Abstract
In this study, biomass was tried to be liquefied by catalytic hydrothermal process. In addition, the effects of waste process water on growth of beneficial-pathogenic fungi and algae were investigated. Ammi visnaga as biomass source; Cu, W, and Fe metal powders as catalysts; Trichoderma harzianum, Trichoderma virens, and Verticillium dahliae as fungus species; and Chlorella minutissima as algae were used. In trials, 250, 275, 300, and 325 °C temperatures and 0, 15, 30, and 45 min parameters were determined. GC–MS, TOC, XRD, and elemental analysis methods were used for characterization. The optimum temperatures for light bio-oil and heavy bio-oil were 300 °C and 325 °C, respectively. The highest HHV value was obtained as 30.30 Mj/kg in the presence of Fe catalyst. It was determined that waste process waters support beneficial fungus and algae growth and suppress pathogen growth.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The depletion of fossil resources and the environmental problems associated with the production and consumption of fossil fuels has increased the interest in alternative fuel production research. Biomass energy, among the renewable energy sources, can be an alternative to fossil fuels due to its features such as less pollution, sustainability, and low cost. Biomass is a material that is indirectly or directly derived from plant life and renewable in less than 100 years. Animal wastes are considered biomass material because they are obtained from biomass directly or through the food chain. Biomass consists of cellulose, hemicellulose, starch, lignin, protein polymers, and different concentrations of triglycerides, terpenes, and other compounds. In general dry biomass consists of 30–60% carbon, 30–40% oxygen, 5–6% hydrogen, < 1% nitrogen, chlorine, and inorganic compounds [1, 2]. The advantage of biomass is its renewable, low sulfur content, regrowth cycle, and near-zero CO2 emissions. Biomass is converted into liquid, solid, and gaseous products by physical, chemical, and biological conversion methods [3]. The hydrothermal liquefaction method (HTL), one of the conversion methods used to obtain products with high energy value from biomass, seems to have a more environmentally efficient conversion method than other methods [4].
The water is used as a solvent in the hydrothermal liquefaction process. Hydrothermal water is valid at temperatures above 100 °C, while supercritical water is valid at temperatures above 374 °C and pressure values above 22 MPa. The water’s properties and usage conditions change in near-critical, critical, and above-critical situations. Water is a suitable solvent for ionic compounds under normal conditions and becomes a suitable solvent for apolar molecules under critical and near-critical conditions. Under supercritical conditions, the dipole moment value of water decreases and water changes character from a polar to a non-polar state. As the temperature rises to 250 °C, much more H3O+ ions form; the pH value decreases at this temperature, and the pH decreases by about three units, which can provide a suitable reaction environment for acid-catalyzed reactions. This ionization rate decreases at critical and supercritical temperatures. The reactivity of water increases as the temperature approaches the critical [5].
The oxygen rate in commercial liquid fuels is around 1%; while obtaining bio-fuel from biomass, oxygen is removed to obtain liquid fuel of similar quality because the oxygen rate in biomass is around 40–60%. In hydrothermal liquefaction of biomass, the reaction occurs in an aqueous medium. In the liquefaction of biomass, 3 product phases were obtained: liquid phase (compounds insoluble in oil and water), aqueous phase (water-soluble part), and gas phase (containing gaseous products). This process is relatively simple, as no single product is obtained in the liquefaction process [6].
Using catalysts in the hydrothermal liquefaction process significantly impacts product yield and quality. Catalysts suppress the formation of coal and tar in the hydrothermal process, reducing the oxygen content and increasing the product yield. Many studies have been conducted using different types of catalysts for efficient and effective conversion of biomass [7, 8]. It has been determined that the alkaline and acidic type catalysts used in the research generally increase the yield and quality of the products. The most significant disadvantage of homogeneous type catalysts used in hydrothermal liquefaction is that they dissolve in the reaction medium and remain in the aqueous phase. The presence of catalysts in the aqueous phase and on the solid residue causes recovery and reuse problems and chemical accumulation in the environment [9]. Heterogeneous catalysts have more significant advantages than homogeneous catalysts due to their properties, such as recovery and separation from the aqueous phase. Metal, supported metal structures, molecular sieves, and metal oxide structures are used in new studies on heterogeneous catalysts [10,11,12].
In many studies, Fe metal powder has been used as a catalyst in the HTL. In these studies, cellulose [13], Cladophora socialis macroalgae [14], Lactuca scariola [10], and pinewood sawdust [15] were used as a biomass source. At the end of the trials, Fe metal powder catalyst positively affected on the conversion and production of liquid products with low oxygen content in all studies. Cu metal powder has been used as a catalyst in hydrothermal liquefaction studies. In these studies, formic acid was converted to methanol [16] and ethanol converted to H2, CO, and CH4 [17]. At the end of the experiments, it was found that Cu metal powder supports the decomposition reactions under hydrothermal conditions and can be used as a catalyst. W metal powder has been used as a catalyst in some hydrothermal process studies. In the studies, W metal powder or W support material catalyst was used in the production of phenolic compounds from Sinocalamus oldhamii alkaline lignin [18], in the direct conversion of cellulose [19], deoxygenation of methyl oleate, and commercial biodiesel [20]. As a result of the studies, it has been found that W is effective in decomposition reactions and has the potential to be used. In some other HTL studies, direct metal powder catalysts were used. In a studies, cellulose-Ni/Fe/Zn [13], oak wood-Ni [21], and Lactuca scariola-Zn/Fe/Zn + Fe [10] were converted by HTL. It was determined that all metals have good hydrogenation degrees. The addition of metallic Ni catalysts (commercial Ni powder and nano-structured surface modified Ni particle) increased the oil yield and prevented hyro-char through the hydrogenation action.
Fe, W, and Cu metal powders were used as catalysts in this study, as they are effective in obtaining liquid products with low oxygen content in literature studies. The most important advantage of these catalysts is that they do not dissolve in water and are not included in the process water at the end of the trials. The reason for choosing Ammi visnaga as a biomass source is that it grows spontaneously in nature is resistant to natural conditions and does not have any usage area. These advantageous features keep the cost at the lowest level. Among the thermochemical conversion technologies, HTL is preferred because the raw material does not need to be dried; it can be applied to wet biomass. It can be carried out at a lower temperature (250–374 °C) than other conversion methods, and water is used as a solvent.
Many different chemical compounds in the waste process water were left at the end of the HTL trials. These chemical compounds cannot be recovered by chemical methods. It causes environmental problems and chemical accumulation. In this study, the release of chemical compounds in the waste process water to the environment was prevented; in addition, a new usage area was investigated by examining the effects on the growth of fungi and algae. Trichoderma virens, Trichoderma harzianum, and Verticillium dahliae species were selected for fungus, and Chlorella minutissima species were selected for algae. In this study, it has been shown that biofuel can be produced as a result of the HTL process and that fungi and algae can be grown from waste process water that can be used for different purposes. GC–MS and elemental analysis methods were used for heavy and light bio-oils content and energy values. XRD analysis was used to characterize catalysts, and the waste process waters used in growing algae and fungi were analyzed by TOC analysis.
2 Materials and methods
2.1 Materials and catalysts
Ammi visnaga plant was collected in July 2020 in the city of Hatay, located in the Mediterranean region of Turkey (36° 21′ 47″ N–36° 27′ 03″ E). The collected plants were separated from their roots and leaves and dried in the shade in an airy place. Then, the plant was ground in size small enough to pass through a 0.425-mm mesh sieve in the grinder. LECO CHNS 932 Elemental analyzer was used for the ultimate analysis of Ammi visnaga. According to the elemental analysis results, raw material C, H, N, and O ratios were 43.15%, 6.49%, 0.15%, and 50.21%, respectively. Dulong’s formula [15] was used to calculate the higher heating value of feedstock as 14.24 MJ/kg. Tappi test methods, Tappi T222, and Tappi T202 were used to determine the main characteristics, lignin, and cellulose contents, while holocellulose was found by the chloride method of the Ammi visnaga [22, 23]. Tappi T264 and Tappi T211 tests were used to determine the moisture and ash contents [22]. According to the component analysis result of the feedstock, lignin 20.81%, cellulose 44.20%, hemicellulose 16.50%, moisture 6.96%, ash 4.23%, and Soxhlet extractors 7.30% by weight as a percentage of dry feedstock. Cu (< 45 µm), W (12 µm), and Fe (6–8 µm) metal powders were used as catalysts in this study. These metal powders were obtained from Sigma-Aldrich and Merck.
In the other part of the study, the effects of the waste process water obtained at the end of the trials on Fungi and microalgae cultivation were examined. Trichoderma virens and Trichoderma harzianum were beneficial fungi, and Verticilium dahliae was used as a pathogen. This fungus was obtained from Mycology Laboratory in Van Yuzuncu Yil University, Faculty of Agriculture, Department of Plant Protection. Chlorella minutissima microalgae were used to examine the effect of waste process waters on algae growth. Chlorella minutissima microalgae were chosen to be used in the experiments. This culture was obtained from the Algae Culture Collection at the University of Göttingen.
2.2 Experimental procedures
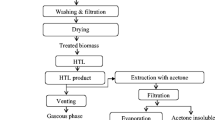
The methods applied in the experiments and the experimental steps followed are shown in Fig. 1. HTL experiments are carried out at high pressure and temperature. For this purpose, special metals and alloys are used in the HTL reactor. The reactor chamber and fittings are 316 stainless steel and 4140 alloy metals. The reactor is resistant to a temperature of approximately 400 °C and a pressure of 26 MPa. The reactor was used with a separate furnace system and an air-cooling system included in this furnace. Temperature control was done with a thermocouple and kept constant with a processor. In studies where metal powders are used as direct catalysts, the amount of catalyst is higher than in catalyst studies with support materials, considering the surface area and bulk formation. In studies in which metal powders are used as catalysts in the literature, the amount of catalyst varies between 5 and 50%. The amount of catalyst was determined by considering these literature studies [14,15,16]. In each experiment, 5 g of Ammi visnaga powder was first added to the reactor, for trials with catalysts, Cu 2.5 g, W 2.5 g, Fe 2.5 g, Cu + W 5 g, Cu + Fe 5 g, W + Fe 5 g, and after 65 mL distilled water added and mixing was done. The feedstock powder/catalyst ratio was defined as 2:1 in the trials where a single catalyst was used. The feedstock powder/catalyst ratio was determined as 1:1 in the trials using double catalysts to examine the synergistic effect. Before starting the trials, the reactor was tightly sealed and placed in the furnace, after which the air was purged with nitrogen for 10 min to maintain an inert reaction medium inside the reactor chamber.
Trials were performed at four different temperatures (225, 250, 275, and 300 °C) without and with the catalysts (Cu, W, Fe, Cu + W, Cu + Fe, W + Fe). The experimental stages followed in the studies were explained in detail in the previous research, with the working steps and chemical processes [3, 10]. Bolt connections of the reactor were made before each heating step and its tightness was checked with a manometer. The reactor was then placed in the oven and allowed to reach the desired reaction temperature at a heating rate of 10 °C/min. After completing the reaction (0, 15, 30, and 45 min), in the reactor’s cooling, an air-cooling system integrated into the furnace was used and cooling to room temperature was provided. After the reactor cooled down, the gas in the reactor was removed from the environment with the outlet valve, the reactor cover was opened, and the slurry consisting of liquid and solid was spilled into a 300-mL glass container. The solid residue was separated from the liquid using a vacuum gooch crucible. The liquid fraction was extracted with diethyl ether, and the ether solution was dried with anhydrous sodium sulfate and then filtered to remove residual water and the solvent was evaporated with a rotary evaporator; this liquid was named light bio-oil. The solid residue was washed with acetone, and this acetone was evaporated; the resulting liquid product was named heavy oil. The solid residue remaining after washing with acetone was called hydro-char. The solid obtained at the end of the trials was dried in an oven at 105 °C for 2 h, brought to room temperature and weighed to determine the amount.
In the study, microorganisms’ development was investigated using waste process water with potato dextrose agar (PDA) nutrient medium. The waste process waters used in the study, in which the fungal growth was investigated, belong to the experiments with Cu, W, and Fe catalysts and without catalyst, which were carried out at 300 °C and 30 min. In preparing the nutrient medium, 200 mL of waste process water was made up to 400 mL of pure water. Sixteen grams of PDA was poured into each 400 mL wastewater mixture. The prepared mixture was autoclaved at 121 °C for 1 h. Then, the mixtures were poured into 5-cm petri dishes. Fungi previously developed in each petri dish were taken with a fungi drill, transferred to the medium, and allowed to grow. Microorganisms planted in the petri center were measured with a ruler every 2 days. The study was established by creating 9 different applications according to the randomized plots experimental design, and each application was established in 4 replications and 1 microorganism in each replication. Fungi studies were carried out in the Mycology Laboratory in vitro environment in Van Yuzuncu Yil University, Faculty of Agriculture, Department of Plant Protection.
Chlorella minutissima microalgae species were grown in BG-11 standard medium at room temperature under continuous white illumination for 15 days to prepare stock microalgae culture in Yıldız Technical University Algal Biotechnology and Bioprocess Laboratory. Process waters and distilled water were mixed at different rates (2%, 4%, 8%, and 16% process water), and the pH values were adjusted as 7.2. For the cultivation experiments of each microalgae species, 15 mL of microalgae stock culture and 135 mL of process water–distilled water or standard medium (2% BG-11) were used in 250 mL (working volume 150 mL) flasks. Cultivation experiments were carried out in a shaking incubator (Nüve ST 30, Turkey) at 25 ± 3 °C for 15 days under continuous white fluorescent light (8000 lx). For simultaneous observation of microalgae growth, optical density at 680 nm was measured at specific intervals using a UV–Vis spectrophotometer (PG Instruments T60, UK). Doubling time (TD) and specific growth rate (μ) of microalgae cultures were determined with the help of Eqs. (1) and (2), respectively:
OD0 is the optical density for the first day, ODt is the optical density measured at day t, and μ is the specific growth rate of the algae culture.
2.3 Calculation of liquid, solid, gas products, and HHV values
Liquid and gaseous products were considered in the transformation of Ammi visnaga. The same method was used for the amounts of the products obtained at the end of the HTL process. The amount of gaseous substance was found by subtracting the initial amount (5 g) of liquid and solid residue.
where WBiomass, db and WSolid, db are the weights of the initial biomass and the remaining solid respectively on a dry basis.
3 Results and discussion
3.1 Effect of temperature on product yields
In high temperature liquefaction, macromolecules in the structure of biomass are hydrolyzed and fragmented into smaller molecules; this process occurs at high pressure. Some intermediates are unstable and reactive and recombine to form larger molecules. These molecules are generally insoluble or turn into gas in the reaction medium. A significant portion of the oxygen in the biomass is removed by dehydration and decarboxylation [6]. The components of biomass decompose at different temperatures, and the unstable products formed to come together again to form different compounds. The effect of temperature on the products obtained at the end of the hydrothermal liquefaction trials of Ammi visnaga is shown in Fig. 2. At the end of the trials, the highest efficiency for light bio-oil was obtained at 300 °C, for heavy bio-oil at 325 °C.
In the studies carried out, it was determined that after reaching the maximum liquid product yield, the increasing temperature supports the secondary decomposition and gasification reactions. These reactions suppress the formation of liquid products and support the formation of gas and coal. The products formed in hydrothermal liquefaction should be evaluated by considering these reaction types [24]. Similar results were obtained in this study, and it is thought that with increasing temperature, liquid and solid residues turn into gaseous products.
3.2 Effect of catalyst on product yields
The addition of minerals, salts, and catalysts to the reaction medium in hydrothermal liquefaction leads to major changes in the reactivity of hydrocarbons [25, 26]. During hydrolysis, which is one of the reactions in hydrothermal liquefaction, water acts as a solvent, reactant, and catalyst with self-decomposition. Acids, bases, transition metal salts, and heterogeneous catalysts are used as catalysts in the hydrothermal liquefaction process [27, 28].
The main purpose of the hydrothermal liquefaction process is to increase the energy value of the newly obtained products by removing the oxygen in the raw material. The oxygen ratio of the products obtained by hydrothermal liquefaction decreases, the ratio of H and C increases, and thus, a higher energy liquid product is obtained. Catalyst reduces the oxygen in the raw material and increases the ratio of H and C in the structure. It has been shown that the catalysts used in the studies accelerated the water–gas shift reaction, reducing the amount of solid residue and increasing the yield of liquid products.
Esters are formed by decarboxylation reactions between the hydroxyl groups from the biomass’s biopolymers and the ions formed in the alkali carbonates with the catalyst effect. Ester formation is followed by dehydration, deoxygenation, decarboxylation reactions, and dehydrogenation of small molecular compounds [29]. Alkaline salts increase the pH value in the reaction medium, reduce dehydration reactions, and cause the formation of unstable molecular units. The production of char at a lower rate with NaOH is due to the neutralization of the molecules that cause polymerization by the OH− ion. The char is formed by the polymerization reaction between the hydroxyl groups on the solid surface and the ester bonds formed in the aqueous medium, and NaOH prevents the polymerization reactions by neutralizing the carboxylic acids, thus suppressing the formation of char [30].
Fe was used as a catalyst in a study in which oil palm empty fruit bunch was converted with HTL. In the study, it was suggested that the Fe catalyst had a twofold effect. One of these effects is that the Fe catalyst supports the formation of precursors bio-oil the formation. The other effect is that it leads to the formation of H2 due to the reaction between Fe and H2O and prevents the formation of char [31]. According to the experimental results, the catalysts showed different effects with different reaction mechanisms in forming liquid products. In light bio-oil formation, an increase from 250 to 300 °C was observed in all trials, followed by a decrease in product yield at 325 °C. The highest light bio-oil yields were determined as 8.32%, 8.04%, 7.80%, and 7.31% for Fe, without catalyst, and Cu and W catalyst experiments at 325 °C. In heavy bio-oil formation, product yield increased with decreasing slope for all trials as the temperature increased. It can say that the most effective catalysts in the formation of heavy bio-oil are W, Cu, and Fe, respectively. The highest liquid product was obtained in the experiments at 325 °C without catalyst, 32.64% and 31.48% in W catalyst, 29.69% in Cu catalyst, and 27.38% in Fe catalyst. Solid residue yield decreased with temperature, and decomposition rate increasing with increasing temperature is expected. The lowest solid residue values were determined as 6.10%, 8.59%, 9.52%, and 10.80% for the without catalyst, W, Cu, and Fe catalyst trials at 325 °C, respectively. There was no significant increase in yield values with an increase in temperature from 300 to 325 °C, when all trials total liquid product yields were examined. The highest total bio-oil yields were determined as 40.30%, 38.12%, 37.01%, and 35.36% for the without catalyst, W, Cu, and Fe trials at 325 °C, respectively. According to the results of the trial with catalyst, W is the most effective catalyst for total bio-oil yield, Fe is the most effective catalyst for light bio-oil, and W is for heavy bio-oil.
Catalysts greatly influence biomass conversion and the quality and yield of the products obtained. According to literature studies, the conversion of lignocellulosic biomass into liquid products includes the following steps. In the first stage, the biomass is hydrolyzed to form water-soluble sugars and phenols. In the second stage, these compounds’ decomposition and bio-oil formation depend on the catalysts removal oxygen ability [32]. In the experiments, the catalyst was used as the only variable; temperature and reaction time were used at constant values to examine the effect of the catalyst on the conversion. The catalyst experiments results are graphed in Fig. 3. The experiment investigated the synergistic effects by using the catalysts used in binary combinations (Cu + W, Cu + Fe, W + Fe) at 325 °C for 30 min to determine how the catalysts affect each other in the production of liquid products. According to the trial results, the double catalyst trials showed a synergistic effect on the formation of some products and positively supported the product formation compared to the use alone.
For the Cu + W catalyst system, the light bio-oil yield was 8.96% and the heavy bio-oil yield was 29.67%. The light bio-oil value is higher than the trials in which both catalysts are used alone. On the liquid product yield, Cu and W catalysts showed a synergistic effect on each other and increased the yield value. The light bio-oil yield for the Cu + Fe catalyst system was 10.64%, and the heavy bio-oil yield was 24.89%. The light bio-oil value is higher than the yield values obtained when both catalysts are used alone. On the light bio-oil yield, Cu and Fe catalysts showed a synergistic effect on each other and increased the yield value, but this positive effect was not observed on the heavy bio-oil yield. For the Fe + W catalyst system, the light bio-oil yield was 10.03% and the heavy bio-oil yield was 28.72%. The light bio-oil value is higher than the yield values obtained when both catalysts are used alone. While Fe and W catalysts showed a synergistic effect on each other on the light bio-oil yield, this positive effect was not observed on the heavy bio-oil. Catalysts support certain reaction types and the formation of certain chemical compounds, and catalyst yields may differ according to the catalyst-free trials.
3.3 Examination and characterization of the liquid and solid residue
3.3.1 GC–MS
The main intermediate monomeric products of biomass depolymerization are glucose, xylose, amino acids, fatty acid, 5-hydroxymethyl furfural (5-HMF), furfural, and furfural phenolic compounds. Cellulose, with its solid crystal structure, swells in water and is resistant to the attacks of enzymes. Cellulose is decomposed into oligomer sugars and then monomer sugars and secondary decomposition products, pyruvaldehyde, HMF, lcarboxylic acids, etc., by the hydrothermal process in high temperature and critical water. Hemicellulose is a heteropolymer with side chains and consists of pentoses, alternating mannose and glucose units, or galactose units. Hemicellulose is amorphous, not as crystalline or as cellulose, and therefore susceptible to hydrothermal reactions and hydrolysis. Lignin contains oxygen functional groups such as hydroxyl, carboxyl, carbonyl, and hydroxyphenyl propane units such as trans-p-coumaryl alcohol, coniferol alcohol, and sinapyl alcohol. Due to its complex composition and structure, thermal and hydrothermal decomposition of lignin occurs over a wide temperature range [9]. The GC–MS analyses were performed on Agilent GC–MS 7890A/5975C series. The column (HP–INNOWAX, length: 60 m, I.D.: 0.250 mm, film: 0.25 m and temperature limits: from 40 to 260 °C) and widget temperatures were equivalent to those for rate. Chemical constituents were known by comparing their retention indices with literature values and their mass spectral knowledge with those from the Wiley7n.1, ADAMS.1, and NIST05a.L mass spectral databases. GC–MS analysis of the liquid products at 325 °C-30-min trials were performed. At the end of the experiments, the GC–MS analysis method determined many different compound groups in liquid products. These compounds are seen in Table 1, and in Fig. 4, these analysis results were examined as compound groups.
In the study, according to the results of the analysis, a total of 45 different chemical compounds were obtained. It can be said that a high rate of monoaromatic compounds is obtained in the experiments with catalyst when the compound groups detected in the light bio-oil are examined. These values were determined as 57.28%, 55.89%, 48.54%, and 18.84% for Cu, W, Fe, and catalyst-free trials, respectively. The polyaromatic compound ratios were examined; 22.23% with Fe catalyst, 22.08% with Cu catalyst, and 14.85% with W catalyst were obtained. These values are higher than the 2.18% value obtained in the trials without catalyst. It is seen that the catalysts cause the formation of oxygenated compounds at a lower rate than to the experiments without catalyst when the oxygenated compound ratios are examined. In without catalyst trials, 51.03% ratio was obtained; 23.13%, 18.14%, and 1% ratios were obtained with Fe, W, and Cu catalysts, respectively. Cu is the most effective catalyst with a value of 10.22% in forming aliphatic compounds. Values 5.41%, 5.36%, and 2.88% were obtained in Fe, W, and uncatalyzed trials, respectively.
According to the heavy bio-oil GC–MS results, different catalysts supported the formation of different compound groups. Catalysts are effective in the formation of monoaromatic compounds. The highest mono aromatic compounds were determined as Cu (38.87%), W(26.86%), Fe(14.63%), and (4.16%) for the uncatalyzed trial. Catalysts are effective in the formation of polyaromatic compounds. It was found as 23.23% with W catalyst, 21.06% with Fe catalyst, 13.97% with Cu catalyst, and 3.17% in the uncatalyzed trial. A similar situation was observed in the formation of aliphatic compounds. Catalysts are effective in the formation of aliphatic compounds. It was found as 29.69% with Cu catalyst, 28.46% with W catalyst, 11.02% with Fe catalyst, and 3.51% in the uncatalyzed trials. In without catalyst trials, 28.92% ratio was obtained; 19.17%, 10.66% and 10.21% ratios were obtained with Fe, Cu, and W catalysts, respectively.
It was determined that the catalysts suppressed the formation of oxygenated compounds and increased the formation of polyaromatic, monoaromatic, and aliphatic compounds. It was detected that the most effective catalysts in forming polyaromatic compounds were Cu, W and Fe, and Cu and Fe in the formation of monoaromatic compounds when the light bio-oil content was examined.
In the formation of aliphatic compounds, the effective catalyst is Cu. It was found that Cu in the formation of monoaromatic compounds and W in the formation of polyaromatic compounds, Cu and W catalysts in the formation of aliphatic compounds were found to be effective when the heavy bio-oil content was examined.
3.3.2 Elemental
The elemental analysis method investigated the C, H, N, and O ratios of the liquid products obtained at the end of the HTL trials. The energy values were calculated with the Dulong formula using these analysis results. Elemental contents and energy values of the products obtained at the end of 325 °C-30-min trials were calculated. The elemental content and energy values of the liquid products obtained are shown in Table 2.
According to the elemental analysis results, the highest C values are between 68.53–70.47 in heavy bio-oil and 60.03–63.56 in light bio-oil. The highest energy value was determined as 30.30 Mj/kg in Fe catalyst, 28.99 Mj/kg in W catalyst, 28.92 Mj/kg in Cu catalyst, and 28.68 Mj/kg in the without catalyst trials when the energy values were examined.
According to the elemental analysis results, Fe is the most effective catalyst in the formation of the liquid product with the highest energy value.
3.3.3 XRD
The crystal structure of the films was determined by Rigaku-SmartLab (X-ray diffraction, XRD) using Cu-Kα radiation with a wavelength of 1.5408 Å. The diffraction patterns were collected at 25 °C and over an angular range of 10 to 70° with a step size of 0.02° per step and a dwell time of 3 min per increment. XRD analysis was used to examine the structures of the catalysts used in the trials at the end of the trial. Figure 5 shows the XRD graphs of the catalysts used in the experiments. No changes in the surfaces of Cu and W catalysts were observed. These catalysts can be reused without any treatment after the trial. Surface changes were observed in Fe catalyst. Fe3O4 compound was detected on the Fe catalyst surface. In order for the Fe catalyst to be reused, it must be renewed with acidic, basic, or alcohol solutions.
3.3.4 Total carbon and inorganic carbon analysis
Total carbon (TOC) and inorganic carbon (IC) analysis was per-formed using a Shimadzu TOC-L analyzer equipped. Measurement range values vary between 4 µg/L and 30.000 mg/L. All samples were analyzed with 6 replicates, and the results were averaged. Injections of 21 mL were analyzed in the TOC using catalytically aided combustion oxidation at 720 °C. Inorganic carbon was measured separately by reacting the sample with phosphoric acid and measuring the resultant CO2. TOC and IC analysis of the waste process waters obtained at the end of the trials at 325 °C can be listed as follows: for without catalyst TOC: 40.2 mg/mL, IC: 2.18 mg/mL; Cu catalyst TOC: 72.12 mg/mL, IC: 5.20 mg/mL; Fe catalyst TOC: 63 mg/mL, IC: 5.78 mg/mL; and W catalyst TOC: 81.3 mg/mL, IC: 2.48 mg/mL. All catalysts have a higher TOC value than the uncatalyzed trial.
3.4 Growing fungi from wastewater
Fungi are significant in agricultural practices, plant cultivation, and soil structure. Chemical pesticides are used in traditional agriculture and crop production to control plant diseases. The widespread use of pesticides leads to dangerous situations for the environment and human health. Microbial biocontrol agents/biopesticides are an alternative to chemical pesticides to prevent these environmental damages. Plant growth stimulants and natural immune supplements include biopesticides and biofertilizers. Biological management methods for plant pathogenic fungi include several species of opportunistic, mycoparasitic fungi from the genera Clonostachys (Gliocladium) and Trichoderma. Fungi belonging to the genus Trichoderma (especially T. harzianum) have been known as biological control agents against plant diseases since the 1930s, and it has been widely used in commercial agriculture for this purpose in both developed and developing countries since the 1990s. Trichoderma-based solutions are sold all over the world for crop protection against a variety of plant diseases as well as to improve plant production and growth. Biological control of plant diseases by microbial antagonists is projected to grow in popularity and become normal practice in agricultural and horticultural products in the future. Various control methods are applied to reduce plant diseases, and the most common of these is chemical control. However, with the widespread use of chemicals, both plant resistance and environmental problems have emerged. Researchers are working on environmentally friendly and sustainable methods since other control methods are insufficient. Biological control is one of the most important ones of these methods. A biological control agent is an organism that inhibits the spread of a pest or pathogen. Trichoderma is a fungus genus found in a wide range of soils, roots, and foliar environments. Many studies have demonstrated the ability of Trichoderma spp. to act as biocontrol agents against a variety of plant diseases. Trichoderma species suppress pathogens by using various mechanisms: antibiosis, parasitism, inducing host-plant resistance, and competition. In addition to the antagonistic effect of these fungi, they also promote plant growth. Especially, T. harzianum species is the most effective and studied bio-agent [33]. Verticillium dahliae Kleb. is a fungus that causes plant disease on a wide range of host plants. Wilt infections, leaf spots, vascular discoloration, yield losses, reduced growth, poor fruit quality, and plant death are all caused by the pathogenic fungus V. dahliae. Verticillium dahliae can live in the soil for years as microsclerotia. The pathogen is soil-borne so the management of the disease difficult. Pesticides, crop rotation, resistant cultivars, and certified seeds are not enough to keep disease at bay [34]. Researchers are working on ecologically friendly and sustainable approaches because other control methods are insufficient.
In the study, the waste process water obtained at the end of the catalyzed trials (325 °C) was used to cultivate of fungi. The fungal growth rates are shown in Fig. 6 together with the control groups; wastewater was not used in the control groups. The most surprising result was in Verticillium dahliae when the fungal growths were examined. Verticillium dahliae is a pathogen, it damages plants, and it is complicated to control this pathogen. The improvement in the control group is perfect, but the nutrient medium using wastewater stopped the development of this pathogen. This is an excellent behavior; this system could be a new approach to struggling pathogens.
The development of Trichoderma harzianum, one of the beneficial pathogens, is even more interesting. The medium that inhibits the growth of pathogen is a suitable environment for beneficial pathogen growth. The medium containing wastewater positively affected the development of Trichoderma harzianum and provided a high degree of reproduction.
It was determined that the substances added to the medium prevented the growth of the pathogen, but the bio-agent (T. harzianum, T. virens) increased the growth of both hyphae and spores (Fig. 6). The other bio-agent showed limited spore and hyphae growth. There was no development of the pathogen, whereas complete development of a bio-agent is most desirable in biological control. As a result, all of the waste process waters formed at the end of the trials with and without catalysts were effective in the growth of beneficial fungi and prevented the growth of pathogens. Organic compounds in water affect the growth of fungi. TOC values can give an idea for the ratio of organic compounds in water. Organic compounds in water are proportional to TOC values. According to the TOC values, it can be said that there are high levels of organic compounds in the water.
3.5 Growing microalgea from wastewater
Algae can be used in food and biofuel applications. Chlorella minutissima is an eukaryotic green unicellular microalga with a cell diameter of about 5 to 10 µm [35]. Although there are many different biomolecules such as protein, lipid, carbohydrates, minerals, pigments, and vitamins in the biochemical content of this microalgae, the significant components are protein, fat, and carbohydrates. This biochemical content varies according to microalgae cultivation method, environmental conditions, amount, and type of nutrient used. Considering the biochemical content of Chlorella minutissima, it is understood that this microalga can be used as a biofuel, supplementary food, animal feed, or fertilizer. Especially since biodiesel is produced from the lipid content of this microalga [36] and biogas is produced from the carbohydrate and protein content [37], these microalgae are very suitable for the production of various biofuels with the biorefinery approach. There are many studies on obtaining various metabolites in the literature, producing biofuels and biofertilizers from C. minutissima with the biorefinery approach [38,39,40].
According to GC–MS results, many different chemicals, consisting of monoaromatic, polyaromatic, aliphatic, and oxygenated compounds, were detected in light bio-oil. Light bio-oil is obtained by extraction from process water. Many compounds cannot be extracted from water. Compound groups in the waste process water are also seen in the total organic carbon (TOC) analysis results. TOC analysis results indicate the biomass-based compounds in the waste process water.
The growth curves of Chlorella minutissima microalgae grown in different concentrations of aqueous phases and BG-11 nutrient solution (control) obtained from different hydrothermal liquefaction processes are shown in Figs. 7–8. The specific growth rate and doubling time values derived from these curves are also shown in Table 3. The main conclusion that can be drawn from this figure is that microalgae are mostly in the lag phase for 1–2 days. It is in the log phase between the 3rd and 11th days. In the experiments, it is seen that the microalgae grown in the control group grew in the highest amount after 15 days. In hydrothermal process waters, microalgae growth is observed to reach up to a maximum absorbance of 0.4 (in A-2 coded process water). It was observed that the lowest growth in process waters numbered A-1, A-2, and A-7 was realized in the levees with 16% process water. From this result, it can be interpreted that more than 8% of the process water types used negatively affect microalgae growth. For A-3, A-4, A-5, and A-6 samples, it was observed that microalgae growth increased when the process water ratio increased. It is examined that microalgae, which reach the highest specific growth rate, grow in A-6 wastewater as examined in Table 1. However, the very low total growth in this wastewater showed that the microalgae log phase was very short and that microalgae growth was inhibited in this short period. It was determined that the most suitable process waters for Chlorella minutissima microalgae were A-1, A-2, A-3, and A-4. At the end of the experiments, it was determined that the waste process water obtained in the trials with and without catalysts can be used to cultivate Chlorella minutissima, and the waste process water obtained in the trials where dual catalysts were used, provided a lower growth for microalgae.
4 Conclusion
It has been determined that metal catalysts are effective in biomass conversion. In addition, it has been detected that catalysts have a synergistic effect and increase the liquid product yield when used in binary groups. According to the GC–MS results of liquid products, some of the oxygen in the biomass structure was removed. Fe and Cu catalysts support the formation of monoaromatic compounds, W and Cu in the formation of polyaromatic compounds, and W and Cu catalysts support the formation of low oxygen compounds. Fe was the most effective catalyst in light bio-oil production, and W was the most effective catalyst in heavy bio-oil production. The highest HHV value was obtained with of Fe catalyst. According to the results obtained, the chemical components in the wastewater supported the growth of beneficial fungi (Trichoderma harzianum, Trichoderma virens) and limited the growth of pathogens (Verticillium dahliae) by suppressing it. The waste process waters can be used to grow Chlorella minutissima microalgae.
Data availability
Not applicable.
References
Peterson AA, Vogel F, Lachance RP et al (2008) Thermochemical biofuel production in hydrothermal media: a review of sub-and supercritical water technologies. Energy Environ Sci 1:32–65
Briens C, Piskorz J, Berruti F (2008) Biomass valorization for fuel and chemicals production--a review. International Journal of Chemical Reactor Engineering 6:
Durak H (2019) Characterization of products obtained from hydrothermal liquefaction of biomass (Anchusa azurea) compared to other thermochemical conversion methods. Biomass Convers and Biorefin 9:459–470. https://doi.org/10.1007/s13399-019-00379-4
Chen J (2018) Bio-oil production from hydrothermal liquefaction of Pteris vittata L.: effects of operating temperatures and energy recovery. Bioresource Technology 265:320–327. https://doi.org/10.1016/j.biortech.2018.06.019
Brunner G (2009) Near critical and supercritical water. Part I. Hydrolytic and hydrothermal processes. J Supercrit Fluids 47:373–381
Toor SS, Rosendahl L, Rudolf A (2011) Hydrothermal liquefaction of biomass: a review of subcritical water technologies. Energy 36:2328–2342. https://doi.org/10.1016/J.ENERGY.2011.03.013
Kumar M, Olajire Oyedun A, Kumar A (2018) A review on the current status of various hydrothermal technologies on biomass feedstock. Renew Sust Energ Rev 81:1742–1770. https://doi.org/10.1016/j.rser.2017.05.270
Cheng F, Tompsett GA, Murphy CE, Magg AR, Carabillo N, Bailey M, Hemingway JJ, Romo CI et al (2020) Synergistic effects of ınexpensive mixed metal oxides for catalytic hydrothermal liquefaction of food wastes. 9ACS Sustain Chemi Eng. 8:6877–6886. https://doi.org/10.1021/acssuschemeng.0c02059
Brunner G (2014) Hydrothermal and supercritical water processes. Elsevier
Durak H, Genel S (2020) Catalytic hydrothermal liquefaction of Lactuca scariola with a heterogeneous catalyst: the investigation of temperature, reaction time and synergistic effect of catalysts. Biores Technol 309:123375. https://doi.org/10.1016/J.BIORTECH.2020.123375
Gollakota ARK, Kishore N, Gu S (2018) A review on hydrothermal liquefaction of biomass. Renew Sustain Energy Rev 81:1378–1392. https://doi.org/10.1016/J.RSER.2017.05.178
Li N, Wei L, bibi R et al (2016) Catalytic hydrogenation of alkali lignin into bio-oil using flower-like hierarchical MoS2-based composite catalysts. Fuel 185:532–540. https://doi.org/10.1016/J.FUEL.2016.08.001
de Caprariis B, Scarsella M, Bavasso I et al (2021) Effect of Ni, Zn and Fe on hydrothermal liquefaction of cellulose: impact on bio-crude yield and composition. J Anal Appl Pyrol 157:105225. https://doi.org/10.1016/J.JAAP.2021.105225
Nguyen ST, Le TM, Nguyen H van (2021) Iron-catalyzed fast hydrothermal liquefaction of Cladophora socialis macroalgae into high quality fuel precursor. Bioresource Technology 337: https://doi.org/10.1016/J.BIORTECH.2021.125445
Zhao B, Li H, Wang H et al (2021) Synergistic effects of metallic Fe and other homogeneous/heterogeneous catalysts in hydrothermal liquefaction of woody biomass. Renewable Energy 176:543–554. https://doi.org/10.1016/J.RENENE.2021.05.115
Liu J, Zeng X, Cheng M et al (2012) Reduction of formic acid to methanol under hydrothermal conditions in the presence of Cu and Zn. Biores Technol 114:658–662. https://doi.org/10.1016/J.BIORTECH.2012.03.032
Sall ED, Morgenstern DA, Fornango JP et al (2013) Reforming of ethanol with exhaust heat at automotive scale. Energy Fuels 27:5579–5588. https://doi.org/10.1021/ef4011274
Lin X, Chen L, Li H et al (2021) Mild depolymerization of the sinocalamus oldhami alkali lignin to phenolic monomer with base and activated carbon supported nickel-tungsten carbide catalyst composite system. Biores Technol 333:125136. https://doi.org/10.1016/J.BIORTECH.2021.125136
Baek IG, You SJ, Park ED (2012) Direct conversion of cellulose into polyols over Ni/W/SiO2-Al2O3. Biores Technol 114:684–690. https://doi.org/10.1016/j.biortech.2012.03.059
Dhillon GS, Vasudevan PT (2021) Deoxygenation of methyl oleate and commercial biodiesel over W and Ni-W catalysts. Waste Biomass Valorization 12:2357–2364. https://doi.org/10.1007/s12649-020-01146-7
de Caprariis B, Bracciale MP, Bavasso I et al (2020) Unsupported Ni metal catalyst in hydrothermal liquefaction of oak wood: effect of catalyst surface modification. Sci Total Environ 709:136215. https://doi.org/10.1016/j.scitotenv.2019.136215
Technical Association of the Pulp and Paper Industry (1998) TAPPI test methods, 1998–1999. TAPPI Press, Atlanta, Ga
Wise Jahn Edwin C. Brauns F. E. American Chemical Society. LE (1952) Wood chemistry. Volume 1 / edited by Louis E. Wise and Edwin C. Jahn; contributors to the monograph, F.E. Brauns [et al.]. Reinhold, New York
Akhtar J, Amin NAS (2011) A review on process conditions for optimum bio-oil yield in hydrothermal liquefaction of biomass. Renew Sustain Energy Rev 15:1615–1624
Katritzky AR, Nichols DA, Siskin M et al (2001) Reactions in high-temperature aqueous media. Chem Rev 101:837–892
McCollom TM, Seewald JS, Simoneit BRT (2001) Reactivity of monocyclic aromatic compounds under hydrothermal conditions. Geochim Cosmochim Acta 65:455–468. https://doi.org/10.1016/S0016-7037(00)00533-0
Baiker A (1999) Supercritical fluids in heterogeneous catalysis. Chem Rev 99:453–473
Savage PE (2009) A perspective on catalysis in sub- and supercritical water. J Supercrit Fluids 47:407–414. https://doi.org/10.1016/J.SUPFLU.2008.09.007
Arturi KR, Kucheryavskiy S, Søgaard EG (2016) Performance of hydrothermal liquefaction (HTL) of biomass by multivariate data analysis. Fuel Process Technol 150:94–103
Sugano M, Takagi H, Hirano K, Mashimo K (2008) Hydrothermal liquefaction of plantation biomass with two kinds of wastewater from paper industry. J Mater Sci 43:2476–2486
Miyata Y, Sagata K, Hirose M et al (2017) Fe-assisted hydrothermal liquefaction of lignocellulosic biomass for producing high-grade bio-oil. ACS SUSTAIN CHEM ENG 5:3562–3569. https://doi.org/10.1021/acssuschemeng.7b00381
Eager RL, Mathews JF, Pepper JM, Zohdi H (1981) Studies on the products resulting from the conversion of aspen poplar to an oil. Can J Chem 59:2191–2198. https://doi.org/10.1139/v81-316
Demirer-Durak E, Demirci E (2018) Anastomosis groups and pathogenicity of Rhizoctonia species from strawberry plants in Erzurum Province, Turkey. Fresenius Environ Bull 27:4206–4211
Pegg GF, Brady BL (2002) Verticillium wilts. CABI
Ferreira SP, Holz JCP, Costa JA v (2018) Influence on cultivation conditions in the heterotrophic lipid production of the microalga Chlorella minutissima. International Food Research Journal 25:
Chandra R, Amit GUK (2019) Effects of various abiotic factors on biomass growth and lipid yield of Chlorella minutissima for sustainable biodiesel production. Environ Sci Pollut Res 26:3848–3861. https://doi.org/10.1007/s11356-018-3696-1
Koçer AT, Özçimen D (2018) Investigation of the biogas production potential from algal wastes. Waste Manage Res 36:1100–1105. https://doi.org/10.1177/0734242X18798447
Khan SA, Sharma GK, Malla FA et al (2019) Microalgae based biofertilizers: a biorefinery approach to phycoremediate wastewater and harvest biodiesel and manure. J Clean Prod 211:1412–1419. https://doi.org/10.1016/J.JCLEPRO.2018.11.281
Khan SA, Malla FA, Rashmi, et al (2018) Potential of wastewater treating Chlorella minutissima for methane enrichment and CO2 sequestration of biogas and producing lipids. Energy 150:153–163. https://doi.org/10.1016/J.ENERGY.2018.02.126
de Bhowmick G, Sarmah AK, Sen R (2019) Performance evaluation of an outdoor algal biorefinery for sustainable production of biomass, lipid and lutein valorizing flue-gas carbon dioxide and wastewater cocktail. Biores Technol 283:198–206. https://doi.org/10.1016/J.BIORTECH.2019.03.075
Funding
This research was supported by the Van Yüzüncü Yıl University Research Fund (No. FBA-2020–9228).
Author information
Authors and Affiliations
Contributions
Halil Durak: working method, experimental planning, interpretation of results.
Salih Genel: hydrothermal trials.
Emre Demirer Durak: planning of fungi experiments, examining the results of fungi.
Didem Özçimen: planning of algae experiments and interpretation of results.
Anıl Tevfik Koçer: growing algae.
Corresponding author
Ethics declarations
Ethical approval.
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Durak, H., Genel, S., Durak, E.D. et al. Hydrothermal liquefaction process of Ammi visnaga and a new approach for recycling of the waste process water: cultivation of algae and fungi. Biomass Conv. Bioref. 14, 7149–7165 (2024). https://doi.org/10.1007/s13399-022-03221-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-022-03221-6