Abstract
Background
No predictive biomarker of immune checkpoint inhibitors in head and neck squamous cell carcinoma (HNSCC) has been well established. The impact of programmed death-ligand 1 (PD-L1) expression, CD8+ tumor-infiltrating lymphocytes (TILs), and p16 status in HNSCC is unclear and may vary according to ethnicity.
Methods
HNSCC patients treated between 2007 and 2013 were reviewed. Archival tissues were retrieved for PD-L1, CD8+ TILs, and p16 analyses. PD-L1 expression was evaluated by using the validated SP142 assay on the VENTANA platform. CD8+ TILs were defined by using semiquantitative scoring.
Results
A total of 203 patients were analyzed. PD-L1 expression was observed in 80% of patients and was significantly associated with older age (P < 0.001). A high CD8+ TIL score (≥ 6) was significantly associated with never-smoking (P = 0.020), oral cavity cancer (P < 0.001), and stage M0 at presentation (P = 0.025). The p16 status was positive in 12% of patients. Patients with a high TIL score had a significantly longer OS (P = 0.032). Patients with PD-L1 expression of 1–49% and ≥ 50% were associated with a significantly shorter OS compared with those with PD-L1 < 1% (P = 0.027 and P = 0.011, respectively). Multivariate analysis showed that PD-L1 ≥ 50% was significantly associated with a poor OS. (HR 2.98 [95% CI 1.2–7.39]; P = 0.019.)
Conclusions
A high prevalence of PD-L1 expression was observed in HNSCC using the validated SP142 assay. PD-L1 expression was associated with older age, while highly PD-L1 expression (≥ 50%) was an independent prognostic factor for poor OS in anti-PD1/PD-L1 untreated HNSCC patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
During recent years, cancer immunotherapy has developed rapidly for many types of malignancy. Moreover, the identification of immune checkpoint inhibitors has changed the standard of care for some deadly cancers such as melanoma, lung cancer, urothelial carcinoma, renal cancer, and some gastrointestinal cancers thought to have high mutational burdens. The immune microenvironment of head and neck squamous cell carcinoma (HNSCC) is defined by alterations in the population of immune cells, immune checkpoints, and the balance of microenvironmental factors [1]. Immunogenicity in HNSCC induces disease development and progression, while immune suppression leads to tumor evasion and escape from immune surveillance [2, 3].
The immune checkpoint inhibitors, pembrolizumab and nivolumab, were approved for treatment of recurrent/metastatic (R/M) non-nasopharyngeal HNSCC after progression or platinum-containing chemotherapy [4,5,6,7,8]. A phase 2 single arm study of pembrolizumab (KEYNOTE-055) in platinum- and cetuximab-refractory R/M HNSCC demonstrated clinically meaningful antitumor activity [7]. Moreover, in CHECKMATE-141, a randomized phase 3 study for patients with R/M HNSCC with disease progression after platinum-based chemotherapy, patients receiving nivolumab had longer overall survival (OS) than those receiving standard of care chemotherapy [6, 8]. This study showed no significant interaction between programmed death-ligand 1 (PD-L1) expression and the benefit of nivolumab [6], although most studies reported a trend towards a greater magnitude of effect from immunotherapy in higher PD-L1-expressing tumors [4,5,6,7]. No established predictive biomarker for these agents has yet been identified for use in clinical practice.
Tumor-infiltrating lymphocytes (TILs), which include all immune cells inside tumors, have previously been examined as possible biomarkers. In some studies of patients with NSCLC and melanoma, significant survival benefits were reported in patients with CD4+ and CD8+ lymphocytes in the tumor area [9,10,11]. However, few studies of TILs in HNSCC have been performed. Moreover, the prognosis of patients with high and low immune receptor expression, and the correlation between immunogenicity and HPV status remain uncertain [12, 13]. Therefore, we aimed to evaluate characteristics of the immune biomarker profile, including PD-L1 expression, CD8+ TILs, and the p16 status of HNSCC patients, and their prognostication.
Materials and methods
Patient and specimen characteristics
Patients with histologically confirmed HNSCC who received treatment at the Ramathibodi Cancer Center between January 2007 and December 2013 were identified. Patient characteristics, pathological features, treatments, and outcomes were retrospectively abstracted from medical records. Archival formalin-fixed, paraffin-embedded (FFPE) samples of HNSCC tissues obtained at diagnosis were retrieved from the pathology department. Patients with nasopharyngeal carcinoma, squamous cell carcinoma of unknown primary, and with insufficient or unavailable FFPE samples and/or incomplete or unavailable medical records were excluded. Deaths were crosschecked with the National Security Death Index. The study was approved by the Ramathibodi Ethics Committee.
Assay methods: quantification of HPV status, PD-L1 expression, and CD8+ TILs
HPV status was defined using p16 immunohistochemistry (IHC). The IHC of the expression of HPV status were characterized by CINtec® p16 histology, consisting of a mouse monoclonal primary antibody against p16INK4a (E6H4™; Roche, USA). VENTANA detection kits and a VENTANA BenchMark XT automated slide stainer were used to detect p16 expression from archived FFPEs. p16 expression was categorized as positive or negative according to the manufacturer’s instructions.
IHC analysis for PD-L1 used 4-µm-thick FFPE tissue sections and a validated anti-human PD-L1 rabbit monoclonal antibody (clone SP142; Ventana, Tucson, AZ) on an automated staining platform (Benchmark; Ventana) using a concentration of 4.3 mcg/ml, with signal visualization by diaminobenzidine; sections were counter-stained with hematoxylin [11]. PD-L1 expression was evaluated on tumor cells and tumor-infiltrating immune cells. Specimens were scored using established criteria, which was defined as ≥ 5% of tumor-infiltrating immune cells (TC) or tumor cells (TC) to determine PD-L1 positivity [11].
TIL scoring was performed semiquantitatively by measuring CD8 cell densities as previously described: (i) no, or sporadic cells; (ii) moderate numbers of cells; (iii) an abundance of cells; and (iv) highly abundant cells [14, 15]. TILs were evaluated in three different tumor areas: the intraepithelial compartment, the stroma, and the tumor periphery. The CD8 total score was measured as the sum of individual scores from these three tumor areas and ranged from 3 to 12. The CD8+ TIL score cut-off for further correlations of the study was statistically analyzed and defined using death as the gold standard.
Study design and statistical analysis
The study aimed to evaluate whether PD-L1 expression could predict OS in the tested population as a primary objective. Exploratory objectives include associations of between PD-L1 expression, p16 status, and CD8+ TIL score. Sample size of the study was justified by using simplest formula for a proportion outcome, assuming alpha of 0.05 and acceptable margin of error 10%. The estimated incidence of PD-L1 expression in the population was between 70 and 80% from previous studies.[4, 5] Therefore, a sample size of at least 165 cases would be adequate for the study.
Although sample size was based on the assumption of normality for the distribution of mean PD-L1 and CD8+ TIL score, the extent of skewness in the observed distribution was such that means could not be assumed to have a normal distribution. Therefore, nonparametric analysis using the Mann–Whitney–Wilcoxon test was employed. Pearson’s correlation was used to assess relationships among the CD8+ TIL score, PD-L1 expression, patient age, and smoking status (pack-years). Continuous variables were summarized as means (± SD) or medians [min, max] and compared using the Student t test or Mann–Whitney–Wilcoxon tests where appropriate. Categorical data were expressed as numbers (percentages) and compared using Fisher’s exact test. The overall model fit was assessed using the Hosmer–Lemeshow test. In analyses based on fully adjusted models, the P-value for interaction was determined to explore the effect modification. To evaluate for multicollinearity, we calculated variance inflation factors and tolerances for each of the independent variables. A multivariable Cox proportional hazards model was performed to evaluate the association of factors with OS. All case survival analyses were performed by constructing Kaplan–Meier curves stratified by PD-L1 level. The log-rank statistic was used to test for differences in survival across strata. A P-value < 0.05 was considered significant.
Results
Patient characteristics
A total of 203 HNSCC patients were identified and stained for IHC analyses. Baseline patient and pathological characteristics are summarized in Table 1. Most patients were male and had an active or former smoking status. The oral cavity was the most common primary tumor site (46%). More than 70% of patients presented with locally advanced disease (stage III/IVab), while fewer than 3% had distant metastasis at diagnosis. p16 was positive in only 24 of 203 patients (12%), representing a low incidence of HPV-associated HNSCC.
PD-L1 expression
PD-L1 expression is shown in Fig. 1a. Using the cut-off of ≥ 5% of TC or IC to determine PD-L1 positivity [11], 163 of 203 HNSCC samples (80%) were classed as positive for PD-L1 expression. PD-L1-positive patients were significantly older than -negative patients (median age, 65 vs 57.5 years; P < 0.001). Older patients (≥ 65 years) also significantly associated with PD-L1 positivity (52.8% vs 22.5%; P = 0.001), while older age significantly correlated with a higher percentage of PD-L1 expression (r = 0.149; P = 0.034) (Fig. 1c). Patient sex, smoking status, site of primary cancer, stage at diagnosis, and p16 status were not associated with PD-L1 expression (Table 1). No correlation of the percentage of PD-L1 expression and smoking pack-years was observed in this population (Fig. 1e). In the subset of primary site, PD-L1 expression was positive in 84%, 83.9%, and 80.9% of tumors in the oral cavity, oropharynx, and larynx, respectively, but in only 61.5% of tumors in the hypopharynx (Supplement 1).
CD8+ TIL score
The mean CD8+ TIL score was 5.49 ± 1.93 (Fig. 1b). To determine the cut-off score for further analyses, a receiver operating characteristic (ROC) curve was drawn using death as the gold standard (Supplement 2). The final cut-off was defined using a score of 6. When compared with low CD8+ TIL score (< 6), high CD8+ TIL score (≥ 6) was significantly associated with patients who never smoked (43% vs 27.5%; P = 0.020), lower amount of smoking per pack-year (median 5 vs 15 pack-years; P = 0.007), those with site of primary cancer (P < 0.001), and those receiving a different definitive treatment (P < 0.001) (Supplement 3). Heavy smokers (per pack-year) were significantly correlated with a lower CD8+ TIL score (r = 0.192; P = 0.010) (Fig. 1f). Patient age, stage at diagnosis, sex, and p16 status were not associated with CD8+ TIL score (Fig. 1d and Supplement 3). No correlation between CD8+ TIL score and PD-L1 status was observed (P = 0.273) (Table 1).
Survivals
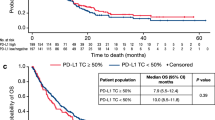
The median follow-up duration was 39.6 months. Using a ≥ 5% cut-off of TC or IC to determine PD-L1 positivity [11], the PD-L1 status was not associated with OS (PD-L1 positive vs negative, 23.6 vs 34.2 months; P = 0.505) (Fig. 2A). However, patients with PD-L1 expression ≥ 50% and 1–49% were significantly associated with a longer OS compared with PD-L1 < 1% patients (P = 0.032) (Fig. 2b). In subgroups of patients according to PD-L1 and p16 status, no association between OS was observed in both oropharyngeal (P = 0.716) and non-oropharyngeal cancers (P = 0.144) (Supplement 4).
Patients with high CD8+ TIL scores (≥ 6) had a significantly longer OS than low scoring patients (48.1 vs 20 months; P = 0.032) (Fig. 3a). Additionally, p16-positive and high TIL scoring patients were associated with the longest OS, while p16-negative and low TIL scoring patients had the shortest OS (P = 0.028) (Fig. 3b). However, this significant correlation of OS between p16 status and CD8+ TIL score was olny limited to patients with non-oropharyngeal cancer (P = 0.015) (Fig. 3c, d). No OS correlation was observed among subgroups of patients based on PD-L1 status and CD8+ TIL score (P = 0.126) (Supplement 4).
In multivariate analysis, ECOG PS ≥ 2, AJCC stage III–IV, type of definitive treatment, and p16 status were significantly associated with OS, while CD8+ TIL score and PD-L1 expression using pre-defined criteria [11] showed no significant association (Table 2). However, PD-L1 expression ≥ 50% was significantly associated with poor prognosis for OS in the multivariate analysis (HR 2.98 [95% CI 1.2–7.39; P = 0.019]).
Discussion
Though PD-L1 expression has been evaluated in many studies using various PD-L1 antibody clones, few investigations have used validated PD-L1 IHC clones developed for specific anti-PD1/PD-L1 drugs. Moreover, different anti-PD1/PD-L1 drugs utilize different compassionate PD-L1 IHC clones, methods, and positivity cut-offs to evaluate PD-L1 status [16]. We reported 80% PD-L1 positivity in our HNSCC patients using the validated SP142 antibody on a VENTANA platform, and previously well-described positivity criteria for atezolizumab [11]. The incidence of PD-L1 positivity in this study was comparable with that reported in a phase 1 study of atezolizumab in HNSCC, in which 25 of 32 patients (78.1%) were PD-L1 positive [17]. However, a retrospective European study evaluating the PD-L1 status of 99 oropharyngeal cancer patients reported low incidences of PD-L1 expression of 23% and 34%, using the SP142 and 22C3 IHC clones, respectively [18]. The 22C3 IHC clone was developed for PD-L1 testing for pembrolizumab. A phase Ib KEYNOTE-012 expansion cohort of 188 R/M HNSCC patients treated with pembrolizumab demonstrated that the combined positive score (CPS) of tumors and inflammatory cells improved the ability to predict the response of pembrolizumab compared with the tumor proportion score (TPS) [4, 5, 19]. In this study, 152 of 188 HNSCC patients (80.8%) had PD-L1-positive tumors using CPS ≥1% as a cut-off [19]. This was confirmed by KEYNOTE-055 and KEYNOTE-040 studies of pembrolizumab in R/M HNSCC, in which PD-L1 positivity using the 22C3 IHC clone and CPS ≥1% as a cut-off was demonstrated in 82% and 78% of patients, respectively [7, 20]. The PD-L1 antibody 28-8 IHC clone was developed along with nivolumab on the Dako platform. A phase III CHECKMATE-141 study reported PD-L1 positivity in 149 of 260 R/M HNSCC patients (57.3%) using tumor PD-L1 membrane expression ≥1% as a cut-off [6]. In a phase I study of 62 R/M HNSCC patients treated with durvalumab using the Ventana PD-L1 SP263 IHC assay, PD-L1-positive tumors were reported in 34% of patients [21]. A large retrospective multicenter study of PD-L1 expression in R/M HNSCC (SUPREME-HN) evaluated PD-L1 expression using the Ventana SP263 assay and scored PD-L1 expression as high (≥ 25% of TCs only) versus low/negative. This study found that 132 of 396 evaluable tumors (33.3%) highly expressed PD-L1 [22]. These various incidences of PD-L1 expression are partially related to different PD-L1 IHC antibodies used, the method of evaluation, and cut-off for positivity. Other retrospective studies using non-validated PD-L1 IHC assays reported widely-ranging PD-L1 expression in HNSCC.[23,24,25].
A Blueprint PD-L1 IHC assay project compared 4 validated PD-L1 IHC assays in 39 NSCLC samples [26]. It found comparable percentages of PD-L1-stained TCs among 22C3, 28-8, and SP263 assays, whereas SP142 exhibited fewer stained TCs overall. All assays demonstrated IC staining, but with greater variability than TC staining. Another larger prospective multicenter study evaluated 90 NSCLC samples stained for 4 PD-L1 platforms (22C3, 28-8, SP142, and E1L3N) [27]. The SP142 antibody was an outlier that detected significantly less PD-L1 expression in TCs and ICs compared with other assays. Though these two studies obtained consistent findings when evaluating four validated PD-L1 assays in NSCLC, non-NSCLC samples have not been tested [26, 27]. Therefore, future non-NSCLC studies are warranted.
We observed no correlation between p16 status and PD-L1 expression, although data regarding this are conflicting [6, 7, 24, 28]. We also found that PD-L1 expression ≥ 50% was significantly associated with poor OS in multivariate analysis. A retrospective study of SP263 IHC PD-L1 expression reported that a PD-L1 ≥ 1% cut-off was associated with a significantly longer OS [29]. These results demonstrated the prognostic activity of PD-L1 using different IHC clones and cut-off values in immunotherapy-naïve HNSCC patients.
Unlike PD-L1 expression, CD8+ TIL measurements have not been determined during clinical development of anti-PD1/PD-L1 drugs. This partially reflects their challenging evaluation in HNSCC because tumors arising from different anatomical sites of the head and neck region contain various numbers of TILs from background lymphoid tissue [30]. The International Immuno-Oncology Biomarkers Working Group proposed a standardized method for assessing TILs, but this recommendation for HNSCC was unclear because of a lack of data support [30]. Balermpas et al. proposed a semiquantitative IHS scoring method to describe TILs [14, 15]. We validated this semiquantitative scoring method but redefined the cut-off using the ROC graph to evaluate sensitivity and specificity for predicting death. Interestingly, we found that a similar score cut-off of 6 was significant. We showed that a high CD8+ TIL score was associated with lower levels of smoking (per pack-year), primary tumor site, and a longer OS in univariate analysis. These results were consistent with the literature [15, 23]. However, an association between p16 expression/HPV positivity and high CD8+ TIL tumors was previously reported [15, 28], but this was not observed in our present study. This could reflect the low incidence of p16-expressing/HPV-associated tumors (12–17.5%), as seen in previous studies from Thailand [31, 32].
PD-L1 expression has been incorporated in the clinical development of all immune checkpoint inhibitors. However, the PD-L1 status has not been used as a predictive biomarker for any indication of immunotherapy in HNSCC to date [5,6,7,8]. Both pembrolizumab and nivolumab improved survival in platinum-refractory R/M HNSCC, regardless of PD-L1 status, although the objective response rate (ORR) was higher in PD-L1-expressing tumors [5,6,7,8]. In a phase Ib KEYNOTE-012 expansion cohort, a significant increase in ORR was observed for PD-L1 positive vs negative patients (22% vs 4%; P = 0.021) [5]. Similarly, a phase II KEYNOTE-055 study reported an overall ORR of 16% [7]. When using a CPS score of ≥50% as a cut-off, the ORR was improved from 13% in patients with CPS < 50–27%. A phase III KEYNOTE-040 study demonstrated that pembrolizumab improved ORR and OS in patients with CPS ≥ 1% compared with chemotherapy. This benefit was more pronounced in patients with a TPS ≥ 50% [20]. Additionally, a phase III CHECKMATE-141 study demonstrated a significant improvement in OS from 5.1 months in the chemotherapy group to 7.7 months in the nivolumab group [6]. This OS benefit was observed regardless of PD-L1 status [8]. Because HNSCC is an area of interest for the development of immune checkpoint inhibitors, numerous clinical studies are ongoing [33]. These are evaluating various approaches in different settings, and are assessing potential biomarkers as single agents, or in combination with radiotherapy or immune checkpoint inhibitors targeting PD-1, PD-L1, CTLA-4, OX40, CD27, and LAG-3.
Conclusions
A high prevalence of PD-L1 expression (80%) was observed in HNSCC using the validated SP142 assay and previously described cut-off value. PD-1L expression was associated with older age, while highly expressed PD-L1 (≥ 50%) was an independent prognostic factor for poor OS in anti-PD1/PD-L1 untreated HNSCC patients. No correlation among PD-L1, CD8+ TIL score, and p16 status was observed in this study.
References
Ling DC, Bakkenist CJ, Ferris RL, Clump DA. Role of immunotherapy in head and neck cancer. Semin Radiat Oncol. 2018;28(1):12–6. https://doi.org/10.1016/j.semradonc.2017.08.009.
Solomon B, Young RJ, Rischin D. Head and neck squamous cell carcinoma: genomics and emerging biomarkers for immunomodulatory cancer treatments. Semin Cancer Biol. 2018. https://doi.org/10.1016/j.semcancer.2018.01.008.
Qi X, Jia B, Zhao X, Yu D. Advances in T-cell checkpoint immunotherapy for head and neck squamous cell carcinoma. OncoTargets Therapy. 2017;10:5745–54. https://doi.org/10.2147/ott.S148182.
Seiwert TY, Burtness B, Mehra R, Weiss J, Berger R, Eder JP, et al. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): an open-label, multicentre, phase 1b trial. Lancet Oncol. 2016;17(7):956–65. https://doi.org/10.1016/S1470-2045(16)30066-3.
Chow LQM, Haddad R, Gupta S, Mahipal A, Mehra R, Tahara M, et al. Antitumor activity of pembrolizumab in biomarker-unselected patients with recurrent and/or metastatic head and neck squamous cell carcinoma: results from the phase Ib KEYNOTE-012 expansion cohort. J Clin Oncol. 2016;34(32):3838–45. https://doi.org/10.1200/JCO.2016.68.1478.
Ferris RL, Blumenschein G Jr, Fayette J, Guigay J, Colevas AD, Licitra L, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016;375(19):1856–67. https://doi.org/10.1056/NEJMoa1602252.
Bauml J, Seiwert TY, Pfister DG, Worden F, Liu SV, Gilbert J, et al. Pembrolizumab for platinum- and cetuximab-refractory head and neck cancer: results from a single-arm, phase II study. J Clin Oncol. 2017;35(14):1542–9. https://doi.org/10.1200/JCO.2016.70.1524.
Ferris R, Blumenschein G Jr, Fayette J, Guigay J, Colevas AD, Licitra L, et al. Nivolumab vs investigator’s choice in recurrent or metastatic squamous cell carcinoma of the head and neck: 2-year long-term survival update of CheckMate 141 with analyses by tumor PD-L1 expression. Oral Oncol. 2018;81:45–51.
Larkin J, Hodi FS, Wolchok JD. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373(13):1270–1. https://doi.org/10.1056/NEJMc1509660.
Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372(4):320–30. https://doi.org/10.1056/NEJMoa1412082.
Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515(7528):563–7. https://doi.org/10.1038/nature14011.
Heineman TE, Widman A, Kuan EC, St John M. The genetic landscape of programmed death ligand-1 (PD-L1) alterations in head and neck cancer. Laryngoscope Investig Otolaryngol. 2017;2(3):99–103. https://doi.org/10.1002/lio2.79.
Wollenberg B. Cancer Immunology and HPV. Recent results in cancer research Fortschritte der Krebsforschung. Progres dans les recherches sur le cancer. 2017;206:243–8. https://doi.org/10.1007/978-3-319-43580-0_19.
Balermpas P, Michel Y, Wagenblast J, Seitz O, Weiss C, Rodel F, et al. Tumour-infiltrating lymphocytes predict response to definitive chemoradiotherapy in head and neck cancer. Br J Cancer. 2014;110(2):501–9. https://doi.org/10.1038/bjc.2013.640.
Balermpas P, Rodel F, Rodel C, Krause M, Linge A, Lohaus F, et al. CD8+ tumour-infiltrating lymphocytes in relation to HPV status and clinical outcome in patients with head and neck cancer after postoperative chemoradiotherapy: a multicentre study of the German cancer consortium radiation oncology group (DKTK-ROG). Int J Cancer. 2016;138(1):171–81. https://doi.org/10.1002/ijc.29683.
Hansen AR, Siu LL. PD-L1 testing in cancer: challenges in companion diagnostic development. JAMA Oncol. 2016;2(1):15–6. https://doi.org/10.1001/jamaoncol.2015.4685.
Bahleda R, Braiteh FS, Balmanoukian AS, Braña I, Hodi FS, Garbo L, et al. Long-term safety and clinical outcomes of atezolizumab in head and neck cancer: phase ia trial results. Ann Oncol. 2017. https://doi.org/10.1093/annonc/mdx374.001a.
De Meulenaere A, Vermassen T, Aspeslagh S, Deron P, Duprez F, Laukens D, et al. Tumor PD-L1 status and CD8(+) tumor-infiltrating T cells: markers of improved prognosis in oropharyngeal cancer. Oncotarget. 2017;8(46):80443–52. https://doi.org/10.18632/oncotarget.19045.
Chow LQM, Mehra R, Haddad RI, Mahipal A, Weiss J, Berger R, et al. Biomarkers and response to pembrolizumab (pembro) in recurrent/metastatic head and neck squamous cell carcinoma (R/M HNSCC). JCO. 2016;34(15):suppl.6010.
Soulieres D, Cohen EE, Le Tourneau C, Dinis J, Licitra L, Ahn MJ, et al. Updated survival results of the KEYNOTE-040 study of pembrolizumab vs standard-of-care chemotherapy for recurrent or metastatic head and neck squamous cell carcinoma. In: AACR; Chicago, IL; 2018.
Segal NH, Ou SHI, Balmanoukian AS, Massarelli E, Brahmer JR, Weiss J, et al. Updated safety and efficacy of durvalumab (MEDI4736), an anti-PD-L 1 antibody, in patients from a squamous cell carcinoma of the head and neck (SCCHN) expansion cohort. Ann Oncol. 2016. https://doi.org/10.1093/annonc/mdw376.1.
Pai S, Cohen EE, Lin D, Fountzilas G, Kim ES, Mehlhorn H, et al. RetroSpective cohort stUdy of PD-L1 expression in REcurrent and/or MEtastatic squamous cell carcinoma of the head and neck (SUPREME-HN). Ann Oncol. 2017;28(suppl_5):6040.
Ono T, Azuma K, Kawahara A, Sasada T, Hattori S, Sato F, et al. Association between PD-L1 expression combined with tumor-infiltrating lymphocytes and the prognosis of patients with advanced hypopharyngeal squamous cell carcinoma. Oncotarget. 2017;8(54):92699–714. https://doi.org/10.18632/oncotarget.21564.
Oguejiofor K, Galletta-Williams H, Dovedi SJ, Roberts DL, Stern PL, West CM. Distinct patterns of infiltrating CD8+ T cells in HPV+ and CD68 macrophages in HPV− oropharyngeal squamous cell carcinomas are associated with better clinical outcome but PD-L1 expression is not prognostic. Oncotarget. 2017;8(9):14416–27. https://doi.org/10.18632/oncotarget.14796.
Muller T, Braun M, Dietrich D, Aktekin S, Hoft S, Kristiansen G, et al. PD-L1: a novel prognostic biomarker in head and neck squamous cell carcinoma. Oncotarget. 2017;8(32):52889–900. https://doi.org/10.18632/oncotarget.17547.
Hirsch FR, McElhinny A, Stanforth D, Ranger-Moore J, Jansson M, Kulangara K, et al. PD-L1 immunohistochemistry assays for lung cancer: results from phase 1 of the blueprint PD-L1 IHC assay comparison project. J Thorac Oncol. 2017;12(2):208–22. https://doi.org/10.1016/j.jtho.2016.11.2228.
Rimm DL, Han G, Taube JM, Yi ES, Bridge JA, Flieder DB, et al. A prospective multi-institutional, pathologist-based assessment of 4 immunohistochemistry assays for PD-L1 expression in non-small cell lung cancer. JAMA Oncol. 2017;3(8):1051–8. https://doi.org/10.1001/jamaoncol.2017.0013.
Badoual C, Hans S, Merillon N, Van Ryswick C, Ravel P, Benhamouda N, et al. PD-1-expressing tumor-infiltrating T cells are a favorable prognostic biomarker in HPV-associated head and neck cancer. Cancer Res. 2013;73(1):128–38. https://doi.org/10.1158/0008-5472.CAN-12-2606.
Stokes M, Wang R, Wildsmith S, Secrier M, Angell HK, Barker C, et al. Relationship between PD-L1 expression and survival in head and neck squamous cell carcinoma (HNSCC) patients (pts). Ann. Oncol. 2017;28(suppl_5):v372–v394.
Hendry S, Salgado R, Gevaert T, Russell PA, John T, Thapa B, et al. Assessing tumor-infiltrating lymphocytes in solid tumors: a practical review for pathologists and proposal for a standardized method from the international immuno-oncology biomarkers working group: part 2: TILs in melanoma, gastrointestinal tract carcinomas, non-small cell lung carcinoma and mesothelioma, endometrial and ovarian carcinomas, squamous cell carcinoma of the head and neck, genitourinary carcinomas, and primary brain tumors. Adv Anat Pathol. 2017;24(6):311–35. https://doi.org/10.1097/PAP.0000000000000161.
Sritippho T, Pongsiriwet S, Lertprasertsuke N, Buddhachat K, Sastraruji T, Iamaroon A. p16-a possible surrogate marker for high-risk human papillomaviruses in oral cancer? Asian Pac J Cancer Prev. 2016;17(8):4049–57.
Phusingha P, Ekalaksananan T, Vatanasapt P, Loyha K, Promthet S, Kongyingyoes B, et al. Human papillomavirus (HPV) infection in a case-control study of oral squamous cell carcinoma and its increasing trend in northeastern Thailand. J Med Virol. 2017;89(6):1096–101. https://doi.org/10.1002/jmv.24744.
Dogan V, Rieckmann T, Munscher A, Busch CJ. Current studies of immunotherapy in head and neck cancer. Clin Otolaryngol. 2018;43(1):13–21. https://doi.org/10.1111/coa.12895.
Acknowledgements
This work was supported by a grant from the Ramathibodi Cancer Center Grant. N. Ngamphaiboon received funding from the Research University Network (RUN), and the Thailand Research Fund (TRF), and the Research Development Grant from the Ramathibodi Hospital. The authors thank Ms. Dollapas Punpanich for statistical analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare no conflict of interest.
Informed consent
The Ramathibodi Ethic Committee approved a waiver of consent for this study as a retrospective chart review. Archrival tissues used in this study were considered as a leftover specimen. The research involves no more than minimal risk to the subject and is not adversely affect the rights and welfare of the subjects. All patient identifications were protected according to the GCP guideline and not published in the manuscript. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ngamphaiboon, N., Chureemas, T., Siripoon, T. et al. Characteristics and impact of programmed death-ligand 1 expression, CD8+ tumor-infiltrating lymphocytes, and p16 status in head and neck squamous cell carcinoma. Med Oncol 36, 21 (2019). https://doi.org/10.1007/s12032-018-1241-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-018-1241-1