Abstract
The hydrothermal liquefaction (HTL) of mixed household waste (MHHW) was carried out at varying temperatures from 260 to 360 °C, residence times of 30–90 min, and catalyst loads of 2.5–12.5 wt. %, in the presence of four heterogeneous catalysts, namely, bentonite clay, diatomaceous earth (DE), nanoporous ZnO (nano ZnO), and zeolite ZSM-5. Maximum bio crude yield of 48.10% was obtained at 360 °C and 75 min, in the presence of 5 wt. % nano ZnO. Among the naturally occurring catalysts, DE yielded a bio crude percentage of 48.52% at 12.5 wt. %, 360 °C, and 75 min. The addition of catalysts increased the bio crude yield and inhibited the formation of bio char and gaseous products. Also, the bio char and bio crude obtained from catalytic HTL (cat-HTL) presented with higher carbon and hydrogen content, thus possessing a significantly higher H/C ratio and HHV. Among cat-HTL, DE and nano ZnO yielded higher carbon and energy recovery percentage for bio char and bio crude, respectively. Also qualitatively, cat-HTL in the presence of DE yielded a higher fraction of hydrocarbons, when compared with non-cat HTL and cat-HTL in the presence of nano ZnO. N-containing compounds, phenols, furfurals, etc., were found to be present in the range of 16.2 to 18.6% for all bio crudes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The study of renewable energies has become significant in the present scenario of continuous economic development, rapid progress of society, depletion of traditional fossil fuel resources, and changing climate patterns. Biofuels, produced from renewable sources, have the potential to accommodate 30% of the world’s energy demand by the year 2050 [1, 2]. Accordingly, many biomasses from different origins, possessing varying biochemical compositions, such as algae, lignocellulosic wastes, agricultural residues, and aquatic trashes, have been investigated for their prospect as practical alternatives for fossil fuels [3]. In this regard, municipal solid waste (MSW) holds within itself, huge untapped energy, which is intentionally squandered into open dumps and landfills due to mishandling solid waste management policies. The mixed household waste (MHHW) forms a major portion of MSW, which retains within it both organic and inorganic portions in the form of food waste, plastics, and paper. And MHHW from residential households, with high energy potential, is disposed off in an unsegregated manner into community bins, which is then transported to transfer stations and/or finally disposed into open dumps and landfills. Thus, this MHHW biomass from community bins, prior to their transportation for final disposal, is a valuable bio-resource that, in addition to posing no competition to food resources, is abundantly and readily available, environmentally friendly, and CO2 neutral, thus making it a sustainable and economical feedstock for bioenergy and bio refinery. Many researchers have worked on effective energy retrieval and safe disposal of MSW, including methods such as landfills, incineration, and pyrolysis [4-6]. These methods present with limited feed variety and extensive pre-treatment methods such as segregation, drying, size reduction, and safe disposal of by-products [7-11].
Hydrothermal liquefaction (HTL) is a promising technology that uses water at subcritical temperatures as a reaction medium to convert biomass into biofuels [12]. This process is specifically useful in the case of raw materials with high moisture content, which can be effectively converted into useful bio-intermediates for the production of high-value-added chemicals and liquid bio fuels. The products of HTL self-separate into solid, liquid, and gaseous phases which are typically called bio char, bio crude, aqueous phase, and gaseous phase products, respectively. Typically, HTL is conducted at medium temperatures of 250–400 °C and moderately high pressures of 5–25 MPa [1, 13, 14] targeting the bio crude component of the liquid product. Owing to its advantages over other thermochemical conversions like pyrolysis and gasification on biomass selectivity and feedstock heterogeneity, HTL has become one of the most practically implementable technologies demonstrating great potentials for the production of biofuels and solving the biomass choice obstacles. Accordingly, a plethora of biomasses have been analyzed for their capabilities for production of bio crude through HTL technology, both singularly and in combination, which include, but are not limited to, various types of lignocellulosic biomass wastes, numerous species of micro and macro algae, and sludges [15-19]. Also, sequential HTL, or two-stage HTL, has also been garnering immense attention to enhance the bio oil yield and to reduce the occurrence of nitrogen heteroatoms in bio oil that arise due to the high protein content of microalgal feedstocks [20, 21]. Furthermore, artificial intelligence techniques such as machine learning and decision support systems have been employed to predict and optimize bio oil yields in HTL processes [22-24].
The bio crudes obtained from the HTL process are dark brown-colored viscous semiliquids that require a set of down streaming process for its upgradation and final usage. Thus, the viscosity of the final product along with low yields limit the practical application of HTL, warranting the usage of catalysts. Several homogeneous and heterogeneous catalysts have been investigated for their ability to positively impact the overall process and the liquid bio crude in particular. Under homogeneous catalysts, acidic and alkaline ones such as NaOH, KOH, Na2CO3, and K2CO3 have been commonly employed for efficient HTL conversion of biomass into bio crudes [25-27]. These catalysts exhibit high activity due to their superior diffusion properties which at the same time leads to increased corrosion effects due to difficulties in their separation and recycling. To overcome these difficulties, heterogeneous catalysts such as metal oxides, and zeolites are being largely focussed not only for their capabilities of efficient hydrothermal conversion but also their recyclability, thus presenting environmental benignity and economic sustainability [2, 14]. Also, the above-mentioned advantages are incremented many folds in the case of naturally occurring catalysts, which are increasingly safe and accessible than chemically synthesized ones.
Since each catalyst plays a different role in promoting the production of bio crude from the HTL process, the aim of this research lies in figuring out the effect of naturally occurring and chemically synthesized heterogeneous catalysts such as bentonite clay, diatomaceous earth (DE), nanoporous zinc oxide (nano ZnO), and zeolite ZSM – 5. Also, to the best of our knowledge, the use of unsegregated and MHHW, obtained solely from residential dwellings, as biomass feedstock for the HTL process has never been reported before. Thus, to address the above knowledge gap, HTL of MHHW for the production of bio crude in the absence and presence of specified catalysts were investigated at various temperatures and residence times. Also, the properties of bio crude and bio char thus obtained were also analyzed in detail by means of elemental analysis, gas chromatography mass spectroscopy (GC MS), and Fourier transform infrared spectrometry (FTIR).
2 Materials and methods
2.1 Waste feedstock and characterization studies
The MHHW biomass feedstock was collected from community bins of residential establishments around SSN College of Engineering, Kalavakkam, Tamil Nadu, India. The collected waste was separated of inert materials such as glass, ceramics, textiles, construction waste, and hazardous wastes such as batteries and e-waste, and the usable portion was taken for further analysis. The analyzed portion of MHHW constituted majorly of food waste both raw and cooked, plastic waste consisting of packaging and disposal materials, and paper waste from packaging, cleaning, and sanitation applications. Thus, the MHHW biomass feedstock, for all experiments, is a mixture of these sub-portions in the following compositions—food sub-portion 60%, plastic sub-portion 25%, and paper sub-portion 15%. The elemental C, H, N, and S compositions of samples were determined as per ASTM D5291 and D3176 standards directly by means of the Thermo Scientific Flash 2000 auto-analyzer (Thermo Fisher Scientific, USA). All catalysts used were analytical grade and used directly as such without processing.
Table 1 depicts the CHNS elemental analysis, proximate analysis, and biochemical analysis values for the MHHW biomass sample. It can be seen that MHHW biomass contains a substantially high amount of carbon (50.25 ± 2.53%) and a low amount of oxygen (27.93 ± 3.25%) which are in and below par with most of biomasses [28-33]. The MHHW biomass sample also contains a considerably high amount of hydrogen which is found to be 7.26 ± 0.68%, thus resulting in a desirable H/C ratio of 1.73. The HHV of the sample is in the range of 22.06 ± 2 MJ/kg. Also, the noticeable amount of volatiles (60.25 ± 2.2%) present in MHHW along with a low amount of fixed carbon (16.88 ± 1.35 %) render it as a good feedstock choice for the production of biofuels. From the proximate analysis of MHHW biomass, the ash content of the sample was found to be 5.24 ± 0.98%. This is significantly lower than most of algal biomasses [34-37]. Ash content has a negative impact on the overall HTL oil-forming efficiency by causing heat transfer limitations, fouling, and deactivation of catalyst. It also increases the probability of the presence of inorganic substances in the bio oil, thus affecting the quality [38, 39].
The biochemical composition (ash-free basis) is attributed by the food sub-portion of MHHW biomass and was found to contain 17.92 ± 0.98% of lipids and 13.23 ± 2.41% of protein, respectively. The influence of the biochemical composition of feedstock on bio crude production by HTL is highly dependent on the presence of lipids and proteins than carbohydrates [40].
2.2 HTL reactor
HTL was carried out in a custom-made cart-mounted SS316 high-pressure stirred autoclave reactor with a volume of 2 L, ID 80 mm, thickness 30 mm, and height 400 mm, designed for a pressure and temperature of 200 bar and 450 °C. The schematic diagram of the reactor is shown in Fig. 1. The heat supply of the reactor is ensured by the SS-coated ceramic strip heaters around its surface. Reactor agitation is handled by a 100–1400 rpm three-blade turbine impeller connected to zero-flux magnetic duty switches with a torque of 2 Nm.
The pressure and temperature of the reactor are monitored by an SS316 pressure regulator with a manometer and a K-type thermocouple connected to the temperature regulator (placed in a heat well), both attached to the end of the reactor. Cooling water is supplied by an SS304 automatic cooling system consisting of a circulating water pump, hose disconnect switch, and cooling coils and is connected to both the reactor and mixing system magnetic drive to initiate automatic cooling above 450 °C. The reactor system also consists of a high-pressure reflux condenser with a reservoir to facilitate the regeneration of gaseous products. The recycling of liquid and solid products is made possible by a 10 mm diameter hole equipped with a bottom flow valve.
2.3 HTL experiments
For all HTL experiments, 120 g of MHHW biomass was loaded into the reactor with 1600 ml of water. The temperature of the reactor contents is raised by heating them at a rate of 10 °C/min and was held at the required final operating temperature for the desired residence time. Then, the reactor was cooled down to ambient conditions before collecting the products. The experiments to determine the effect of operating temperature and residence time were analyzed in the range of 260–360 °C and 30–90 min, respectively. For catalytic HTL (cat-HTL) experiments, respective catalysts were added in varying ratios of 2.5–12.5 wt. % of feed. After the stipulated time, a solid and liquid product mixture was obtained from the bottom flush valve, and both phases were separated by means of filtration. The obtained solid phase was then dried to remove moisture, and the recovered liquid phase was taken to solvent extraction. Polar solvent such as dichloromethane (DCM) was used in a ratio of 3:1 to separate the hydrocarbon-rich organic phase and water-rich aqueous phase.
2.4 Product yields
All experiments were triplicated, and the obtained product streams were quantitatively analyzed. Using Eqs. (1) and (2), the specific product yield was determined as the ratio of the total mass of the product phase to the total mass of MHHW biomass feedstock.
2.5 GC MS and FTIR analysis
The liquid products were analyzed for their composition by gas chromatography mass spectroscopy (GCMS) by means of Agilent 7890 GC equipped with Agilent 7683A auto-injector and flame ionization detector (FID). Here, 1 μL of the diluted sample that is to be tested was injected into the system with Helium (99.9995% purity) as the carrier gas. The initial temperature of the column was maintained at 50 °C and was increased to 300 °C at the rate of 10 °C/min, and the sample was held for 5 min at this temperature.
The Fourier transform infrared (FTIR), Perkin Elmer FTIRC 100566, UK, analysis was used to determine and elucidate the major organic constituents of bio char samples, based on the absorption peaks for functional groups between 400 to 4000 cm−1.
2.6 Thermo gravimetric analysis and boiling point distribution of bio crude
The thermo gravimetric analysis (TGA) of MHHW was analyzed using a Shimadzu Thermogravimetric Analyzer (50H) at a heating rate of 10 °C.min−1 using nitrogen ambience. Around 20 mg of waste was placed inside the furnace, and the temperature increased from 100 to 800 °C, and the weight loss was noted. The boiling point distribution of various fractions in bio oil was obtained as per ASTM 7169 standard [41, 42].
2.7 Carbon and energy recovery
The recovery percentages of carbon in bio crude and bio char were calculated by Eq. (3) [43, 44]:
The percentage of energy recovery for bio crude and bio char was calculated by Eq. (4) [27, 42].
3 Results and discussions
3.1 MHHW biomass characterization
3.1.1 Effect of operating temperature and residence time
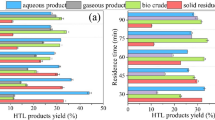
The effect of operating temperature and residence time on different product streams for HTL of MHHW biomass is depicted in Fig. 2a and b. From Fig 2a, it can be seen that the formation of bio crude increases from 13.68 to 37.60% as the temperature increases from 260 to 360 °C. The same trend of increase can be noted in the formation of gaseous products. On the contrary, a 67.24% decrease is noted in the amount of bio char, indicating that the reaction proceeds in the desired direction as temperature is increased. The formation of the aqueous phase varies in the range of 24.20 to 41.83 % as the temperature is varied from 260 to 360 °C.
The operating temperature for HTL highly depends on the type of feedstock used and also highly impacts the formation of various HTL products [45]. In this present work, the MHHW biomass feedstock is a consortium of three major sub-portions—food, plastic, and paper—due to which slightly higher temperatures are required for the occurrence of effective fragmentation and degradation reactions. Having said that, any further increase in temperature fails to maintain the hydrothermal medium (water) in subcritical temperatures, driving it to supercritical conditions [46]. This above state critically affects the HTL product yields, thus making the reliance on operating temperature for an efficient HTL process a paramount one.
From Fig. 2b, it can be seen that the bio crude formation increases initially with an increase in residence time up to 75 min and then falls with a further increase to 90 min. In this regard, the highest yield of bio crude for HTL of MHHW biomass (40.82%) is obtained at a residence time of 75 min, beyond which an increase of residence time to 90 min decreases the bio crude yield falls by almost 16%. Similar to the trend followed in the previous section, the yields of bio char and gaseous products fall and rise, respectively, with an increase in residence time from 30 to 90 min. As stated above, the feedstock investigated in this work is a mixture of various individual sub-portions; a slightly increased residence time is needed for effective HTL of all components. This residence time of 75 min is on the higher side when compared with other existing works in literature [1, 47]. Also, as the feedstock contains a substantial amount of plastics, high operating temperatures such as 360 °C are favorable for the HTL process due to their heat-resisting properties [48]. Also, such severe conditions can be moderated by the usage of liquefaction solvents instead of pure water as hydrothermal medium that can beneficially aid not only in the fragmentation and decomposition of MHHW feedstock but also in increasing the yield of bio crude [49-51]. Furthermore, the bio crude yield obtained is in par and below to other works in literature for HTL of food waste, in spite of high operating conditions, is due to the presence decreased of lipids in MHHW feedstock and its inherent complexity [52-55]. Since, in this present work, an operating temperature of 360 °C and a residence time of 75 min presented with the highest bio crude yield, the same was used for further investigations.
3.2 Effect of usage of catalysts
Non-catalytic HTL of a heterogeneous feedstock may lead to operation at higher temperatures and the formation of non-desired products that reduce the overall yield of the process. This disadvantage can be overcome by the usage of catalysts which can provide a beneficial pathway to attain specific product yields. Previous studies on catalytic co-liquefaction have concluded that the type of catalyst used is very feedstock-specific and has a high impact on the distribution and composition of products obtained. Zeolite-based catalysts are most commonly used in the thermochemical conversion of plastics, owing to their high thermal stability and efficient cracking of large hydrocarbons leading to the formation of branched, cyclic, and smaller ones by accelerating dehydration and deoxygenation reactions [56-60]. Metal oxide-based catalysts have provide efficiency and low cost for catalytic thermochemical conversion of various biomasses and plastic feedstock [61, 62]. These nanoporous metal oxides possess a high surface area to volume ratio and form complexes with the liquefaction solvents aiding in the supply of electrons [63]. Clay or silica-based catalysts serve as green catalysts that can be used for the production of high-grade bio oils and heterogeneous bio chars with improved adsorption capacity. In addition to being inexpensive, they also produce a positive effect on the boiling point distribution of the obtained bio oil, by increasing medium-temperature distillates, energy recovery, and heating value [43, 64, 65]. Owing to the heterogeneous nature of the feedstock, a right sample set of catalysts that cater to both food waste fraction and plastic waste fraction were thoroughly analyzed.
In the present work, four catalysts—bentonite clay, diatomaceous earth, Zeolite ZSM-5, and nanoporous ZnO—were tested for their capability for the production of liqud products in HTL of MHHW biomass. Figure 2c depicts the effect of usage of various catalysts on formation of HTL products. It can be seen that, among the catalysts used, nano ZnO presented with highest bio crude yield of 49.58%, which is 17.5% higher than the no catalyst condition. The bio crude yields for the analyzed catalysts followed the trend: nano ZnO > zeolite ZSM-5 > diatomaceous earth > bentonite clay. The addition of catalyst during HTL of MHHW biomass resulted in a higher portion of liquid products with less bio char and gaseous product formation.
Also, among the naturally occurring catalysts, DE presented with a high bio crude yield of 48.52%, which is 9% lower than the yield obtained by nano ZnO, a chemical-based catalyst. Naturally occurring catalysts pose multiple advantages over chemical ones, including safety and accessibility, along with being economical and environmentally benign. Also, the choice of catalyst depends on the desired composition of obtained bio crude, which plays a crucial role in its further upgradation and end-usage. Due to the above reasons, both diatomaceous earth and nano ZnO were used for further investigations.
The effect of the amount of catalyst (wt. %) for both DE and nano ZnO in cat-HTL mode was tested under previously optimized reaction temperature and residence time conditions, and the results are depicted in Fig. 2d and e, respectively. It is clear that, in the case of both catalysts, as the amount of catalyst used is increased from 0 to 12.5 wt. %, the yield of bio crude increased by 18.87% and 21.46% for cat-HTL by DE and nano ZnO, respectively. Also, the maximum bio crude yields of 48.52% and 49.58% were obtained for cat-HTL of 12.5 wt % diatomaceous earth and nano ZnO, respectively.
Furthermore, in cat-HTL with diatomaceous earth, usage of 10 wt. % catalyst could be more beneficial economically than 12.5 wt. %, as the bio crude yields for both conditions differ by a miniscule percentage of 1.06. Whereas, in the case of cat-HTL with Nano ZnO, the bio crude yields remain more or less the same over the catalyst range of 7.5 to 12.5 wt %. Thus, in cat HTL with nano ZnO, 7.5 wt % is considered more beneficial economically. Also, though the bio crude yield that was obtained at 10 wt % diatomaceous earth could be achieved with 5 wt. % nano ZnO, it is also important to take note that any chemically synthesized catalyst, apart from being environmentally hazardous, is also costly, thus making the whole process economically less feasible.
Thus, the introduction of a catalyst increased the yield of bio crude and decreased gaseous products and solid residue yield. Also, when a lignocellulosic biomass is co-liquefied with plastics, the decomposition of biomass occurs first, and the products of this reaction positively impact and promote the breakdown of plastics in feedstock by affecting its thermal stability. Also, the quantity of plastics present in the feedstock is also a significant operating parameter for efficient HTL. Studies have shown that there exists a critical plastic concentration in the feedstock, beyond which the bio crude yield decreases, increasing the formation bio char yield [43, 66-68]. Also, the liquefaction products of paper organics reduce the decomposition temperature of plastics promoting its depolymerization. The free radicals from decomposed paper portions are readily taken by the depolymerizing plastic portions, thus enhancing bio crude yield and contributing to the overall synergetic effect [69, 70].
3.3 Elemental composition analysis of bio crude and bio char
The elemental analysis for bio char and bio crude obtained from HTL of MHHW biomass at 360 °C and 75 min, in the presence of diatomaceous earth and nanoporous ZnO as catalyst is presented in Table 2.
It can be seen that the carbon content of bio char and bio crude obtained from HTL of MHHW biomass feedstock in the presence of both catalysts is higher than that obtained from non-cat HTL by 6–10%. The C (wt. %) was found to be highest at 61.23% and 56.69% for bio crude and bio char in the presence of nano ZnO and diatomaceous earth, respectively, which resulted in significantly higher HHV of 29.56 MJ/kg and 25.42 MJ/kg. This significant increase in carbon content can be attributed to enhanced fragmentation resulting in better passage of hydrothermal medium into the matrix of biomass feedstock.
Similarly, the hydrogen content of bio crude and bio char obtained from cat-HTL with diatomaceous earth is substantially higher than ones obtained from cat-HTL with nano ZnO and non-cat HTL, thus leading to an H/C ratio that is 1.02 to 1.08 times higher than the others. Furthermore, the N content of the bio crude and bio char obtained follows the trend cat-HTL nano ZnO > non-cat HTL > cat-HTL DE. This relatively high nitrogen content in bio crude renders it less suitable for transport applications and also has the potential to cause fouling of catalysts causing upgradation processes strenuous. Various treatment techniques, adsorption and extraction techniques have been intensively researched for removing nitrogen from petroleum in addition to catalytic treatments since they are less expensive and operate under benign circumstances [71-73].
The S content of all bio crudes obtained remained constant throughout. The O content of bio crude and bio char is on the higher side for non-cat HTL than cat-HTL by 41.22% and 26.4%, respectively. The lowest O/C ratios of 0.29 and 0.21 for bio char and bio crude, respectively, were obtained for cat-HTL with nano ZnO. The O/C ratios of the bio crude and bio char thus obtained follow the trend non-cat HTL > cat-HTL DE > cat-HTL nano ZnO, indicating efficient deoxygenation of MHHW biomass feedstock by means of catalysts during the HTL process.
Furthermore, the carbon and energy recovery percentages for bio char follow the trend non-cat HTL > cat-HTL DE > cat-HTL nano ZnO. Also, the carbon and energy recovery percentages for bio crude follow the trend cat-HTL nano ZnO > cat-HTL DE > non-cat HTL. This indicates that the usage of catalyst helps retain most of the carbon and energy from the MHHW biomass is retained in the primary product (bio crude) than the secondary (bio char). Thus, in particular, cat-HTL nano ZnO proves advantageous bio crude regime and non-cat HTL for bio char, respectively.
3.4 GC MS analysis
The chemical composition of bio crude analyzed by GC MS is presented in Fig. 3. The organic compounds obtained are classified into 4 major classes—hydrocarbons, oxygenates, esters and fatty acids, and others. From Fig. 3, it can be seen that cat-HTL DE yielded the highest fraction of hydrocarbons (HCs) of 45.57%, which is 6.77% and 39.76% higher than the yield of HCs obtained from cat-HTL nano ZnO and non-cat HTL, respectively. Similarly, the yield of oxygenates and esters and fatty acids followed the trend non-cat HTL > cat-HTL nano ZnO > cat-HTL DE. The others fraction, consisting of N compounds, furfurals, phenols, etc., constitute 18.54% in cat-HTL DE, which is comparatively higher than non-cat HTL and cat-HTL nano ZnO. Thus, the usage of diatomaceous earth and nano ZnO as catalysts aided not only in increasing the yield of desirable HCs, but also in reducing the yield of undesirable products like oxygenates and fatty acids. Furthermore, in view of results obtained in the previous section, though the bio crude obtained from cat-HTL of DE did not present with the highest carbon composition, it possessed the highest amount of hydrocarbons than cat-HTL of nano ZnO and non-cat conditions.
Furthermore, GCMS analysis of the accumulated aqueous phase revealed the presence of acids (40%), alcohols (35%), aldehydes and ketones (15%), and others (10%), attributing its use as a precursor for the production of many platform chemicals [74-77] and also as a hydrothermal agent by means of recirculation [1, 78]. Also, in many occasions, the aqueous phase was tested for its ability as a co-solvent in addition to water as a hydrothermal medium [79-81]. Thus, though the HTL process aims at the production of liquid bio crude, such auxiliary products that are formed during the process augment its overall effectiveness and thus contribute to the bio-refinery aspect of the technology.
3.5 TGA and boiling point distribution of bio crude
To ascertain the thermal stability of the bio crude, TGA test was performed, and the curve is plotted in Fig. 4. It can be seen that bio crude presented with the following thermal degradation patterns: a low temperature decomposition range between 25 and 110 °C with centered peak at 70 °C—corresponding to loss of moisture and degradation of low molecular weight organic acids and oxygenates, a broad moderate temperature decomposition range between 120 and 360 °C with centered peak at 240 °C—corresponding to decomposition of medium to high molecular weight compounds and final decomposition range 380 °C and 480 °C with peak at 440 °C—corresponding to degradation of PAHs and long chained polymers. Beyond 500 °C, no notable change in mass was deducted.
Fractional distillation was performed to identify the boiling point of bio crude obtained from the HTL process of MHHW biomass, and the results are presented in Table 3. It can be seen that the bio crude is fractionally distilled into four temperature ranges: < 190 °C as gasoline, 190–340 °C as diesel, 340–540 °C as vacuum oil, and > 540 °C as residue [82]. This suggests that HTL bio crude obtained from MHHW biomass resulted in a greater degree of gasoline and diesel fraction formation, with 60% of bio crude was detected in < 340 °C, with 28% in gasoline, and 31.5% in diesel range.
3.6 Analysis of bio char
The bio char obtained from both cat-HTL of MHHW biomass were evaluated for their composition by means of FTIR analysis, and the results are depicted in Fig. 5. For both bio char samples, the peaks centering around 3500–3550 cm−1 is mainly due to the –OH stretching of absorbed water, alcohol, and carboxylic acids. Also, both bio char spectra showed prominent bands by N–H and C–H stretching around 3400 cm−1 and 2950 cm−1, respectively. Also, the peaks around 3000 to 2700 cm−1 could occur due to C–H stretching of aliphatic hydrocarbons. Furthermore, C=C stretching at 1600 cm−1 and C=O stretching at 1750 cm−1 can also be observed in the IR spectra indicating the presence of conjugated acids and aldehydes. In addition to the above, the FTIR spectra of nano ZnO bio char and diatomaceous earth bio char showed pronounced peaks at 461 and 545 cm−1, respectively, corresponding to zinc oxide and Al-Si-O, Si-O-Si presence in them. This detail that the bio char contains the leftover catalyst supports the fact that the obtained bio chars can serve as catalyst for further processes [16, 83-85]. Recyclability and reusability of catalyst play a very crucial role not only in the economic aspect of the process but also in its bio-refinery feature.
The bio char obtained from cat-HTL of MHHW biomass in the presence of diatomaceous earth and nanoporous ZnO was subjected to SEM imaging to analyze the surface characteristics as depicted in Fig. 6a and b. From the images, it is clear that, at a 100 nm length scale, the bio char possessed irregularly shaped pores exhibiting wide pore size distribution. The average pore sizes for both bio chars were 130.94 nm and 120.89 nm for cat-HTL with ZnO and cat-HTL of DE, respectively. These pores may be a result of moisture and volatile degradation reactions [86]. Also, intense aggregate formation is also visible from the FESEM images. This may be a result of finer inorganic particles binding with each other at severe operating conditions forming aggregate complexes [87].
4 Conclusions
Catalytic hydrothermal liquefaction of mixed household waste resulted in high bio crude yields with significant hydrocarbon fractions. The maximum bio crude yield of 49.58% was obtained from cat HTL at a reaction temperature of 360 °C, for a residence time of 75 min, and catalyst loading of 12.5 wt. % nanoporous ZnO. The bio crude thus obtained possessed an HHV of 29.6 MJ/kg, with carbon and energy recovery of 58.61% and 64.42%. Solid residue obtained from HTL possesses an HHV of 25.42 MJ/kg and also contained remaining catalyst presenting economical and reuse benefits. Mixed household waste can be considered a valuable biomass feedstock for the production of high-quality bio crude and bio char by means of catalytic hydrothermal liquefaction, thus paving a path for the possible role of mixed household waste in bio-refinery application in the future.
Data availability
Not applicable.
References
Hong C, Wang Z, Si Y et al (2021) Effects of aqueous phase circulation and catalysts on hydrothermal liquefaction (HTL) of penicillin residue (PR): characteristics of the aqueous phase, solid residue and bio oil. Sci Total Environ 776:145596. https://doi.org/10.1016/j.scitotenv.2021.145596
Zhao B, Li H, Wang H et al (2021) Synergistic effects of metallic Fe and other homogeneous/heterogeneous catalysts in hydrothermal liquefaction of woody biomass. Renew Energy 176:543–554. https://doi.org/10.1016/J.RENENE.2021.05.115
Li Y, Zhu C, Jiang J et al (2021) Catalytic hydrothermal liquefaction of Gracilaria corticata macroalgae: effects of process parameter on bio-oil up-gradation. Bioresour Technol 319:124163. https://doi.org/10.1016/J.BIORTECH.2020.124163
Chand Malav L, Yadav KK, Gupta N et al (2020) A review on municipal solid waste as a renewable source for waste-to-energy project in India: current practices, challenges, and future opportunities. J Clean Prod 277:123227. https://doi.org/10.1016/J.JCLEPRO.2020.123227
Varjani S, Shahbeig H, Popat K et al (2022) Sustainable management of municipal solid waste through waste-to-energy technologies. Bioresour Technol 355:127247. https://doi.org/10.1016/J.BIORTECH.2022.127247
Hoang AT, Varbanov PS, Nižetić S et al (2022) Perspective review on municipal solid waste-to-energy route: characteristics, management strategy, and role in circular economy. J Clean Prod 359:131897. https://doi.org/10.1016/J.JCLEPRO.2022.131897
Zadeh ZE, Abdulkhani A, Aboelazayem O, Saha B (2020) Recent insights into lignocellulosic biomass pyrolysis: a critical review on pretreatment, characterization, and products upgrading. Process 799(8):799. https://doi.org/10.3390/PR8070799
Kumar R, Strezov V, Weldekidan H et al (2020) Lignocellulose biomass pyrolysis for bio-oil production: a review of biomass pre-treatment methods for production of drop-in fuels. Renew Sustain Energy Rev 123:109763. https://doi.org/10.1016/J.RSER.2020.109763
Lu Y, Tian A, Zhang J et al (2020) Physical and chemical properties, pretreatment, and recycling of municipal solid waste incineration fly ash and bottom ash for highway engineering: a literature review. Adv Civ Eng 2020. https://doi.org/10.1155/2020/8886134
Khanh Nguyen V, Kumar Chaudhary D, Hari Dahal R et al (2021) Review on pretreatment techniques to improve anaerobic digestion of sewage sludge. Fuel 285:119105. https://doi.org/10.1016/J.FUEL.2020.119105
Atelge MR, Atabani AE, Banu JR et al (2020) A critical review of pretreatment technologies to enhance anaerobic digestion and energy recovery. Fuel 270:117494. https://doi.org/10.1016/J.FUEL.2020.117494
Vaishnavi M, Gopinath KP, Ghodke PK (2022) Recent advances in hydrothermal liquefaction of microalgae. In: Micro-algae: Next - generation Feedstock for bio refinerieseedstock for bio refineries. Springer, Singapore, pp 97–127
Tyagi VK, Lo SL (2013) Sludge: a waste or renewable source for energy and resources recovery? Renew. Sustain. Energy Rev 25:708–728
de Caprariis B, Bracciale MP, Bavasso I et al (2020) Unsupported Ni metal catalyst in hydrothermal liquefaction of oak wood: effect of catalyst surface modification. Sci Total Environ 709:136215. https://doi.org/10.1016/j.scitotenv.2019.136215
Arun J, Gopinath KP, Sivaramakrishnan R et al (2021) Hydrothermal liquefaction of Prosopis juliflora biomass for the production of ferulic acid and bio-oil. Bioresour Technol 319. https://doi.org/10.1016/j.biortech.2020.124116
Arun J, Gopinath KP, SundarRajan PS et al (2020) Hydrothermal liquefaction of Scenedesmus obliquus using a novel catalyst derived from clam shells: solid residue as catalyst for hydrogen production. Bioresour Technol 310. https://doi.org/10.1016/j.biortech.2020.123443
Arun J, Shreekanth SJ, Sahana R et al (2017) Studies on influence of process parameters on hydrothermal catalytic liquefaction of microalgae (Chlorella vulgaris) biomass grown in wastewater. Bioresour Technol 244:963–968. https://doi.org/10.1016/J.BIORTECH.2017.08.048
SundarRajan PS, Gopinath KP, Arun J et al (2020) An insight into carbon balance of product streams from hydrothermal liquefaction of Scenedesmus abundans biomass. Renew Energy 151:79–87. https://doi.org/10.1016/J.RENENE.2019.11.011
Huang Y, Chen Y, Xie J et al (2016) Bio-oil production from hydrothermal liquefaction of high-protein high-ash microalgae including wild Cyanobacteria sp. and cultivated Bacillariophyta sp. Fuel 183:9–19. https://doi.org/10.1016/j.fuel.2016.06.013
Usami R, Fujii K, Fushimi C (2020) Improvement of bio-oil and nitrogen recovery from microalgae using two-stage hydrothermal liquefaction with solid carbon and HCl acid catalysis. ACS Omega 5:6684–6696. https://doi.org/10.1021/ACSOMEGA.9B04468/SUPPL_FILE/AO9B04468_SI_001.PDF
Chen J, Zhang J, Pan W et al (2022) A novel strategy to simultaneously enhance bio-oil yield and nutrient recovery in sequential hydrothermal liquefaction of high protein microalgae. Energy Convers Manag 255:115330. https://doi.org/10.1016/J.ENCONMAN.2022.115330
Gopirajan PV, Gopinath KP, Sivaranjani G, Arun J (2023) Optimization of hydrothermal liquefaction process through machine learning approach: process conditions and oil yield. Biomass Convers Biorefinery 13:1213–1222. https://doi.org/10.1007/S13399-020-01233-8/METRICS
Zhang W, Chen Q, Chen J et al (2023) Machine learning for hydrothermal treatment of biomass: a review. Bioresour Technol 370:128547. https://doi.org/10.1016/J.BIORTECH.2022.128547
Li J, Zhang W, Liu T et al (2021) Machine learning aided bio-oil production with high energy recovery and low nitrogen content from hydrothermal liquefaction of biomass with experiment verification. Chem Eng J 425:130649. https://doi.org/10.1016/J.CEJ.2021.130649
Nazari L, Yuan Z, Souzanchi S et al (2015) Hydrothermal liquefaction of woody biomass in hot-compressed water: catalyst screening and comprehensive characterization of bio-crude oils. Fuel 162:74–83. https://doi.org/10.1016/J.FUEL.2015.08.055
Mahmood N, Yuan Z, Schmidt J, Xu CC (2015) Hydrolytic depolymerization of hydrolysis lignin: effects of catalysts and solvents. Bioresour Technol 190:416–419. https://doi.org/10.1016/J.BIORTECH.2015.04.074
Ross AB, Biller P, Kubacki ML et al (2010) Hydrothermal processing of microalgae using alkali and organic acids. Fuel 89:2234–2243. https://doi.org/10.1016/J.FUEL.2010.01.025
Fan Q, Fu P, Song C, Fan Y (2023) Valorization of waste biomass through hydrothermal liquefaction: a review with focus on linking hydrothermal factors to products characteristics. Ind Crops Prod 191. https://doi.org/10.1016/J.INDCROP.2022.116017
Gollakota ARK, Kishore N, Gu S (2018) A review on hydrothermal liquefaction of biomass. Renew Sustain Energy Rev 81:1378–1392. https://doi.org/10.1016/J.RSER.2017.05.178
Martins-Vieira JC, Lachos-Perez D, Draszewski CP et al (2023) Sugar, hydrochar and bio-oil production by sequential hydrothermal processing of corn cob. J Supercrit Fluids 194. https://doi.org/10.1016/j.supflu.2023.105838
Sahoo A, Saini K, Jindal M et al (2021) Co-hydrothermal liquefaction of algal and lignocellulosic biomass: status and perspectives. Bioresour Technol 342:125948. https://doi.org/10.1016/J.BIORTECH.2021.125948
Nallasivam J, Prashanth PF, Vinu R (2022) Hydrothermal liquefaction of biomass for the generation of value-added products. Biomass, Biofuels, Biochem Circ Bioeconomy Technol Waste Remediat 65–107. https://doi.org/10.1016/B978-0-323-88511-9.00018-5
Mathanker A, Pudasainee D, Kumar A, Gupta R (2020) Hydrothermal liquefaction of lignocellulosic biomass feedstock to produce biofuels: parametric study and products characterization. Fuel 271. https://doi.org/10.1016/J.FUEL.2020.117534
Chen WT, Zhang Y, Zhang J et al (2014) Co-liquefaction of swine manure and mixed-culture algal biomass from a wastewater treatment system to produce bio-crude oil. Appl Energy 128:209–216. https://doi.org/10.1016/j.apenergy.2014.04.068
Chen WT, Zhang Y, Zhang J et al (2014) Hydrothermal liquefaction of mixed-culture algal biomass from wastewater treatment system into bio-crude oil. Bioresour Technol 152:130–139. https://doi.org/10.1016/j.biortech.2013.10.111
Chen WT, Ma J, Zhang Y et al (2014) Physical pretreatments of wastewater algae to reduce ash content and improve thermal decomposition characteristics. Bioresour Technol 169:816–820. https://doi.org/10.1016/j.biortech.2014.07.076
Rojas-Pérez A, Diaz-Diestra D, Frias-Flores CB et al (2015) Catalytic effect of ultrananocrystalline Fe3O4 on algal bio-crude production via HTL process. Nanoscale 7:17664–17671. https://doi.org/10.1039/c5nr04404a
Chen WT, Qian W, Zhang Y et al (2017) Effect of ash on hydrothermal liquefaction of high-ash content algal biomass. Algal Res 25:297–306. https://doi.org/10.1016/j.algal.2017.05.010
Audu M, Wang H, Arellano D et al (2021) Ash-pretreatment and hydrothermal liquefaction of filamentous algae grown on dairy wastewater. Algal Res 57:102282. https://doi.org/10.1016/j.algal.2021.102282
Aierzhati A, Stablein MJ, Wu NE et al (2019) Experimental and model enhancement of food waste hydrothermal liquefaction with combined effects of biochemical composition and reaction conditions. Bioresour Technol 284:139–147. https://doi.org/10.1016/j.biortech.2019.03.076
Mancio AA, da Mota SAP, Ferreira CC et al (2018) Separation and characterization of biofuels in the jet fuel and diesel fuel ranges by fractional distillation of organic liquid products. Fuel 215:212–225. https://doi.org/10.1016/J.FUEL.2017.11.029
Mahima J, Sundaresh RK, Gopinath KP et al (2021) Effect of algae (Scenedesmus obliquus) biomass pre-treatment on bio-oil production in hydrothermal liquefaction (HTL): biochar and aqueous phase utilization studies. Sci Total Environ 778:146262. https://doi.org/10.1016/J.SCITOTENV.2021.146262
Arun J, Gopinath KP, SundarRajan PS et al (2019) Co-liquefaction of Prosopis juliflora with polyolefin waste for production of high grade liquid hydrocarbons. Bioresour Technol 274:296–301. https://doi.org/10.1016/J.BIORTECH.2018.11.102
Pedersen TH, Grigoras IF, Hoffmann J et al (2016) Continuous hydrothermal co-liquefaction of aspen wood and glycerol with water phase recirculation. Appl Energy 162:1034–1041. https://doi.org/10.1016/J.APENERGY.2015.10.165
Mahesh D, Ahmad S, Kumar R et al (2021) Hydrothermal liquefaction of municipal solid wastes for high quality bio-crude production using glycerol as co-solvent. Bioresour Technol 339:125537. https://doi.org/10.1016/J.BIORTECH.2021.125537
Saengsuriwong R, Onsree T, Phromphithak S, Tippayawong N (2021) Biocrude oil production via hydrothermal liquefaction of food waste in a simplified high-throughput reactor. Bioresour Technol 341:125750. https://doi.org/10.1016/j.biortech.2021.125750
Nunes VO, Fraga AC, Silva RVS et al (2021) Chemical characterisation of sugarcane bagasse bio-oils from hydrothermal liquefaction: effect of reaction conditions on products distribution and composition. J Environ Chem Eng 9:106513. https://doi.org/10.1016/J.JECE.2021.106513
Akhtar J, Amin NAS (2011) A review on process conditions for optimum bio-oil yield in hydrothermal liquefaction of biomass. Renew Sustain Energy Rev 15:1615–1624
Cao L, Zhang C, Hao S et al Effect of glycerol as co-solvent on yields of bio-oil from rice straw through hydrothermal liquefaction. Elsevier
Singh R, Prakash A, Dhiman SK et al (2014) Hydrothermal conversion of lignin to substituted phenols and aromatic ethers. Bioresour Technol 165:319–322. https://doi.org/10.1016/J.BIORTECH.2014.02.076
Isa KM, Abdullah TAT, Ali UFM (2018) Hydrogen donor solvents in liquefaction of biomass: a review. Renew Sustain Energy Rev 81:1259–1268. https://doi.org/10.1016/J.RSER.2017.04.006
Aierzhati A, Watson J, Si B et al (2021) Development of a mobile, pilot scale hydrothermal liquefaction reactor: food waste conversion product analysis and techno-economic assessment. Energy Convers Manag X 10:100076. https://doi.org/10.1016/j.ecmx.2021.100076
Chen WH, Lin YY, Liu HC et al (2019) A comprehensive analysis of food waste derived liquefaction bio-oil properties for industrial application. Appl Energy 237:283–291. https://doi.org/10.1016/j.apenergy.2018.12.084
Bayat H, Dehghanizadeh M, Jarvis JM et al (2021) Hydrothermal liquefaction of food waste: effect of process parameters on product yields and chemistry. Front Sustain Food Syst 5:658592. https://doi.org/10.3389/fsufs.2021.658592
Motavaf B, Engineering PS-AE, 2021 undefined (2021) Effect of process variables on food waste valorization via hydrothermal liquefaction. ACS Publ 1:363–374. https://doi.org/10.1021/acsestengg.0c00115
Zheng Y, Tao L, Yang X et al The thermal behavior, kinetics, and product characterization of biomass and low-density polyethylene co-pyrolysis by thermogravimetric analysis and pyrolysis-GC/MS. Elsevier
Bhoi PR, Ouedraogo AS, Soloiu V, Quirino R (2020) Recent advances on catalysts for improving hydrocarbon compounds in bio-oil of biomass catalytic pyrolysis. Renew Sustain Energy Rev 121. https://doi.org/10.1016/J.RSER.2019.109676
Hoff TC, Thilakaratne R, Gardner DW et al (2016) Thermal stability of aluminum-rich ZSM-5 zeolites and consequences on aromatization reactions. J Phys Chem C 120:20103–20113. https://doi.org/10.1021/ACS.JPCC.6B04671
Zhou G, Li J, Yu Y et al Optimizing the distribution of aromatic products from catalytic fast pyrolysis of cellulose by ZSM-5 modification with boron and co-feeding of low-density polyethylene. Elsevier
Li X, Li G, Li J et al Producing petrochemicals from catalytic fast pyrolysis of corn fermentation residual by-products generated from citric acid production. Elsevier
Iliopoulou E, Stefanidis S, … KK-ACB, 2012 undefined Catalytic upgrading of biomass pyrolysis vapors using transition metal-modified ZSM-5 zeolite. Elsevier
Wang Z, Liu G, Shen D et al (2020) Co-pyrolysis of lignin and polyethylene with the addition of transition metals - part I: thermal behavior and kinetics analysis. J Energy Inst 93:281–291. https://doi.org/10.1016/J.JOEI.2019.03.003
Wang Z, Burra K, Lei T et al Co-pyrolysis of waste plastic and solid biomass for synergistic production of biofuels and chemicals-a review. Elsevier
Ma Q, Wang K, Sudibyo H et al (2021) Production of upgraded biocrude from hydrothermal liquefaction using clays as in situ catalysts. Energy Convers Manag 247:114764. https://doi.org/10.1016/J.ENCONMAN.2021.114764
Rajagopal J, Gopinath KP, Neha R et al (2022) Processing of household waste via hydrothermal gasification and hydrothermal liquefaction for bio-oil and bio-hydrogen production: comparison with RSM studies. J Environ Chem Eng 10:107218. https://doi.org/10.1016/J.JECE.2022.107218
Raikova S, Knowles TDJ, Allen MJ, Chuck CJ (2019) Co-liquefaction of macroalgae with common marine plastic pollutants. ACS Sustain Chem Eng 7:6769–6781. https://doi.org/10.1021/ACSSUSCHEMENG.8B06031/ASSET/IMAGES/LARGE/SC-2018-06031T_0013.JPEG
Baloch HA, Nizamuddin S, Siddiqui MTH et al (2020) Co-liquefaction of synthetic polyethylene and polyethylene bags with sugarcane bagasse under supercritical conditions: a comparative study. Renew Energy 162:2397–2407. https://doi.org/10.1016/J.RENENE.2020.10.008
Baloch HA, Siddiqui MTH, Nizamuddin S et al (2021) Catalytic co-liquefaction of sugarcane bagasse and polyethylene for bio-oil production under supercritical conditions: effect of catalysts. J Anal Appl Pyrolysis 153. https://doi.org/10.1016/j.jaap.2020.104944
Wang Y, Wang Y, Zhu Y et al (2021) Interactions of the main components in paper-plastic-aluminum complex packaging wastes during the hydrothermal liquefaction process. Chem Eng Technol 44:1519–1527. https://doi.org/10.1002/ceat.202100124
Zhang L, Champagne P, (Charles) Xu C (2011) Bio-crude production from secondary pulp/paper-mill sludge and waste newspaper via co-liquefaction in hot-compressed water. Energy 36:2142–2150. https://doi.org/10.1016/j.energy.2010.05.029
Chen WT, Tang L, Qian W et al (2016) Extract nitrogen-containing compounds in biocrude oil converted from wet biowaste via hydrothermal liquefaction. ACS Sustain Chem Eng 4:2182–2190. https://doi.org/10.1021/ACSSUSCHEMENG.5B01645/SUPPL_FILE/SC5B01645_SI_001.PDF
Leng L, Zhou J, Li T et al (2020) Nitrogen heterocycles in bio-oil produced from hydrothermal liquefaction of biomass: a review. Fuel 335:126030
Leng L, Zhou J, Li T et al (2023) Nitrogen heterocycles in bio-oil produced from hydrothermal liquefaction of biomass: a review. Fuel 335:126995
El-Hefnawy ME, Alhayyani S, Ismail A et al (2023) Integrated approach for enhanced crude bio-oil yield from microalgae cultivated on the aqueous phase of hydrothermal co-liquefaction with agar-free seaweed residues. J Clean Prod 392. https://doi.org/10.1016/j.jclepro.2023.136286
Zoppi G, Tito E, Bianco I et al (2023) Life cycle assessment of the biofuel production from lignocellulosic biomass in a hydrothermal liquefaction – aqueous phase reforming integrated biorefinery. Renew Energy. https://doi.org/10.1016/j.renene.2023.02.011
Swetha A, ShriVigneshwar S, Gopinath KP et al (2021) Review on hydrothermal liquefaction aqueous phase as a valuable resource for biofuels, bio-hydrogen and valuable bio-chemicals recovery. Chemosphere 283. https://doi.org/10.1016/j.chemosphere.2021.131248
Yuan C, Zhao S, Ni J et al (2023) Integrated route of fast hydrothermal liquefaction of microalgae and sludge by recycling the waste aqueous phase for microalgal growth. Fuel 334. https://doi.org/10.1016/j.fuel.2022.126488
SundarRajan P, Gopinath KP, Arun J et al (2021) Insights into valuing the aqueous phase derived from hydrothermal liquefaction. Renew Sustain Energy Rev 144:111019. https://doi.org/10.1016/J.RSER.2021.111019
Leng S, Leng L, Chen L et al (2020) The effect of aqueous phase recirculation on hydrothermal liquefaction/carbonization of biomass: a review. Bioresour Technol 318:124081
Zhu Z, Rosendahl L, Toor SS et al (2015) Hydrothermal liquefaction of barley straw to bio-crude oil: effects of reaction temperature and aqueous phase recirculation. Appl Energy 137:183–192. https://doi.org/10.1016/j.apenergy.2014.10.005
Chen X, Ma X, Peng X et al (2018) Effects of aqueous phase recirculation in hydrothermal carbonization of sweet potato waste. Bioresour Technol 267:167–174. https://doi.org/10.1016/j.biortech.2018.07.032
Biller P, Sharma BK, Kunwar B, Ross AB (2015) Hydroprocessing of bio-crude from continuous hydrothermal liquefaction of microalgae. Fuel 159:197–205. https://doi.org/10.1016/J.FUEL.2015.06.077
Yameen MZ, AlMohamadi H, Naqvi SR et al (2023) Advances in production & activation of marine macroalgae-derived biochar catalyst for sustainable biodiesel production. Fuel 337. https://doi.org/10.1016/J.FUEL.2022.127215
Arun J, Varshini P, Prithvinath PK et al (2018) Enrichment of bio-oil after hydrothermal liquefaction (HTL) of microalgae C. vulgaris grown in wastewater: bio-char and post HTL wastewater utilization studies. Bioresour Technol 261:182–187. https://doi.org/10.1016/j.biortech.2018.04.029
Lee J, Kim KH, Kwon EE (2017) Biochar as a catalyst. Renew Sustain Energy Rev 77:70–79. https://doi.org/10.1016/J.RSER.2017.04.002
Arun J, Gopinath KP, Vigneshwar SS, Swetha A (2020) Sustainable and eco-friendly approach for phosphorus recovery from wastewater by hydrothermally carbonized microalgae: study on spent bio-char as fertilizer. J Water Process Eng 38:101567. https://doi.org/10.1016/j.jwpe.2020.101567
Dandamudi KPR, Murdock T, Lammers PJ et al (2021) Production of functionalized carbon from synergistic hydrothermal liquefaction of microalgae and swine manure. Resour Conserv Recycl 170:105564. https://doi.org/10.1016/j.resconrec.2021.105564
Author information
Authors and Affiliations
Contributions
Mahadevan Vaishnavi: methodology, analysis and investigation, writing—original draft
K Sathish Kumar: supervision, writing—review and editing
Kannappan Panchamoorthy Gopinath: conceptualization, writing—review and editing
Corresponding author
Ethics declarations
Ethics approval
Not applicable
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Vaishnavi, M., Kumar, K.S. & Gopinath, K.P. Comparative studies on catalytic hydrothermal liquefaction of mixed household waste into bio crude. Biomass Conv. Bioref. 13, 14253–14265 (2023). https://doi.org/10.1007/s13399-023-04489-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-023-04489-y