Abstract
Polygalacturonase is a pectinolytic enzyme with potential applications in the food, chemical, textile, and paper industries. Herein, extracellular Exo-polygalacturonase (Exo-PG) was biosynthesized from Penicillium fellutanum by harnessing the potential of wheat bran as an agricultural substrate via solid-state fermentation (SSF). Optimization protocol revealed the maximum production of the enzyme at 40% moisture level, pH 4.0, temperature 40 °C, and fermentation duration of 96 h. The partially purified enzyme derivative exhibited its maximum biocatalytic activity at pH 4.0 and 40 °C. It retained appreciable stability over a wide temperature range of 30–70 °C and a pH range of 3.0–8.0. Values of Km and Vmax, i.e., Michaelis–Menten constants, were calculated to be 12.5 mg/mL and 22.22 μmol/mL/min, respectively. The activation energy for thermal denaturation was determined to be 128.8 kJ/mol. Exposure to a hydrophobic environment, i.e., urea, considerably suppressed the enzymatic activity. A significant increase in the clarity and viscosity reduction was achieved for all fruit juices after treatment with Exo-PG. Conclusively, the current findings may represent Exo-PG as a prospective and promising candidate for diverse bio-applications in the food and beverage industries.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
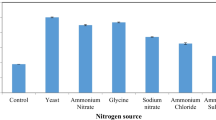
Microbial pectinases are a group of fascinating food enzymes that catalyze pectin breakdown in the plant cells walls [11, 48]. Pectin is a high molecular weight biomacromolecule present in the cell wall and middle lamella of plants. It is a jell-like material acting as cement for plant cells. The structural organization of pectin is formed by alpha-1,4-linked galacturonic acid (Gummadi and Panda, 2003). Pectinolytic enzymes from microbial origin hold a 25% of share in the global market of food enzymes. Pectinases have a broad range of industrial applications, e.g., in extraction and purification of fruit juices, tea and coffee fermentation, retting of fiber crops, and textile and paper industry [24, 38, 50]. Pectinases are responsible for splitting glycosidic bonds of long-chain galacturonic acid residues of the pectinaceous substances [26]. They are mainly classified into pectin esterases, depolymerizing enzymes, and proto pectinases.
Enzymes are procured from various natural sources, i.e., plants and animals; however, microbial fermentation has gained the utmost attention due to a lot of advantages compared with other sources because of a higher metabolic assortment of microorganisms, the regular availability of biocatalyst/biomolecule in all seasons, and its production at optimized levels in labs in less time [41, 51, 52]. The most famous microorganisms reported so far for enzyme production are filamentous fungi, bacteria, and yeasts (Bilal and Iqbal, 2019; [6, 7, 20].
Fermentation is a process that involves the biological interconversion of the complex substrates into simpler compounds by microorganisms, such as fungi, bacteria, and yeast. The submerged fermentation (SmF) and solid-state fermentation (SSF) can be successfully employed to produce pectinases. However, the submerged fermentation technique is economically least favorable. In a more economical SSF process, microbial development and product formation occur on solid substrates’ surface without free water, which is a natural habitat for fungal growth (Zheng et al., 2000; [33, 45]. The solid substrate provides an attachment area for fungi, which is beneficial for fermentation with low moisture levels [25]. Due to these advantages, pectinases are currently being produced from various fungal strains under SSF.
Various studies have been conducted to purify and characterize fungal polygalacturonases from various microorganisms [1, 34]. Partial purification of pectinases from microbial sources is usually carried out via the ammonium sulfate precipitation technique [14]. Characteristic study of pectinases can be carried out by observing the effect of pH and temperature on enzyme activity by applying various conditions. All enzymatic proteins possess an optimum temperature and pH at which they show the highest activity, e.g., a commercial pectinase might be activated within a temperature and pH range of 45–55 °C and 3.0–6.5, respectively.
Pectinases are an integral part of many industrial processes. In 1930, they were used for the first time in the alcohol and beverage industries, playing a vital role in fruit and vegetable extraction and having an imperative contribution to alcohol and beverage manufacturing at a commercial scale. In addition, they are also used to clear the cloudiness of citrus juices, as the removal of haze from juices has been a matter of great concern on a commercial scale [6, 21, 29].
Many enzymes from the hydrolases family, i.e., pectinase, hemicellulase, and xylanase, have been reported so far for their promising applications in the modern fruit juice processing industries leading us towards more stable and clarified juices of different seasonal fruits. Global account of pectinases is more than 10% of the total industrial-scale enzymes produced commercially. Most pectinases originating from microbial sources are implemented in the beverage industries for juice extraction and clarification purpose. These pectinases are responsible for the breakdown of structural polysaccharides of pulpy fruits and decline in turbidity, viscosity, and improvement in consistency [28, 40]. Additionally, biocatalytic clarification is advantageous over conventional methods in terms of high catalytic efficiency, more specificity, and low energy consumption [28, 40].
A wide range of applications of this enzyme provides different studies aiming at its production for its potential use in various industrial practices. Lu et al. [28] found a purified polygalacturonase to have a stimulatory and significant effect in the juice clarification of four different fruits. Results showed that the purified polygalacturonase had a remarkable impact on the clarification of tangerine and apple juices. In another finding, Patil et al. [39] reported a significant reduction in viscosity for apple, orange, banana, and sapota juices via treatment with polygalacturonase of Paecilomyces variotii NFCCI 1769 as these fruits are naturally enriched with pectin, thereby showing a marked reduction in viscosity. In comparison to commercial pectinase, the results obtained by polygalacturonase from P. variotii NFCCI 1769 were observed to be substantial.
Keeping in view the commercial significance of this important industrial enzyme, the present study intends the production of Exo-polygalacturonase from Penicillium fellutanum using wheat bean as an agricultural waste in solid-state fermentation. The optimally produced enzyme was partially purified and characterized concerning various parameters, including thermodynamic and kinetic characteristics. Finally, the industrial potential of Exo-PG was assessed via studying the treatment of different fruit juices with Exo-PG to observe the viscosity, haze, and clarification parameters.
2 Materials and methods
2.1 Preparation of substrates
Wheat bran used for Exo-PG biosynthesis was obtained from a local commercial market in Faisalabad, Pakistan. It was oven-dried until a constant weight was attained. The dried sample was ground in an electric mill and sieved to obtain homogenous particle size using an Octagon Sieve (OCT-Digital 4527–01). Dried and ground substrate was stored in an air-tight jar for further study.
2.2 Fungal strain and preparation of inoculum
Penicillium fellutanum was obtained from the department of plant pathology, University of Agriculture, Faisalabad. The strain was sustained on potato dextrose agar (PDA) slants and revived every week, then stored at 4 °C in a refrigerator. Sporulation media was prepared by taking 39 g of PDA in 1000 mL of distilled water. The solution was then simmered for about 5–7 min with constant stirring. The cooled PDA solution was equally distributed in sterilized test tubes, plugged with cotton and aluminum foil and immediately sterilized in an autoclave at 121 °C for 15 min. These test tubes were placed on a clean surface in a slanting position to increase the surface area until solidification and inoculated with spores of the fungus under sterilized conditions in a laminar airflow followed by incubation at 30–35 °C till sporulation and stored at 4 °C in a refrigerator.
For inoculum preparation, 250-mL Erlenmeyer flasks containing 100 mL of the medium, consisting of (g/L) (NH4)2SO4, 4.0; MgSO4.7H2O; KH2PO4, 5.0; NH4NO3, 2.0; 0.2 g/L; trisodium citrate, 2.5 g/L; peptone, 2 g/L; and yeast extract 0.1 g/L were used as inoculum medium with a 2.0% final glucose concentration. Small gravels (5–6) washed with chromic acid were added into flasks to break mycelia for obtaining the homogenous suspension of spores, followed by autoclaving at 121 °C for 15 min before inoculation. Seed culture (inoculum) was prepared by inoculating a loop full of the stock culture from the slants to autoclaved media under sterilized conditions, following the incubation at 30–35 °C with an agitation speed of 120 rpm. A 3-day-old culture with a spore count of 1 × 108 spores per mL was used as an inoculum.
2.3 Exo-polygalacturonase production and harvesting of the growth media
Ten grams of the substrate (wheat bran) were transferred in a series of Erlenmeyer flasks (250 mL) humidified with the distilled water (40–80%) and then sterilized at 15 psi for 20 min at 121 °C. After sterilization, the cooled flasks were inoculated with 2–3-mL spore suspension, cotton plugged and incubated at 30–35 °C for 72–96 h. Optimization of media for the maximum Exo-PG production was conducted following a classical approach by varying one factor while keeping other variables constant. Fundamental factors influencing Exo-PG production studied were moisture (20–80%), pH (2–8), and time of incubation (24–120 h). After the completion of fermentation, the crude enzyme was extracted by mixing the fermented substrate with 70 mL of 0.1 M phosphate buffer (pH 7.0) and then shaking the mixture in an orbital shaker at 130 rpm. The crude extract was collected, and then 30 mL of buffer was again mixed in residue followed by shaking at 150 rpm. A 100 mL of crude extract collected was filtered by ultra-vacuum filtration and subjected to Exo-PG assay and protein estimation.
2.4 Enzyme assay and estimation of protein contents
The activity of Exo-PG (Exo-polygalacturonase) was determined by measuring reducing groups released from pectin solution using the 3,5-dinitrosalicylic acid (DNS) method [30]. In this method, D-Galacturonic acid monohydrate was used as a standard reference. A suitably diluted enzyme solution (0.1 mL) was then added to the reaction mixture containing the pectin solution (1%) and acetate buffer (50 mM at pH 5.5) and immediately incubated at 40 °C for 30 min. Later, this enzymatic reaction was terminated by adding DNS reagent (1 ml) followed by heating in a boiling water bath for 5–10 min. The reducing sugars formed in the solution were observed at 535 nm. The enzyme activity units were determined by Eq. 1
A unit (U) of the Exo-PG, defined as a quantity of enzyme that liberates one micromole of the galacturonic acid per minute at 40 °C at a pH of 5.5. Enzyme production in SSF was expressed in the units per gram of the dry substrate (U/gds).
Total protein content was estimated using the method of Bradford [17]. The standard reference used was bovine serum albumin (BSA). In the test tube containing 100 μL amount of the enzyme, 100 μL of the Bradford reagent was added, the mixture was incubated at room temperature for 10–15 min, and absorbance was recorded on a spectrophotometer at 595 nm. Control blank was made using an equal volume of distilled water as taken for the enzyme. For the standard curve, 25 mg BSA was dissolved in 25 mL of distilled water. The BSA solution was diluted ten times to prepare a standard stock solution (0.1 mg/ml). Different dilutions, e.g., 0.02, 0.03, 0.04, 0.05, 0.06, 0.07, 0.08, 0.09, and 0.1 mg/mL, were prepared. Then, 100 μL was added to a test tube containing 900 μL of Bradford reagent from each solution. 100-μL distilled water was then added into a test tube with 0.9 mL of Bradford reagent for the blank. The absorbance of all the samples was observed at 595 nm after 10–15 min of incubation at room temperature [7, 8]. A standard curve was plotted between absorbance and concentration of protein, and the standard factor was found to be 0.36 (Fig. S1).
2.5 Partial purification of Exo-polygalacturonase via (NH4)2SO4 precipitation
Ammonium sulfate precipitation method was adopted to partially purify the enzyme and reduce fermented broth volume, followed by dialysis at 4 °C [6]. Different quantities of solid ammonium sulfate were separately added to 10 mL of the crude enzyme to obtain 25% to 90% saturation at 4 °C. Test tubes were kept overnight at a 4 °C, and the residue was collected by centrifugation at 10,000 rpm for 10 min at 4 °C. The supernatant and residue both were assayed for Exo-PG activity after dialysis. Eighty percent saturation level was found to be the best to get the maximum enzyme as a palette residue. So, ammonium sulfate at 80% saturation level was added to the crude Exo-PG solution, gradually in small amounts, in an ice-cold water bath (4 °C) with mild shaking. The solution was left overnight at 4 °C following the subsequent centrifugation at 10,000 rpm for about 15 min at 4 °C. The non-active supernatants, for enzyme activity, were immediately discarded, and the palette in the form of residue was dissolved in a small amount of 50 mM of phosphate buffer (pH 7.0) and dialyzed overnight against 25-mM phosphate buffer (pH 7.0) [7, 8, 15].
2.6 Characterization of Exo-polygalacturonase
Partially purified Exo-PG was biochemically characterized for activity and stability profiles comparative to temperature, pH, effects of metal ions, chemicals, inhibitors, etc. [14, 15, 28].
2.6.1 Effect of temperature on the activity of Exo-PG
Effect of temperature for hydrolysis of pectin was determined by incubating the enzyme and substrate mixture at various temperatures (25–90 °C) under standard assay conditions [30]. Arrhenius plot was used to calculate the activation energy (Ea). The effect of temperature on the reaction rate was expressed as temperature quotient (Q10), which is the factor for which the reaction rate increases with every 10 °C rise in the temperature. Q10 was then calculated by rearranging Eq. 2 [43].
where E = Ea, the energy of activation.
2.6.2 Thermodynamic and kinetic study of Exo-PG for substrate hydrolysis
The values of Michaelis–Menten kinetic constants (km, Vmax) were obtained by incubating the fixed amount of enzyme (Exo-PG) with varied pectin substrate concentrations. Lineweaver Burk plot was plotted between the inverse of substrate concentration [1/S, %] and the inverse of velocity [1/V], taking 1/[S] along X-axis and 1/[V] along Y-axis [2]. From the graph, the values of Vmax and km (Michaelis–Menten constant) were calculated.
Thermodynamic parameters for the substrate hydrolysis were determined by the rearrangement of Eyring’s absolute rate equation, derived from transition state theory [43]:
∆G# is the free energy of activation.
where kcat can be described as
where kb is Boltzmann’s constant (R/N) = 1.38 × 10−23 JK−1,
T is the absolute temperature (K).
h is Planck’s constant = 6.626 × 10−34 J s.
N is Avogadro’s number = 6.02 × 1023 mol−1.
R is the gas constant = 8.314 JK−1 mol−1.
∆H# is the enthalpy of activation.
∆S# is the entropy of activation for hydrolysis of substrate
2.6.3 Effect of the pH on activity and stability of Exo-PG
Optimum pH for Exo-PG production was established by incubating the purified enzyme using a pH range of 2.5–10 under the standard assay conditions [13]. Following buffers were used for the said purpose: glycine/HCl (pH 2.5–3.5), sodium-acetate/acetic acid (pH 4–5.5), sodium-phosphate buffer (6.0–7.5), and glycine/NaOH (pH 8.0–10). The stability of the enzyme at various pH was determined by measuring the residual enzyme activity after overnight incubation of Exo-PG with buffers having different pH ranging from 2.5 to 10 for 24 h at 4 °C.
2.7 Applications of Exo-PG in fruit juice clarification
The potential of Exo-PG was tested for fruit juice clarification. Different fruits juices, like apples, grapes, and peach, were treated with different amounts of the Exo-PG [7, 8, 49]. Different units of enzymes depending on the pulp of juice (40–200 Units) (40 Units for apple juice, 200 Units for peach juice and 30 units for grapes juice) were used to treat juice samples following incubation in an orbital shaker at 120 rpm for 60 min at 50 °C followed by centrifugation for 20 min at 10,000 rpm. A control experiment was also conducted, and the same volume of distilled water was used for the enzyme. The clarification parameter of juices following each treatment was evaluated using a spectrophotometer by taking absorbance at 660 nm [10]. The viscosity parameter of the juices was evaluated in 50 mL of juice samples at room temperature using a digital viscometer (Brookfield model DV-E, USA), and results were expressed in centipoise units.
3 Results and discussion
3.1 Exo-polygalacturonase production and optimization
3.1.1 Effect of fermentation duration on Exo-PG production
The fermentation period is an important factor affecting the production of Exo-PG obtained from any fungal source. Incubation time is a statistically significant & critical parameter as it affects the growth rate of microorganisms leading to enzyme biosynthesis. During the incubation time, the enzyme can react with the substrate for a given time when the reaction is concentrated towards producing the bioproducts effectively [5]. Different incubation periods were employed in the present study, i.e., 24, 48, 72, 96, and 120 h, and the highest Exo-PG production (74 U/gds) was obtained at 96 h of incubation (Fig. 1). Similar results have been reported by Mrudula and Anithraraj [31], who obtained the optimum enzyme production at 96 h of incubation.
3.1.2 Effect of moisture content on Exo-PG production
The moisture level is also one of the most important and major factors having a crucial role in SSF to attain the maximum production of the enzyme. Very low or very high substrate humidity levels tend to reduce the fungal enzyme production due to disruption in growth and less penetration of the microorganisms in the substrate bed, making the nutrients less accessible for fungus to grow. The microorganisms need optimum moisture levels for proper growth to induce and biosynthesize the molecule of interest [4]. Exo-PG production was observed to be reduced when the level of moisture exceeds or decreases the optimum level. At a higher moisture level, the substrate prevents the penetration of the oxygen and proper diffusion of nutrients, while on the other hand, low moisture levels can lead towards inhibition of the enzyme activity and inadequate access of microorganisms to the nutrients [22]. Wheat bran was moistened with different moisture levels for examining the impact of water level on Exo-PG production. The maximum enzymatic activity (198 U/gds) was obtained at 40% moisture content (Fig. 2). Similar results were documented by Castilho (2000), where the highest enzyme activities were achieved at a 40% moisture level.
3.1.3 Effect of medium pH on Exo-PG production
Medium pH is one of the pivotal factors influencing the growth and metabolism of enzymes in the SSF. Every microorganism has an optimal working level of pH and a range of pH for activity and growth [32]. pH was determined using different buffers in the range of 2.0–8.0. The maximum Exo-PG production was achieved at pH 4.0 with an activity of 190 U/gds (Fig. 3). Pagarra (2019) reported that pH 4.0 was the optimal pH for the utmost Exo-PG activity. Comparable results were achieved by Rashmi et al. [42] and reported Exo-PG production by Aspergillus niger at pH optima of 4.0. Nazir et al. [35] demonstrated the optimal activity of the purified enzyme at pH 4.5 with a decrease in the activity at increased pH value. pH optima value contradicts the findings of Amin et al. [6], who reported the optimum pH to be 6.0.
3.1.4 Effect of incubation temperature on Exo-PG production
Incubation temperature is a crucial factor to produce enzymes by SSF because it affects the enzymatic structure and growth of microorganisms (Roses and Guerra et al., 2009; [27]. Temperature is significant for developing different biological processes, as temperature affects enzyme inhibition and protein folding and determines cell death. By keeping the incubation time and pH of medium optimum, SSF was performed at different temperatures ranging from 30 to 60 °C. The maximum activity (208 U/gds) was observed at 40 °C (Fig. 4). Maximum Exo-PG activity found in the present study agrees with the findings of Gomes et al. [23], who determined the maximum activity for Exo-PG production at 37 °C. Comparable results were reported by Anand et al. [10]. However, the present results contradicted Ahmed et al. [3], who reported the maximum activity of Exo-PG at 55 °C. These findings could arise because different fungal strains may yield different enzymatic proteins with some different biochemical behaviors and properties (Adedeji et al., 2019).
3.2 Partial purification of Exo-PG
Exo-PG was partially purified using the ammonium sulfate precipitation technique. Exo-polygalacturonase exhibited increased activity on the addition of solid ammonium sulfate (NH4)2SO4 until a 75% saturation was reached. The supernatant of Exo-PG showed no activity. Therefore, after 75% ammonium sulfate precipitation, enzymatic pallets were collected and dialyzed against the distilled water for salts removal. The summary of the purification of Exo-PG from P. fellutanum is given in Table 1.
3.3 Biochemical characteristic study of Exo-PG
3.3.1 Optimum pH and pH stability
Enzyme’s tertiary and quaternary structures and level of organizations are affected mainly with different pH values because of the involvement of weak forces of attraction. Enzymes possess amphoteric nature and possess both basic and acidic amino acid residues. These amino acids substantially affect overall charge distribution because of the involvement of electrostatic attraction, thereby immensely influencing the enzyme stability, activity, and solubility [35]. The activity of Exo-PG was investigated over a range of pH (2.5–10.0), and the results are shown in Fig. 5a. Activity profile revealed that the Exo-PG from P. fellutanum functioned actively under the acidic pH range and showed the maximum activity at pH 4.0. Likewise, enzymatic stability against various pH values was evaluated using different buffers (within a range of 3.0–10.0) for a period of 24 h. The purified enzyme was found to be stable in the pH range of 4–6 (Fig. 5b). Comparable results were also recorded by Amin et al. (2017a, b. Pagarra (2019) reported that an Exo-PG by A. niger was stable in the pH range of 3–7. Nazir et al. [35]. observed that the enzyme showed greater stability over the pH range of 3.5–5.5, decreasing the activity above or below this range. Likewise, these enzymes are reported stable over a broad pH range with stability over 3.0–9.0 pH for polygalacturonase enzyme from Solanum macrocarpum [19].
3.3.2 Optimum temperature and thermal stability
Temperature is one of the central factors of extreme importance in regulating the activity of the enzyme. Characteristic properties of the proteins, such as the hydrogen bonding and hydrophobic interactions, help resist enzymes’ thermal denaturation [35]. Exo-PG was assayed at various temperatures, i.e., 30, 35, 40, 45, 50, 60, and 70 °C, at its optimum pH. As shown in Fig. 6, Exo-PG exhibited the maximum activity at 40 °C, which was the optimum temperature of the enzyme, while a considerable loss of activity was observed to be at temperatures above 40 °C because of protein denaturation reactions at higher temperatures. Above 70 °C and below 40 °C, the enzyme activity was reduced substantially, more likely because of structural modification of enzyme that disrupts its interaction with the substrate. Pagarra et al. [37] found an optimum temperature for Exo-PG to be 50 °C. Amin et al. [6] found 37 °C as the optimal temperature. Enzymes with a wide temperature stability range are suitable for numerous biotechnological applications, as many biotechnological processes need high-temperature values [12]. To evaluate the thermo-stability of polygalacturonase, the enzyme was incubated at different temperatures between 50 and 70 °C at different time intervals. Residual activity at various temperatures is given in Figs. 7 and S2. Exo-PG obtained from different sources have a temperatures range of 35–55 °C. According to Ázar et al. [13], the maximum activity of the enzyme was observed between 55 and 65 °C.
3.4 Thermodynamic characterization
Thermodynamic characterization is very important for determining the relation between the structure and function of the enzymes. ∆H* and ∆S* are the most significant parameters in structure–activity relation. ∆S* and ∆H* provide a change in entropy and heat to convert the enzyme from native to transition state during the thermal inactivation. Large values of ∆H* are representative of the protein denaturation reaction. The thermo-stabilization of the enzyme is accompanied by a decrease in ∆H* and ∆S* [6]. Exo-PG from P. fellutanum was incubated at a range of 50–70 °C to determine the thermal inactivation (Table 2). For computing different thermodynamic parameters, the energy of the activation (Ea) was calculated by plotting the Arrhenius plot (Fig. 8).
3.4.1 Activation energy (E a )
The activation energy (Ea) was calculated using the Arrhenius plot. In the Arrhenius plot, the slope of Lnk was plotted against 1/T. The activation energy (Ea) for thermal denaturation was observed to be 128.8 kJ/mol. The activation energy value reported by Sharma and Satyanarayana [48] for alkaline pectinases was found to be 74.7 kJ/mol.
3.4.2 Determination of kinetic constants
Michaelis–Menten kinetic constants (Vmax, Km) were evaluated using various concentrations of pectin from 0.2–5 M. Stock solution of pectin (5 M) was prepared using volumetric flask, km was found to be 12.5 mg/mL, and Vmax was 22.22 μmol/mL/min (Fig. 9). Similar findings have been documented by Amin et al. (2017a, b. A Km value was calculated to be 2.3 mg/mL for the Exo-PG from A. niger, while an enzyme obtained from A. tubingensis was observed to exhibit Km value of 3.2 mg/mL [10].
3.5 Effect of urea on Exo-PG activity
As urea destroys hydrophilic sites of enzymes, it denatures protein, which has less hydrophobic amino acid than hydrophilic amino acid residues [6]. The long-term exposure to urea inhibits the catalytic performance drastically. In the present case, the results showed that an activation trend was observed with urea (Fig. 10). Niture [36] documented a deactivating effect of the urea when present in the higher concentrations. However, when urea was at lower concentrations, it had no considerable effect on Exo-PG activity.
3.6 Exo-PG biocatalyst-assisted fruit juice clarification
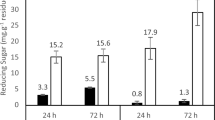
Exo-polygalacturonases are fundamental elements of the fruit juice industry. These are being employed in the clarification of various fruit juices [47]. This application of Exo-PG in fruit juice clarification establishes a huge prospect for bio-industrial exploitation [6]. Treatment of crude enzyme was carried out with peach, apple, and grape juices, and treatment efficiency was estimated by measuring the clarification and viscosity of juices. Treated fruit juice samples were found to have decreased viscosities compared with those possessed by raw juice samples (Fig. 11). Enzymatically treated samples of fruit juices showed more clarity than untreated samples as observed at 660 nm. Pectinaceous material offers a high capacity of holding water and develops a network structure. Pectin material is directed towards the degradation by a pectinolytic enzyme, reducing the pectin’s water holding capacity, subsequently the free water released in the system, thereby decreasing the viscosity level [7, 8]. The viscosities for juices such as apple, grape, and peach were calculated to be 0.87, 1.013, and 0.797 centipoise. A profound increase in the clarity and viscosity reduction was achieved for the fruit juices after treating with Exo-PG. Our results agree with Patil et al. [39], who reported 42%, 50%, 23%, 44%, 28%, 48%, and 18% reductions in viscosities of orange, apple, pomegranate, sapota, grape, banana, and guava fruit juices, respectively, after treatment with a polygalacturonase of Paecilomyces variotii. Similarly, Lu et al. [28] examined the % transmittances at 660 nm in the tangerine, orange, grapefruit, and apple juice clarification and reported 3.51, 4.36, 8.04, and 12.2% increase in transmittance, respectively. Their results showed that the purified polygalacturonase had a more remarkable and noticeable effect on apple juice clarification, where the % transmittance at 660 nm increased from 78.7 to 88.31%. Fruit juice clarification is observed because the pectin degrading enzymes can break the insoluble pectin into soluble galacturonic acid molecules leading towards an increased and improved sense of color and taste of fruit juice products [28].
4 Conclusion
An Exo-polygalacturonase from Penicillium fellutanum was produced using wheat bran and purified and characterized in kinetic and thermodynamic parameters. The purified enzyme exhibited optimum pH and temperature of 4.0 and 40 °C, respectively. The enzyme was found stable over a wide pH range from 4.0 to 6.0. Exposure to urea drastically suppressed the biocatalytic activity of the enzyme. Depending upon the nature of the enzyme, the purified enzyme was further processed towards determining its potential for the clarification of fruit juice. Exo-polygalacturonase from P. fellutanum is an excellent and promising candidate for the applications in the fruit juice industry.
References
Abdulaal WH, Almulaiky YQ (2020) Purification and Thermodynamic Characteristics of an Exo-Polygalacturonase from Trichoderma Pseudokoningii. J Chem Soc Pakistan 42(5)
Adedeji OE, Ezekiel OO (2019) Pretreatment of selected peels for polygalacturonase production by Aspergillus awamori CICC 2040: Purification and application in mango juice. Extraction. Biores Technol Rep 7:100306
Ahmed I, Zia MA, Hussain MA, Akram Z, Naveed MT, Nowrouzi A (2015) Bioprocessing of citrus waste peel for induced pectinase production by Aspergillus niger; its purification and characterization. J Radiat Res Appl Sci 9(2):148–154
Ahmed SA, Mostafa FA (2013) Utilization of Orange Bagasse and Molokhia Stalk for Production of Pectinase Enzyme. Braz J Chem Eng 30(3):449–456
Akhter N, Morshed MA, Uddin A, Begum F, Sultan T, Azad KA (2011) Production of Pectinase by Aspergillus niger Cultured in Solid-state Media. Int J Biosci 1(1):33–42
Amin F, Mohsin A, Nawaz H, Bilal M (2020) Production, thermodynamic characterization, and fruit juice quality improvement characteristics of an Exo- polygalacturonase from Penicillium janczewskii. BBA-Prot Proteom 1868:140379
Amin F, Bhatti HN, Bilal M, Asgher M (2017) Multiple parameter optimizations for enhanced biosynthesis of exo-polygalacturonase enzyme and its application in fruit juice clarification. Int J Food Eng 13(2)
Amin F, Bhatti HN, Bilal M, Asghar M (2017) Improvement of activity, thermo-stability and fruit juice clarification characteristics of fungal exo-polygalacturonase. Int J Biol Macromol 95:974–984
Anand G, Yadav S, Yadav D (2017) Production, purification, and biochemical characterization of an exo-polygalacturonase from Aspergillus niger MTCC 478 suitable for clarification of orange juice. 3 Biotechnology 7:122
Anand G, Yadav S, Yadav D (2017) Purification and biochemical characterization of an exo-polygalacturonase from Aspergillus flavus MTCC 7589. Biocatal Agric Biotechnol 10:264–269
Anisa SK, Ashwini S, Girish K (2013) Isolation and screening of Aspergillus spp For pectinolytic activity. Electron J Biol 9(2):37–41
Antranikian G (2009) Extremophiles and biotechnology. In: Encyclopedia of life sciences (ELS). John Wiley & Sons, Ltd., Chichester
Ázar RLSL, da Morales LM, Alfenas GPM, Falkoski DL, Alfenas RF, Guimarães VM (2020) Apple juice clarification by a purified polygalacturonase from Calonectria pteridis. Food Bioprod Process. 119:238–245
Bhatti HN, Asgher M, Abbas A, Nawaz R, Sheikh MA (2006) Studies on kinetics and thermostability of a novel acid Invertase from Fusarium solani. J Agric Food Chem 54:4617–4623
Bhatti HN, Rashid MH, Nawaz R, Perveen R, Jabbar A, Asgher M (2007) Purification and characterization of a novel glucoamylase from Fusarium solani. Food Chem 103:338–343
Bilal M, Iqbal HM (2020) State-of-the-art strategies and applied perspectives of enzyme biocatalysis in food sector—current status and future trends. Crit Rev Food Sci Nutr 60(12):2052–2066
Bradford M (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Castilho LR, Medronho RA, Alves TLM (2000) Production and extraction of pectinases obtained bu solid state fermentation of agroindustrial residues with Aspergillus niger. Bioresour Technol 71:45–50
Chinedu SN, Dayo-Odukoya OP, Iheagwam FN (2017) Partial purification and kinetic properties of polygalacturonase from Solanum macrocarpum L. fruit. Biotechnology 16:27–33
Cipolatti EP, Pinto MCC, Henriques RO, da Silva Pinto JCC, de Castro AM, Freire DMG, Manoel EA (2019) Enzymes in green chemistry: the state of the art in chemical transformations. Adv Enzyme Technol 137–151
de Marco M, Souza RC, da Silva LM, de Bastos G, Dallago RM, Valduga E, ..., Zeni J (2020) In Situ and Contact Immobilization/Adhesion of Penicillium brasilianum in Polyurethane Foam for Production of Exo-Polygalacturonase. Industr Biotechnol 16(4), 241–246
El-Shishtawy RM, Mohamed SA, Asiri AM, Gomaa AM, Ibrahim IH, Al-Talhi HA (2014) Solid Fermentation of Wheat Bran for Hydrolytic Enzymes Production and Saccharification Content by a Local Isolate Bacillus megatherium. BMC Biotechnol 14(29):1–8
Gomes J, Zeni J, Cence K, Toniazzo G, Treichel H, Valduga E (2011) Evaluation of Gummadi, S. N. and T. Panda. 2003. Purification and biochemical properties of microbial pectinases - A review. Process Biochem 38:987–996
Jayani RS, Saxena S, Gupta R (2005) Microbial pectinolytic enzymes: A review. Process Biochem 40:2931–2944
John J, Kaimal KKS, Smith ML, Rahman PKSM, Chellam PV (2020) Advances in upstream and downstream strategies of pectinase bioprocessing: a review. Int J Biol Macromol 162:1086–1099
Kashyap DR, Vohra PK, Chopra S, Tewari R (2001) Applications of pectinases in commercial sector: a review. Biores Technol 77:215–227
Kumar YS, Varakumar S, Reddy OVS (2010) Production and optimization of polygalacturonase from mango (Mangifera indica L.) peel using Fusarium moniliforme in solid state fermentation. World J Microbiol Biotechnol 26:1973–1980
Lu X, Lin J, Wang C, Du X, Cai J (2016) Purification and characterization of exo-polygalacturonase from Zygoascus hellenicus V25 and its potential application in fruit juice clarification. Food Sci Biotechnol 25(5):1379–1385
Majumder K, Paul B, Sundas R (2020) An analysis of exo-polygalacturonase bioprocess in submerged and solid-state fermentation by Pleurotus ostreatus using pomelo peel powder as carbon source. Journal of Genetic Engineering and Biotechnology 18(1):1–8
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31(3):426–428
Mrudula S, Anithraraj R (2011) Pectinase production in solid-state fermentation by Aspergillus niger using orange peel as substrate. Glob J Biotechnol Biochem 6(2):64–71
Munir N, Asgher M, Tahir IM, Riaz M, Bilal M, Shah SM (2015) Utilization of agro-wastes for production of ligninolytic enzymes in liquid state fermentation by Phanerochaete chrysosporium-IBL-03. Int J Chem Biochem Sci 7:9–4
Naveed M, Nadeem F, Mehmood T, Bilal M, Anwar Z, Amjad F (2020) Protease—A versatile and ecofriendly biocatalyst with multi-industrial applications: an updated review. Catalysis Lett 1–17
Nawaz A, Sameer M, Akram F, Tahir SF, Arshad Y, Haq IU, Mukhtar H (2021) Kinetic and thermodynamic insight of a polygalacturonase: A biocatalyst for industrial fruit juice clarification. Revista Mexicana de Ingeniería Química 20(2):1029–1045
Nazir Z, Ijaz S, Gul R, Saleem M (2019) Purification and characterization of an acidic polygalacturonase from grapes and its potential to juice quality. Pakistan J. Zool 51(4):1387–1402
Niture KS (2008) Comparative biochemical and structural characterizations of fungal Polygalacturonases. Biologia 63:1–19
Pagarra H, Rahman RA, Illias RM, Ramli NA (2019) Screening of factors influencing exo-polygalacturonase production by Aspergillus niger atcc 120120 using two-level fractional factorial design. Jurnal Teknologi (Sciences & Engineering) 81(6):73–80
Patidar MK, Nighojkar S, Kumar A, Nighojkar A (2020) Production of polygalacturonase using Carica papaya peel biowaste and its application for pomegranate juice clarification. Environ Sustainab 3(4):509–520
Patil NP, Patil KP, Chaudhari BL, Chincholkar SB (2012) Production, purification of exo-polygalacturonase from soil isolate Paecilomyces variotii NFCCI 1769 and its application. Indian J Microbiol 52(2):240–246
Prajapati J, Dudhagara P, Patel K (2021) Production of thermal and acid-stable pectinase from Bacillus subtilis strain BK-3: optimization, characterization, and application for fruit juice clarification. Biocatalysis and Agricultural Biotechnology, 102063. production and characterization of polygalacturonase by Aspergillus niger ATCC9642
Qamar SA, Asgher M, Bilal M (2020) Immobilization of alkaline protease from Bacillus brevis using Ca-alginate entrapment strategy for improved catalytic stability, silver recovery, and dehairing potentialities. Catal Lett 150:3572–3583
Rashmi R, Murthy SK, Sneba S, Syama A, Radhika VS (2008) Partial Purification and Biochemical Characterization of Extracellular Pectinase from Aspergillus niger Isolated from Groundnut Seeds. J Appl Biosci 9(1): 378-384. 20093254516
Riaz M, Perveen R, Javed MR, Nadeem HU, Rashid MH (2007) Kinetics and thermodynamics of a novel glucoamylase from Humicola sp. Enzyme Microbiol Technol 41(5):558–564
Roses RP, Guerra NP (2009) Optimization of Amylase Production by Aspergillus niger in Solid-state Fermentation using Sugarcane Bagasse as Solid Support Material. World J Microbiol Biotechnol 25:1929–1939
Saeed S, Bibi I, Mehmood T, Naseer R, Bilal M (2020) Valorization of locally available waste plant leaves for production of tannase and gallic acid by solid-state fermentation. Biom Convers Bioref 1–8
Sandri IG, Lorenzoni CMT, Fontana RC, Silveira MM (2013) Use of pectinases produced by a new strain of Aspergillus niger for the enzymatic treatment of apple and blueberry juice. LWT-Food Sci Technol 51(2):469–475
Sandri IG, Fontana RC, Barfknecht DM, da Silveira MM (2011) Clarification of fruit juices by fungal pectinases. LWT Food Sci Technol 44(10):2217–2222
Sharma DC, Satyanarayana T (2020) Thermostable and alkalistable exopolygalacturonase of Bacillus pumilus dcsr1: characteristics and applicability. Int J Biol Macromol 164:3340–3348
Tu T, Meng K, Bai Y, Shi P, Luo H, Wang Y, Yao B (2013) High-yield production of a low-temperature-active polygalacturonase for papaya juice clarification. Food Chem 141(3):2974–2981
Uzuner S, Cekmecelioglu D (2021) Biochemical characterization and stability of Bacillus subtilis polygalacturonase produced using hazelnut shells by submerged fermentation. Biocatal Biotransform 1–6
Vilar DS, Fernandes CD, Nascimento VR, Torres NH, Leite MS, Bharagava RN, ... Ferreira LF (2020) Hyper-production optimization of fungal oxidative green enzymes using citrus low-cost byproduct. J Environ Chem Eng 105013
Zhan S, Bilal M, Zdarta J, Cui J, Kumar A, Franco M, ... Iqbal HM (2020) Biopolymers and nanostructured materials to develop pectinases-based immobilized nano-biocatalytic systems for biotechnological applications. Food Res Int 109979
Zheng Z, Shetty K (2000) Solid state production of polygalacturonase by Lentinus edodesws using fruit processing wastes. Process Biochem 35:825–830
Acknowledgements
The authors are grateful to the Government College Women University, Faisalabad, Pakistan, for financial support under in-house research grant program.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Amin, F., Arooj, T., Nazli, ZiH. et al. Exo-polygalacturonase production from agro-waste by Penicillium fellutanum and insight into thermodynamic, kinetic, and fruit juice clarification. Biomass Conv. Bioref. 13, 11141–11151 (2023). https://doi.org/10.1007/s13399-021-01902-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-021-01902-2