, A. Baccarelli, J. van Meurs, J.T. Bell, A. Peters, I.J. Deary, J.S. Pankow, L. Ferrucci, S. Horvath, DNA methylation-based measures of biological age: meta-analysis predicting time to death. Aging 8, 1844–1865 (2016). https://doi.org/10.18632/aging.101020
S. Horvath, K. Raj, DNA methylation-based biomarkers and the epigenetic clock theory of ageing. Nat. Rev. Genet. 19, 371–384 (2018). https://doi.org/10.1038/s41576-018-0004-3
M. Yu, W.D. Hazelton, G.E. Luebeck, W.M. Grady, Epigenetic aging: more than just a clock when it comes to cancer. Cancer Res. 80, 367–374 (2020). https://doi.org/10.1158/0008-5472.CAN-19-0924
Y. Zheng, B.T. Joyce, E. Colicino, L. Liu, W. Zhang, Q. Dai, M.J. Shrubsole, W.A. Kibbe, T. Gao, Z. Zhang, N. Jafari, P. Vokonas, J. Schwartz, A.A. Baccarelli, L. Hou, Blood epigenetic age may predict cancer incidence and mortality. EBioMedicine 5, 68–73 (2016). https://doi.org/10.1016/j.ebiom.2016.02.008
C. Huang, L. Chen, S. R. Savage, R. V. Eguez, Y. Dou, Y. Li, F. da Veiga Leprevost, E. J. Jaehnig, J. T. Lei, B. Wen, M. Schnaubelt, K. Krug, X. Song, M. Cieślik, H. Y. Chang, M. A. Wyczalkowski, K. Li, A. Colaprico, Q. K. Li, D. J. Clark, Y. Hu, L. Cao, J. Pan, Y. Wang, K. C. Cho, Z. Shi, Y. Liao, W. Jiang, M. Anurag, J. Ji, S. Yoo, D. C. Zhou, W. W. Liang, M. Wendl, P. Vats, S. A. Carr, D. R. Mani, Z. Zhang, J. Qian, X. S. Chen, A. R. Pico, P. Wang, A. M. Chinnaiyan, K. A. Ketchum, C. R. Kinsinger, A. I. Robles, E. An, T. Hiltke, M. Mesri, M. Thiagarajan, A. M. Weaver, A. G. Sikora, J. Lubiński, M. Wierzbicka, M. Wiznerowicz, S. Satpathy, M. A. Gillette, G. Miles, M. J. Ellis, G. S. Omenn, H. Rodriguez, E. S. Boja, S. M. Dhanasekaran, L. Ding, A. I. Nesvizhskii, A. K. El-Naggar, D. W. Chan, H. Zhang, B. Zhang, A. Agarwal, M. L. Anderson, S. C. Avanessian, D. Avtonomov, O. F. Bathe, C. Birger, M. J. Birrer, L. Blumenberg, W. E. Bocik, U. Borate, M. Borucki, M. C. Burke, S. Cai, A. P. Calinawan, S. Cerda, A. Charamut, L. S. Chen, S. Chowdhury, K. R. Clauser, H. Culpepper, T. Czernicki, F. D’Angelo, J. Day, S. De Young, E. Demir, F. Ding, M. J. Domagalski, J. C. Dort, B. Druker, E. Duffy, M. Dyer, N. J. Edwards, K. Elburn, T. S. Ermakova, D. Fenyo, R. Ferrarotto, A. Francis, S. Gabriel, L. Garofano, Y. Geffen, G. Getz, C. A. Goldthwaite, L. I. Hannick, P. Hariharan, D. N. Hayes, D. Heiman, B. Hindenach, K. A. Hoadley, G. Hostetter, M. Hyrcza, S. D. Jewell, C. D. Jones, M. H. Kane, A. Karz, R. B. Kothadia, A. Krek, C. Kumar-Sinha, T. Liu, H. Liu, W. Ma, E. Malc, A. Malovannaya, S. Mareedu, S. P. Markey, A. Marrero-Oliveras, N. Maunganidze, J. E. McDermott, P. B. McGarvey, J. McGee, P. Mieczkowski, S. Migliozzi, R. Montgomery, C. J. Newton, U. Ozbek, A. G. Paulovich, S. H. Payne, D. D. Pazardzhikliev, A. M. Perou, F. Petralia, L. Petrenko, P. D. Piehowski, D. Placantonakis, L. Polonskaya, E. V. Ponomareva, O. Potapova, L. Qi, N. Qu, S. Ramkissoon, B. Reva, S. Richey, K. Robinson, N. Roche, K. Rodland, D. C. Rohrer, D. Rykunov, E. E. Schadt, Y. Shi, Y. Shutack, S. Singh, T. Skelly, R. Smith, L. J. Sokoll, J. Stawicki, S. E. Stein, J. Suh, W. Szopa, D. Tabor, D. Tan, D. Tansil, G. C. Teo, R. R. Thangudu, C. Tognon, E. Traer, S. Tsang, J. Tyner, K. S. Um, D. R. Valley, L. V. Vasilev, N. Vatanian, U. Velvulou, M. Vernon, T. F. Westbrook, J. R. Whiteaker, Y. Wu, M. Xu, L. Yao, X. Yi, F. Yu, K. Zaalishvili, Y. Zakhartsev, R. Zelt, G. Zhao, J. Zhu, Proteogenomic insights into the biology and treatment of HPV-negative head and neck squamous cell carcinoma. Cancer Cell 39, 361–379.e16 (2021). https://doi.org/10.1016/j.ccell.2020.12.007.
G. Marioni, A. Staffieri, E. Manzato, G. Ralli, M. Lionello, L. Giacomelli, V. Prosenikliev, R. Marchese-Ragona, A. Busnardo, F. Bolzetta, S. Blandamura, A higher CD105-assessed microvessel density and worse prognosis in elderly patients with laryngeal carcinoma. Arch. Otolaryngol. Head Neck Surg. 137, 175–180 (2011). https://doi.org/10.1001/archoto.2010.244
Author information
Authors and Affiliations
Contributions
M.F. van der Kamp – acquisition, analysis and interpretation of literature data, drafting the manuscript.
C.J. Verhoeven—concept of design, acquisition, analysis and interpretation of literature data, drafting the manuscript.
G.B. Halmos – concept of design, interpretation of literature data, critical revision the manuscript.
B.E.C. Plaat—interpretation of literature data, critical revision the manuscript.
B.F.A.M. van der Laan—critical revision the manuscript.
P.L. Horvatovich—concept of design, critical revision the manuscript.
V. Guryev—critical revision the manuscript.
E. Schuuring—critical revision the manuscript.
B. van der Vegt—concept of design, critical revision the manuscript.
Corresponding author
Ethics declarations
Ethical approval and Consent to participate
As the manuscript is a review article, neither ethical approval from the Ethical Commission nor consent from patients is needed.
Consent for publication
The authors give consent for publication in Cellular Oncology.
Competing interests
The authors have no competing interests related to this study to declare.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix 1. Literature search
Appendix 1. Literature search
Given the broad scope and aim of this review, an open literature search rather than a systematic literature search was conducted in the database Pubmed/Medline.
Multiple searches using variations of Mesh terms with the following key elements: head and neck squamous cell carcinoma (HNSCC), ageing, physiopathology, and tumor biology. For HNSCC, the Mesh terms included: head and neck cancer, laryngeal carcinoma, laryngeal cancer, LSCC, hypopharyngeal carcinoma, hypopharyngeal cancer, oropharyngeal carcinoma, oropharyngeal cancer, OSCC, tongue carcinoma, and tongue cancer. For physiopathology, the Mesh terms included: physiopathology, tumor biology, molecular biology, molecular pathway, cellular ageing, cellular senescence, inheritance, inherited, familial tumors, and familial cancer syndromes. For ageing, the Mesh terms included: age, ag(e)ing, age-related, age-dependent, young, elderly, old.
Search results were screened on title and abstract to determine if ageing was the main topic or a factor significantly correlating with a pathway or biological marker. If this was the case, manuscripts were selected for full reviewing. Furthermore, to find more potential articles that were not identified by the literature search, citations of relevant articles were screened and when matching the scope of this article, included.
Rights and permissions
About this article
Cite this article
van der Kamp, M.F., Halmos, G.B., Guryev, V. et al. Age-specific oncogenic pathways in head and neck squamous cell carcinoma - are elderly a different subcategory?. Cell Oncol. 45, 1–18 (2022). https://doi.org/10.1007/s13402-021-00655-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13402-021-00655-4