Abstract
Hydrothermal liquefaction (HTL) is a promising route for producing bio-crude from various biomass feedstocks. However, high content of inorganic constituents in biomass like macroalgae Laminaria digitata and spent mushroom compost (SMC) affect the conversion process and the resulting fuel products. This research studied the effects of different acid leaching treatments on such feedstocks, subsequent HTL, and bio-crude properties. Leaching treatments were performed using five different agents: deionized water, acetic acid, citric acid, sulfuric acid, and hydrochloric acid. Performance of leaching was evaluated by analyzing both leached biomass and HTL products by elemental analysis, ash content, inductively coupled plasma (ICP) analysis, and X-ray diffraction (XRD) analysis. Catalytic and non-catalytic HTL of both feedstocks before and after treatment were performed in a 10-mL microreactor at 400 °C with a holding time of 15 min and pressures of 27–30 MPa. For macroalgae, sulfuric acid and hydrochloric acid were found the most effective in reducing the ash content from 30.42 to 20.45 and 20.87%, respectively, followed by acetic and citric acid treatment that could reduce the ash content to 21.5 and 22.15%, respectively. Similarly for SMC, citric acid and acetic acid were found the most effective in reducing the ash content from 50.34 to 37.04 and 39.94%, respectively. Citric acid did not show significant leaching of organic components such as carbohydrates and proteins and represented a less toxic and hazardous option for the leaching. The results from HTL of untreated and citric acid-treated biomass showed that the acid leaching resulted in an increase in bio-crude yields from 20.7 to 29.2% (dry ash-free basis) for macroalgae and from 22.9 to 25.1% for SMC.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Depletion of fossil fuel resources and environmental issues associated with greenhouse gases (GHG) are the main reasons of the increasing attention towards renewable energy resources like biomass. Various thermochemical technologies can be utilized to convert the biomass into biofuels [1]. Among them, hydrothermal liquefaction (HTL) has received increasing interest in the past decades as a process for converting biomass into drop-in biofuels and chemicals [2, 3]. Higher temperatures and pressures to maintain the water as a liquid are generally employed [4]. The biomass feedstock can be processed directly, without an energy-consuming drying step, since water acts both as a solvent and catalyst [5, 6]. High reactivity and superior ionic product (Kw) of supercritical water break down biomass complex polymers including carbohydrates, lipids, and proteins into smaller molecules that can be converted into bio-crude, water-soluble chemicals, solid residue, and gas [4, 7] depending on the catalysts, solvents, feedstock composition, and pre-treatment methods employed [8,9,10].
To avoid negative impacts caused by food production, novel non-food biofuel feedstocks need to be identified and utilized. One option is to utilize marine biomass, notably fast-growing, large marine plants such as macroalgal kelps. Brown macroalgae Laminaria digitata is considered as the potential biomass source for energy production due to their relatively fast growth rates, ease of harvesting, and low pre-production cost [11]. One of the studies reported the use of L. digitata biomass to generate bio-crude via HTL. They reported a bio-crude yield of 17.6 wt% (daf) basis with a higher heating value (HHV) of 32 MJ/kg [12]. Another study on HTL of brown algae Saccharina ssp. reported a yield of 8.7 and 27.7% of bio-crude depending on the harvesting times and conditions of the macroalgae [13]. Spent mushroom compost (SMC) is commonly used as a low-cost commercial and private scale fertilizer. Approximately 5 kg of SMC is generated from each 1 kg of grown button mushrooms on the farm [14] that led to ca. 17 million metric tonnes of the byproduct in 2007 globally. In Europe alone, SMC availability is estimated to be 47 million tonnes per year, which results in a potential of ~ 130,000 t of available feedstock per day [15].
Although HTL has the potential to generate high yields of bio-crude, there are some limitations that need to be addressed if L. digitata and SMC are to be used as feedstocks. One of the major limitations is their high ash content (L. digitata up to 30% and SMC up to 50%). The high ash content is due to the presence of inorganic constituents, mostly alkali and alkaline earth metallic species (AAEMs). This reduces the yield and quality of the generated bio-crude and restricts their alternative usage in direct combustion and gasification processes [9, 16]. Also, physicochemical characteristics of bio-crude can be changed easily during storage due to the presence of higher AAEMs content in the bio-crude because the AAEMs catalyze the polymerization reactions and thereby increase the viscosity [17]. High ash content can bring additional challenges to the catalytic refining of bio-crude such as decrease in catalyst activities, poisoning, and coking [9, 16]. It is reported that AAEMs present in feedstocks inactivated the catalysts used in the downstream upgrading processes of bio-crude [17, 18].
Leaching has been suggested by many authors to be an efficient, fast, and low-cost way to significantly reduce the ash content of a biomass material [19,20,21]. Therefore, many leaching experiments using different agents such as deionized water [22, 23], acetic acid [17, 24], hydrochloric acid [25, 26], sulfuric acid [27, 28], and citric acid [18] had been conducted.
Post-leaching, water washing steps are carried out in order to remove residual acids. It is well known that alkaline HTL media lead to lower amounts of produced char. Meanwhile, HTL of acidic feedstock slurries ends up generating higher amounts of solid residues. This is believed to be brought on by the fact that low pH media promote dehydration, resulting in the production of easily polymerizing unsaturated compounds. HTL of model cellulose shows that acidic conditions lead to lower yields exactly due to the polymerization of 5-HMF [29]. Neutralization step via water washing is a welcome alternative especially when strong acids (e.g., hydrochloric acid and sulfuric acid) are used in order to remove chlorine and sulfates which enhance equipment corrosion and fouling in bio-crude production and refining, respectively.
In this study, we investigate the impacts of different leaching treatments on both macroalgae L. digitata and SMC biomass. Five different treatments were selected for the study: two strong acids (hydrochloric acid and sulfuric acid), two weak acids (citric and acetic acid), and deionized water. The study focused on analysis of the biomass changes in its physical–chemical composition, and the impact of the pre-treatment on the bio-crude yield through the HTL process. The pre-treated and non-treated biomass samples were analyzed and compared for their metal content and organic composition.
2 Materials and methods
2.1 Raw materials
Macroalgae L. digitata and spent mushroom compost (SMC) biomass were selected for this study. The former was collected from Easdale Island, Scotland, and the latter was received from a local Danish mushroom farm (St. Restrup Champignon). The samples were pre-dried at 105 °C for 24 h, pulverized, and stored at room temperature for analysis. The particle size fraction of 200 μm was used for the experiments.
2.2 Leaching process
L. digitata and SMC were leached with five different agents for reduction of inorganics: deionized water, acetic acid, citric acid, hydrochloric acid, and sulfuric acid; all acid agents were purchased from Sigma-Aldrich, which are in analytical grade of 99.9%. The acids were diluted to 1.0 wt% solutions. In a typical leaching treatment, 10.0 g of biomass were soaked in 100 mL of 1.0% acid solution under magnetical stirring (1000 rpm) at 30 °C for 4 h. Lower acid concentration (1.0%) was selected because of lower water consumption for removal of residual acid during post-leaching process. After acid leaching, the biomass residues were subjected to water washing in order to eliminate residual acids. The washing was carried out in several steps, each of which consisted of mixing the leached biomass with 200 mL of deionized water, stirring the mixture well, and separating the two phases gravimetrically after centrifugation. Treated biomass samples were dried in an electric oven at 105 °C for 24 h and then stored prior to be used for analyzing. Leaching treatments and analysis were done in triplicate, and mean values are reported.
2.3 Biomass characterization methods
2.3.1 Proximate and ultimate analysis
Thermogravimetric analysis (TGA) of treated and untreated biomass samples was applied on Simultaneous Thermal Analyzer (STA) 6000 (PerkinElmer) for determination of moisture, ash, and volatile and fixed carbon contents. All samples were heated from 50 to 950 °C at a rate of 10 °C/min under nitrogen atmosphere. Ultimate analysis was carried out by using a vario MACRO cube (Elementar). All the measurements were conducted three times, and the mean values are reported.
2.3.2 Inductively coupled plasma–optical emission spectroscopy (ICP-OES)
The alkali and alkaline earth metallic species (AAEMs) of each biomass sample were quantified using inductively coupled plasma–optical emission spectroscopy (ICP-OES) following microwave-assisted acid digestion. The samples were prepared for analysis according to USEPA SW-846 Method 3051A - Microwave Assisted Acid Digestion of Solids and Oils (US. EPA, 2007). The microwave digestion system was an Anton Paar Multiwave 3000 equipped with high-pressure fluoropolymer-lined ceramic digestion vessels. The digest of each sample was subsequently diluted to 50.0 mL using type 1 ultra-pure water (PURELAB Ultra, Elga LabWater, Glostrup, Denmark).
The ICP-OES was a Thermo iCap 6300 duo ICP-OES equipped with a Cetac ASX-260 autosampler. The spectrometer was operated in radial view mode with a RF power of 1.15 kW. The plasma and auxiliary gas flows were 12 L min−1 and 1.0 L min−1, respectively. The sample introduction system was a Cetac U5000AT+ ultrasonic nebulizer. The nebulization gas pressure was 0.2 MPa, and the sample uptake rate was 2 mL min−1. The ICP-OES was calibrated using matrix matched multi-element external standards (PlasmaCAL, SCP Science, Quebec, Canada). Three standards and one blank were used for calibration of each element using three emission lines. Yttrium was used as internal standard. All standards were traceable to the National Institute of Standards and Technology (NIST).
2.3.3 X-ray diffraction analysis
Pre-treated and untreated samples of L. digitata and SMC were analyzed by X-ray diffraction (XRD) analysis on an Empyrean (PANalytical, Netherlands) system under Cu Kα radiation (λ = 0.1542 nm; 45 kV and 40 mA) in a 2θ range between 5° and 70° at a step size of 0.013°. The measurements were conducted three times, and the mean values are reported.
2.4 Hydrothermal liquefaction methodology
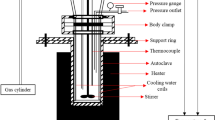
Hydrothermal liquefaction experiments were carried out in a 10-mL stainless steel tubular micro-batch reactor at 400 °C and 25–30 MPa with a holding time of 15 min. Biomass slurry was prepared with a composition of 85% deionized water, 15% dry biomass, and 5% K2CO3 of total biomass (in case of a catalytic run) by weight. In a typical experiment, homogenized biomass slurry (~ 5 g) was loaded in the reactor and sealed. The system was purged twice to pressures of ~ 8 Mpa, flushed, and finally pressurized to 2 ± 0.1 MPa. The reactor was heated in a pre-heated fluidized sand bath (Techne SBL-2D). At the end of the reaction, the reactor was quenched in a cold water bath. Gas products were vented in fume hood. HTL products were separated into bio-crude fraction, water soluble fraction, and solid residues according to the procedure schematically shown in Fig. 1.
The water phase was poured out of the reactor and filtered using pre-weighed Whatman No. 5 filter paper to collect the solid product. The reactor was washed using acetone (Sigma-Aldrich) to recover the bio-crude. The acetone and bio-crude mixture was filtered and evaporated using a rotary evaporator to remove the acetone. The solid residue was oven-dried at 105 °C for 24 h. All HTL experiments were performed in triplicates.
2.5 Analysis of HTL bio-crude
Bio-crude yield was calculated separately on an ash-and-moisture free basis using the following equation (wt%) [30]:
where Y bio-crude is the bio-crude yield (wt%) on a dry weight basis, W bio-crude is the mass of the bio-crude (g), W feedstock is the mass of the biomass used in the reactor, and W moisture and W ash are the moisture and ash content of the feedstock, respectively. The HHVs of the generated bio-crude were calculated by Channiwala and Parikh formula [31].
3 Results and discussions
3.1 Chemical analyses of the samples
The effectiveness of the different biomass leaching treatments was evaluated considering the impact of the treatments on the ash contents of the biomass. The ultimate and proximate analysis results of untreated and treated samples are shown in Table 1.
As shown in Table 1, compared to the untreated L. digitata sample, the ash content of the deionized water leaching sample decreased from 30.42% in the untreated biomass to 28.58% only, and the ash content of the acid leaching samples decreased significantly to 20.45 and even 5.96%, when a 5% citric acid solution was employed. In case of SMC, the ash content of the deionized water leaching sample slightly decreased from 50.34% in the untreated biomass to 47.19%, and the ash content of the acid leaching samples decreased to 37.04 and 16.66% with 1 and 5% citric acid solutions, respectively. The ash content of the samples decreased remarkably, and volatile content increased dramatically after the leaching process, which indicates the leaching process could improve biomass fuel properties such as heating value. Despite showing similar leaching efficiencies in L. digitata and SMC as 1% solution (27.2% and 26.4%), citric acid performed better in macroalgae when used as a 5% leaching agent (80.4% ash reduction compared to 66.9% in SMC). The fivefold increase in acid concentration led to 3 and 2.5 times higher leaching efficiencies in the two biomass feedstocks, respectively.
As mentioned above, after water leaching, the ash content of L. digitata decreased from 30.42 to 28.58%; hence, ~ 6% are water-soluble ash components. After 1% acid leaching, the ash content of the samples decreased to 20.45–22.15%; here, ~ 27–33% of the lost matter consist of both water-soluble and acid-soluble portions. Similarly, the ash content of SMC decreased from 50.34 to 47.19%; hence, ~ 6% are water-soluble. Whereas after 1% acid solution leaching, the ash content of samples decreased to 37.04–44.40%, here the ~ 12–26% of the lost mass is composed of both water- and acid-soluble fractions. Considering the ~ 6% of water-soluble part, the minerals present in the two feedstocks comprise of a significant amount of water-soluble components (mainly in the form of chlorides, nitrates, carbonates, and phosphates) [32, 33]. The experiments with 5% citric acid solutions, however, showed that stronger acid leaching removes more acid solubles, namely, in L. digitata, water solubles added up to just 7.5% of the total removed ash, while in SMC, the same was true for 9.4% of the removed ash. This confirms the previously described higher efficiency of acid leaching.
The organic components of biomass are composed of different hydrocarbons which mainly consist of C and H atoms. The molecular mass of C is much higher than H; thus, the C/H wt% ratio reflects variances in amounts of hydrocarbons. When some hydrocarbons are washed away, the C/H wt% ratio decreases. As shown in Table 2, the C/H wt% ratio of raw and treated samples fluctuates between 7.19 and 7.55 in the case of L. digitata and 7.77 and 9.31 for SMC. The C/H wt% ratio decreased slightly after the leaching processes, which illustrates that a fraction of organic components were removed from samples during the leaching process.
The contents of AAEMs in untreated and treated samples are listed in Table 3. K and Na contents of untreated L. digitata are higher than other metal contents, and this is common in brown macroalgae. After distilled water leaching, very small amount of AAEMs were removed, which suggests that the majority of these AAEMs were present in the form of water-insoluble salts. Compared to water leaching, the amount of AAEMs had different degrees of decline after acid leaching, and such decline was related with acidity of leaching agents. Especially after leaching by strong acids such as HCl and H2SO4, more than 90% AAEMs were removed, and comparing to their reduction by water leaching, it can therefore be deduced that most of AAEMs were present in the form of water-insoluble salts.
Ca and K contents of untreated SMC is higher than other metal content, while this is not surprising because of excess agricultural fertilization with common gypsum and potash fertilizer during cultivation of mushroom compost. After distilled water leaching, K and Na were almost completely removed compared to Ca and Mg, which suggests that the majority of K and Na were present in the form of water-soluble salts. After leaching by strong acid such as HCl and H2SO4, more than 50% of Ca and Mg were removed depending upon the acidity of the agents. It means most of Ca and Mg were present in the form of water-insoluble salts.
Acid especially strong acid leaching is more effective on the removal of AAEMs, but it might introduce a negative impact on the physicochemical structure of the samples. Thus, as a complementary means of investigation, XRD was used to follow the change of crystalline structures as the leaching agents varied.
3.2 Crystalline structure of treated and untreated biomass
Figure 2 shows changes in the X-ray diffraction pattern of untreated and treated biomass samples.
The diffraction pattern of untreated L. digitata in Fig. 2a showed eight peaks of different intensity between 25° and 70° at different 2θ. On diffractograms of the samples after treating with different agents, almost all the peaks disappeared except in the case of deionized water treatment. These results indicated that all the acidic treatments have converted the L. digitata into complete amorphous phases.
In the case of SMC, the diffraction pattern of untreated SMC Fig. 2b showed numerous peaks of different intensity between 20° and 70° at 2θ. Almost all the peaks on diffractograms of the samples remained the same even after treating with different solvents. So leaching treatments had no impacts on crystallization of the SMC samples.
3.3 Hydrothermal liquefaction of untreated and treated biomass
Although strong acids HCl and H2SO4 were better pre-treatment agents for leaching both L. digitata and SMC, it could be associated with a negative impact on the physicochemical structure of the biomass and led to the loss of biochemical composition of the biomass. The yield of bio-crude in HTL depends on the amount of biochemicals such as carbohydrates, proteins, and lipids present in the biomass. The strong acids were not selected due to their toxicity, and also, it is a very high working load to neutralize the biomass during post-leaching water washing step after the use of strong acids like HCl and H2SO4. Although citric acid treatment did not show the highest leaching potential, it was selected for further HTL studies due to its more experimental friendly nature and lower water requirements after post-leaching to neutralize both L. digitata and SMC.
Proximate analysis of the untreated and treated biomasses (treated with citric acid) was performed and resulted in higher fixed carbon percentage and lower ash content than the untreated one. Also, the citric acid-treated biomass had a higher content of volatiles than the untreated biomasses.
3.3.1 HTL product yields
HTL product yields of citric acid-treated and untreated biomasses are presented in Tables 4 and 5.
As anticipated from the proximate analysis, a higher bio-crude yield (29.15%) was obtained from the catalytic HTL of citric acid-treated L. digitata than that of the untreated through catalytic HTL, which had a bio-crude yield of 20.69%. Similarly, in the case of SMC, a higher bio-crude yield (25.06%) was obtained from the non-catalytic HTL of citric acid-treated SMC than that of the untreated SMC through non-catalytic HTL, i.e., 22.86%. There are no significant differences in elemental carbon and hydrogen composition in bio-crude samples obtained from both untreated and treated biomasses (Table 6). High oxygen and nitrogen content in bio-crude suggests that an upgrading step is required after the HTL process to convert the product into a drop-in fuel.
In summary, for the HTL primary fuel target, the bio-crude, we observe an increase in yield and lower ash content, along with a decrease in solid residues after the leaching pre-treatment.
Although assessing process economics is not among the main objectives of this study, it is necessary to discuss the issues of additional costs and resources associated with feedstock leaching inclusion. Due to low acid concentrations necessary for efficient removal of inorganics, the additional amount of water required for the proposed pre-treatment step is the key factor. The economics of acid leaching are highly dependent on local water availability, water costs, and expenses related to process water treatment. Furthermore, costs encountered due to HTL system plugging and fouling are production site specific, namely due to the varying system design and the implemented char evacuation technologies. In the end, case studies shall be carried out to weigh the economic advantages and disadvantages of acid leaching pre-treatment for continuous HTL plants.
As far as the results from this study go, the inclusion of feedstock acid leaching is seen as a HTL enabling strategy. The fact that experiments with macroalgae and SMC at batch scale exhibited signs of potential blockage problems (i.e., major agglomeration of solids in the reactor) raise concern for the viability of continuous HTL with such feedstocks. On the other hand, biomass leaching did not lead to decreased bio-crude yields and quality. Given that cheap biomass often has a low calorific value, is of high humidity, and exhibits a high content of inorganics, system blockages are the major obstacle for large-scale continuous HTL processing. Waste biomass valorization via HTL is known for its high energetic efficiency [34] and capacity to produce high-quality biofuel precursors. The possibility of successfully converting low-cost high-ash sustainable feedstocks might as well lead to enabling acid leaching as an additional pre-treatment step.
3.4 Water demand for biomass neutralization
Water demand was quantified for each leaching procedure with the two studied feedstocks. In the case of L. digitata, the acetic and citric acid leached biomass reached neutral pH after six washing steps, whereas algae exposed to hydrochloric and sulfuric acid leaching remained at pH 5 and 4, respectively, even after eight washing steps. Acetic, citric, and sulfuric acid leached SMC reached neutral pH already after three washing steps, whereas hydrochloric acid pre-treated biomass remained at pH 5 after eight consecutive washing steps. Here, three, six, and eight washing steps signify process water consumption levels of 0.06, 0.12, and 0.16 L for each gram of the initial feedstock.
Water washing as a post acid leaching neutralization technique inherently increases process water demands; hence, future facilities must take this and the raised subsequent waste water treatment requirements into account when carrying out techno-economic analyses of continuous HTL processing. HTL-specific research is needed on the alternative chemical neutralization route. Acid neutralization via the addition of bases is a straightforward reaction chemically; however, the acidic and basic compounds present in the feedstock might have adverse effects on the production of bio-crude due to precipitation, especially in terms of potentially impaired yields and promoted formation of solids. Arguably, when comparing water and chemical neutralization, the effect of superior organic matter preservation might counteract the possible drop-in bio-crude yields caused by acid-driven recondensation reactions.
4 Conclusions
The effects of leaching pre-treatments using five different leaching agents (deionized water, acetic acid, citric acid, sulfuric acid, and hydrochloric acid) on HTL of L. digitata and SMC biomass were studied. It was found that all the leaching pre-treatments decreased the inorganic contents in the biomasses as expected while citric acid was selected as the best leaching agent because it was able to remove maximum AAEMs without using a large amount of water in the post-leaching process to neutralize the biomasses. In addition, it represents a more eco-friendly alternative. The results of HTL in both treated and untreated L. digitata and SMC showed that the leaching treatment resulted in an increase in bio-crude yield from 20.7 to 29.2% (dry ash-free basis) for macroalgae and from 22.9 and 25.1% for SMC. It is obvious that algae produce more bio-crude yield than lignocellulosic biomass like SMC due to higher amount of protein and lipid.
References
Asadieraghi M, Daud WMAW (2014) Characterization of lignocellulosic biomass thermal degradation and physiochemical structure: effects of demineralization by diverse acid solutions, energy convers. And Mgmt 82:71–82
Biller P, Ross AB (2012) Hydrothermal processing of algal biomass for the production of biofuels and chemicals. Biofuels 3:603–623
Barreiro DL, Prins W, Ronsse F, Brilman W (2013) Hydrothermal liquefaction (HTL) of microalgae for biofuel production: state of the art review and future prospects. Biomass Bioenergy 53:113–127
Villadsen SR, Dithmer L, Forsberg R, Becker J, Rudolf A, Iversen SB, Iversen BB, Glasius M (2012) Development and application of chemical analysis methods for investigation of bio-oils and aqueous phase from hydrothermal liquefaction of biomass. Energy Fuel 26:6988–6998
Toor SS, Rosendahl L, Rudolf A (2011) Hydrothermal liquefaction of biomass: a review of subcritical water technologies. Energy 36:2328–2342
Jazrawi C, Biller P, He Y, Montoya A, Ross AB, Maschmeyer T, Haynes BS (2015) Two-stage hydrothermal liquefaction of a high-protein microalga. Algal Res 8:15–22
Peterson AA, Vogel F, Lachance RP, Fröling M, Antal MJ Jr, Tester JW (2008) Thermochemical biofuel production in hydrothermal media: a review of sub-and supercritical water technologies. Energy Environ Sci 1:32–65
Y. Zhuang, J. Guo, L. Chen, D. Li, J. Liu, N. Ye, Microwave-assisted direct liquefaction of Ulvaprolifera for bio-oil production by acid catalysis, Bioresour Technol 116 (2012) 133–139
Neveux N, Yuen AK, Jazrawi C, Magnusson M, Haynes BS, Masters AF, Montoya A, Paul NA, Maschmeyer T, de Nys R (2014) Biocrude yield and productivity from the hydrothermal liquefaction of marine and fresh water green macroalgae. Bioresour Technol 155:334–341
Singh R, Bhaskar T, Balagurumurthy B (2015) Effect of solvent on the hydrothermal liquefaction of macroalgae Ulva fasciata. Process Saf Environ Prot 93:154–160
Adams JMM, Ross AB, Anastasakis K, Hodgson EM, Gallagher JA, Jones JM, Donnison IS (2011) Seasonal variation in the chemical composition of the bioenergy feedstock Laminaria digitata for thermochemical conversion. Bioresour Technol 102:226–234
Anastasakis K, Ross AB (2015) Hydrothermal liquefaction of four brown macro-algae commonly found on the UK coasts: an energetic analysis of the process and comparison with bio-chemical conversion methods. Fuel 139:546–553
Elliott DC, Hart TR, Neuenschwander GG, Rotness LJ, Roesijadi G, Zacher AH, Magnuson JK (2013) Hydrothermal processing of macroalgal feedstocks in continuous-flow reactors. ACS Sustain Chem Eng 2(2):207–215
Williams BC, McMullan JT, McCahey S (2001) An initial assessment of spent mushroom compost as a potential energy feedstock. Bioresour Technol 79(3):227–230
Eurostat, Mushrooms, energy crops, GMO: number of farms and areas by size of farm (UAA), 2005–2013 data, Tech. rep. (2016–09-15)
Bach QV, Sillero MV, Tran KQ, Skjermo J (2014) Fast hydrothermal liquefaction of a Norwegian macroalga: screening tests. Algal Res 6(Pt B):271–276
Liu X, Bi XT (2011) Removal of inorganic constituents from pine barks and switchgrass. Fuel Process Technol 92:1273–1279
Vázquez LMD, Pérez AR, Caraballo MF, Robles IV, Jena U, Das KC (2015) Demineralization of Sargassum spp. macroalgae biomass: selective hydrothermal liquefaction process for bio-oil production. Front Energy Res 3:1–11
Jenkins BM, Bakker RR, Wei JB (1996) On the properties of washed straw. Biomass Bioenergy 10(4):177–200
Turn SQ, Kinoshita CM, Ishimura DM (1997) Removal of inorganic constituents of biomass feedstocks by mechanical dewatering and leaching. Biomass Bioenergy 12(4):241–252
Jiang L, Hu S, Sun L, Su S, Xu K, He L, Xiang J (2013) Influence of different demineralization treatments on physicochemical structure and thermal degradation of biomass. Bioresour Technol 146:254–260
Fahmi R, Bridgwater A, Donnison I, Yates N, Jones JM (2008) The effect of lignin and inorganic species in biomass on pyrolysis oil yields, quality and stability. Fuel 87:1230–1240
Mourant D, Wang ZH, He M, Wang XS, Garcia-Perez M, Ling KC, Li CZ (2011) Mallee wood fast pyrolysis: effects of alkali and alkaline earth metallic species on the yield and composition of bio-oil. Fuel 90:2915–2922
Davidsson KO, Korsgren JG, Pettersson JBC, Jaglid U (2002) The effects of fuel washing techniques on alkali release from biomass. Fuel 81:137–142
Eom IY, Kim KH, Kim JY, Lee SM, Yeo HM, Choi IG, Choi JW (2011) Characterization of primary thermal degradation features of lignocellulosic biomass after removal of inorganic metals by diverse solvents. Bioresour Technol 102:3437–3444
Mayer ZA, Apfelbacher A, Hornung A (2012) Effect of sample preparation on the thermal degradation of metal-added biomass. J Anal Appl Pyrolysis 94:170–176
Fierro V, Torne-Fernandez V, Celzard A, Montane D (2007) Influence of the demineralisation on the chemical activation of Kraft lignin with orthophosphoric acid. J Hazard Mater 149:126–133
Keown DM, Hayashi JI, Li CZ (2008) Effects of volatile–char interactions on the volatilisation of alkali and alkaline earth metallic species during the pyrolysis of biomass. Fuel 87:1187–1194
Yin S, Tan Z (2012) Hydrothermal liquefaction of cellulose to bio-oil under acidic, neutral and alkaline conditions. Appl Energy 92:234–239
Li D, Chen L, Xu D, Zhang X, Ye N, Chen F, Chen S (2012) Preparation and characteristics of bio-oil from the marine brown alga Sargassum patens C. Agardh. Bioresour Technol 104:737–742
Channiwala SA, Parikh PP (2002) A unified correlation for estimating HHV of solid, liquid and gaseous fuels. Fuel 81(8):1051–1063
Knudsen JN, Jensen PA, Dam-Johansen K (2004) Transformation and release to the gas phase of Cl, K, and S during combustion of annual biomass. Energy Fuel 18:1385–1399
Patwardhan PR, Satrio JA, Brown RC, Shanks BH (2010) Influence of inorganic salts on the primary pyrolysis products of cellulose. Bioresour Technol 101:4646–4655
Feng W, van der Kooi HJ, Arons JDS (2004) Biomass conversions in subcritical and supercritical water: driving force, phase equilibria, and thermodynamic analysis. Chem Eng Process 43:1459–1467
Acknowledgements
This work is part of C3BO (Center for BioOil) at the Department of Energy Technology, Aalborg University. The research was financially supported by a grant from the Innovation Fund Denmark Grant No 1305-00030B. The authors are grateful to Patrick Biller and Maika Klemmer from the Department of Chemistry, Arhus University, for their support with some of the analysis. One of the authors, Chunbao (Charles) Xu, would also like to acknowledge the VELUX Visiting Professor Programme.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Toor, S.S., Jasiunas, L., Xu, C.(. et al. Reduction of inorganics from macroalgae Laminaria digitata and spent mushroom compost (SMC) by acid leaching and selective hydrothermal liquefaction. Biomass Conv. Bioref. 8, 369–377 (2018). https://doi.org/10.1007/s13399-017-0290-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-017-0290-6