Abstract
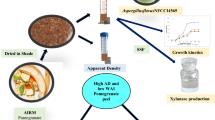
The present study focuses on utilization of papaya peel for polygalacturonase production in solid-state fermentation (SSF). Papaya peel was screened as optimum solid substrate and valorized under SSF for polygalacturonase production by Aspergillus niger AN07 and the effect of different fermentation parameters viz. fermentation time, particle size, moisture content and agitation speed on the enzyme production was investigated. Two fermentation variables viz. moisture content and fermentation time have been identified to significantly affect polygalacturonase production as predicted using Plackett–Burman Design (PBD). It was further optimized by Response Surface Methodology (RSM) using Rotatory Central Composite Design (RCCD). An overall 5.4-fold increase (264.20 U/g dried substrate) in enzyme production was achieved after optimization at fermentation time 144 h and moisture content 90%. The results of RSM showed that the model was in good agreement with experimental results with R2 = 99.6% (P < 0.05). A. niger AN07, A. tubingensis MP30, A. fumigatus M1 and A. sydowii indicated a high growth rate of 0.55, 0.52, 0.39 and 0.25 mm/h, respectively on the optimized solid substrate in SSF. Native PAGE and Zymogram study showed predominant presence of polygalacturonase in the purified preparation. The purified polygalacturonase enzyme significantly increased pomegranate juice clarification by 3.6-fold and prevented haze formation during storage conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Solid State Fermentation (SSF) has several economic advantages that have demanded the researchers’ interest in recent years for the production of industrially important enzymes. The SSF process has been reported to be the most suitable because of its high productivity, easy enzyme recovery and cost effectiveness for the production of fungal enzymes (Viniegra-Gonzále et al. 2003; Pandey et al. 2000). The agro-industrial residues are not only economical solid substrates but also suitable for extracellular enzyme production due to low cost of harvesting and thus are considered best suited for production of enzymes in SSF (Mahmoodi et al. 2019). Pectin is the integral part of middle lamella of plant cell wall (Caffall and Mohnen 2009). It is composed of D-galacturonic acids joined together by α-1, 4 glycosidic linkages, in which a few hydroxyl groups are methylated. Pectin methylesterase, polygalacturonase and pectin lyase completely degrade pectin, releasing galacturonic acid units (Combo et al. 2012). Polygalacturonase is a pectinolytic enzyme that hydrolyses pectic substances randomly and produces oligosaccharides. This enzyme has been reported to be produced by higher plants and microorganisms including bacteria and fungi (Uzuner and Cekmecelioglu 2015; Patidar et al. 2018; Aggarwal et al. 2020). Polygalacturonase is mainly used in beverage industries for extraction and clarification of fruit and vegetable juices. Additionally, it has important role in tea and coffee industry, textile industry, animal feed industry, treatment of waste water, protoplast fusion etc. (Jayani et al. 2005; Nighojkar et al. 2019; Amin et al. 2019).
Pectinolytic enzymes have been produced using agro-industrial wastes like lemon peel, orange peel, strawberry pomace, lemon pulp, orange bagasse, apple pomace, banana peel, grape skin, grape pomace, sunflower head, wheat bran, rice bran, soy bran etc. in SSF (Patidar et al. 2018; Amin et al. 2019). Several fungal sources have been reported earlier for production, purification and characterization of polygalacturonase including Mucor flavus, Rhizopus oryzae, Sclerotium rolfsii, M. circinelloides, A. carbonarius, A. niger, A. awamori, Penicillium sp., Bispora sp., Neosartorya fischeri etc. (Amin et al. 2019; Nighojkar et al. 2019).
Papaya peel has been utilized earlier for extraction of nutritionally valuable compounds and proteases (Chaiwut et al. 2010; Parniakov et al. 2014). However, papaya commonly grown in tropical America and Asian countries (OECD 2005) has not been used earlier for polygalacturonase production in SSF. In India, according to an estimate, 5,382,000 metric tons of papaya is produced every year (IHD 2013). It is commonly consumed as fruit and is also used in juice preparation, salad preparation, cosmetics and medications (Nighojkar et al. 2019).
It is important to note that about 20–25% of papaya fruit is its peel which is discarded as organic pollutant (Koubala et al. 2014). In present study, the utilization of papaya peel for production of polygalacturonase in SSF shall help papaya processing units to curb the pollution problems and increase their income instead.
The current study was carried out to optimize polygalacturonase production by A. niger AN07 in SSF. The SSF variables viz. fermentation time, particle size, moisture content and agitation speed were screened using PBD and significant variables were optimized using RCCD. The purified enzyme was successfully used for clarification of pomegranate juice and inhibition of haze formation.
Materials and methods
Isolation and identification of fungi
A total of 32 samples such as soil from decaying matter, soil from fruit processing sites, compost soil, agriculture soil were collected from different locations of Indore, India and screened for polygalacturonase production using Potato Dextrose Agar (PDA) medium, pH 5.6 containing 1% pectin (Patidar et al. 2016). The fungal isolate S1 used in this study was isolated from Indore region (latitude:longitude:altitude -22.685 N:75.8856 E:553 m). The fungal isolate S1was sequenced for Internal Transcribed Spacer (ITS) region at National Fungal Culture Collection of India, Agarkar Research Institute, Pune, India. The DNA sequence data obtained from ABI3100 automated DNA sequencer was aligned with publically available sequences to check identity. MEGH5 software was used for Maximum Likelihood Tree preparation (Tamura et al. 2011). The isolate was identified as A. niger on the basis of molecular and morphological studies.
A. niger AN07 maintained on PDA slants at 30°C for 4 days was used for inoculum development. Five millilitre of Tween 80 (0.1%) was added to slants and scrapped spores were filtered through sterile glass wool. The spore count was maintained to 1 × 106 spores/ml and used as inoculum.
A. sydowii (Accession No. JF831015.1), A. tubingensis MP30 (KT945096.1) and A. fumigatus M1 (soil isolate) used in this study were maintained in the PI laboratory at Maharaja Ranjit Singh College of Professional Sciences, Indore, M.P., India.
Screening and characterization of solid substrate
Sun-dried wheat bran, corn cob, groundnut shell, orange peel, rice bran, sugarcane bagasse and papaya peel were screened as solid substrates for polygalacturonase production. Autoclaved solid substrate (10 g) was inoculated with 1 ml of spore suspension in a 250 ml Erlenmeyer flask. The initial moisture content was maintained to 80% (v/w) using distilled water and flask was incubated for 5 days.
The total carbohydrate content of papaya peel was estimated using phenol–sulfuric acid method (Kumar et al. 2012a). Reducing sugar in the solid papaya substrate was estimated by Nelson-Somogyi method using D-glucose as a standard. Moisture content of papaya peel was analyzed using Karl Fischer moisture titration method. Total nitrogen content in the dried papaya peel was estimated using Kjeldahl’s method (Kumar et al. 2012a). The ash content was analyzed using combustion method (AOAC 1970). Crude lipid content present in the peel was estimated by solvent extraction method. Pectin content was determined by microwave assisted extraction method (Maran and Prakash 2015).
Pretreatment of papaya peel and scanning electron microscope (SEM) analysis
The dried papaya peel was treated with 1:50 (w/v) 0.1 N HCl and 0.1 N NaOH in glass beaker separately and incubated for 15 min, 30 min and 45 min. The papaya peel was washed repeatedly with excess of distilled water until pH of the filtrate reached to neutral. The treated peel was filtered and dried at 45 °C for 5–7 h in oven.
Untreated papaya peel (10 g) in triplicate was treated with 0.1 N HCl and 0.1 N NaOH and each was inoculated with 1 × 106 spores/ml suspension and incubated for 5 days to study the effect of pretreatment. The structural changes in the treated and untreated papaya peel were observed using SEM type JEOL JSM 5600 at UGC-DAE Consortium, Indore, India . The samples were coated with 5 nm gold particles using Quorum Q150TS.
Enzyme extraction and estimation
Enzyme was extracted from solid medium by adding distilled water in ratio of 1:10 (w/v) and incubated at 25°C for 30 min at 100 rpm. Afterwards, the homogeneous medium was centrifuged at 10,000×g for 20 min and supernatant used for polygalacturonase assay.
Polygalacturonase assay was performed in the crude enzyme extract using Nelson (1944) and Somogyi (1952) method. One millilitre polygalacturonic acid (0.1% w/v) prepared in 0.1 M acetate buffer, pH 5, was incubated with 10 µl enzyme and 990 µl distilled water for 20 min at 55°C. The reducing sugar equivalent liberated as a result of enzyme activity was calculated using the standard curve of galacturonic acid. One unit (U) of the enzyme activity was defined as the amount of enzyme that catalyses the release of one µmol of galacturonic acid equivalent per minute under the standard enzyme assay conditions. Polygalacturonase units are expressed as Unit per gram dried substrate (U/gds). Lowry’s method (Lowry et al. 1951) was used to calculate total soluble protein using bovine serum albumin as the standard.
Optimization of SSF
Screening of important factors using Plackett–Burman Design
Plackett–Burman Design (PBD) was performed using dried papaya peel to screen important variables for polygalacturonase production (Plackett and Burman 1946). Fermentation variables viz. fermentation time, particle size, moisture content and agitation speed were tested using the PBD. Three levels (+ 1, 0, − 1) were considered for each constituent (Table 1) with enough difference in both variables to detect any significant effect, if exists. Total 15 runs were designed with three centre point values using MINITAB 16.
Optimization of significant variable using Rotatory Central Composite Design
Rotatory Central Composite Design (RCCD) was adopted to optimize the significant variables (screened in PBD) for polygalacturonase production by A. niger. The independent variables screened in the PBD were applied in RCCD using statistical software Design Expert 11. The variables, moisture content and fermentation time were tested in set of 13 experiments at 5 levels (− α, − 1, 0, + 1, + α). The experimental enzyme activity in triplicate was recorded with respect to the actual and coded values (Table 2). A second order polynomial equation (Eq. 1) was used to analyze the polygalacturonase production by multiple regression procedure.
where Y is the predicted response; βo is the offset term; βi is the linear effect; βii is the squared effect; βij is the interaction effect, Xi and Xj are independent variables.
Model validation
The derived model was validated by additional trials carried out in triplicate at the optimal fermentation conditions.
Suitability of optimized medium for polygalacturonase production
The suitability of papaya peel as medium for polygalacturonase production by Aspergillus sp. was assayed in SSF under statistically optimized conditions. The water absorption index (WAI) of unprocessed papaya peel was determined (Orzua et al. 2009) and expressed as gram swollen gel/gram dry weight. The growth rate of each A.niger AN07, A. fumigatus M1, A. sydowii and A. tubingensis MP30 was examined by inoculating 0.1 ml of 1 × 106 spore/ml of each on 5 g papaya peel in a petriplate. An initial moisture content of 90% was maintained by adding sterile distilled water and incubated for 144 h at 30°C. The fungal growth rate and polygalacturonase production were measured.
Native PAGE and Zymogram study
The enzyme produced in SSF by A. niger AN07 was precipitated using 90% ammonium sulphate and desalted using Sephadex G25. The desalted enzyme was purified using DEAE-cellulose Ion Exchange Chromatography (IEC). The bound protein was eluted using 0–1 M NaCl gradient prepared in 75 mM phosphate buffer, pH 7.0. The fractions exhibiting enzyme activity were pooled and concentrated by reverse osmosis using solid sucrose.
Native PAGE (Laemmli 1970) was used to check homogeneity of the purified enzyme. It was also used for polygalacturonase activity staining by incorporating polygalacturonic acid in the gel in which a clear zone was obtained upon addition of 1% CTAB.
Application in fruit juice clarification
Pomegranate fruits were collected from local fruit market of Indore, India. Pomegranate juice was prepared using lab mixer and filtered using 10-mesh sieve. The different units of purified enzyme (0, 10, 20, 40, 80 U) were added to the extracted pomegranate juice. The juice was incubated at 30°C for 1 h in water bath. The samples were then heated at 80°C for 1 min to stop the reaction and centrifuged at 15,000×g for 10 min. The clarification of the juice was evaluated by estimating % transmission at 650 nm (%T650) using Thermospectronic UV1 spectrophotometer.
The haze formation in the juice was performed by the method described by Cerreti et al. (2016). Twenty five millilitre each of treated and untreated pomegranate juice was taken separately in 50 ml tubes. Gelatin (0.3%) was added to treated juice only and both the tubes were incubated for 2 weeks at low temperature. The turbidity of the juice was measured after every 24 h interval using methods described by Dongare et al. (2013) and represented as Turbidity Unit (TU).
Results and discussion
Identification of fungal isolate
In the screening step, a total of 57 fungi were isolated from 32 samples of decaying fruit waste, soil from different sites, compost pits and agricultural waste materials. Out of 57 fungal isolates, fungal isolate S1 exhibited maximum polygalacturonase activity. The fungal isolate S1 exhibiting maximum polygalacturonase production was isolated from soil of fruit processing area in Indore. The selected fungal isolate S1 showed 100% sequence similarity with A. niger strain IHEM 22432. The ITS sequence of A. niger AN07 was submitted to NCBI GenBank and accession number assigned was KR908781.1.
Fruit and agricultural wastes have been reported earlier as potential source for isolation of high polygalacturonase producers.
Aspergillus sp. viz. A. pulverulentus (Abd El-Rahim et al. 2020), A. niger (Pagarra et al. 2019; Li et al. 2020), A. aculeatus (de Carvalho Silva et al. 2019), A. tubingensis (Huang et al. 2019), A. fumigatus (Zehra et al. 2020), A. awamori (Marzo et al. 2018), A. sojae (Demir and Tari 2014), A. sydowii (Singh and Mandal 2012) and A. carbonarius (Nakkeeran et al. 2011) have been widely used for polygalacturonase production. The fungal isolates other than Aspergillus sp. such as Trametes hirsuta and Phanerochaete sp. (Vibha and Negi 2018), Penicillium janthinellum (Pagnonceli et al.2019) and Schizophyllum commune (Mehmood et al. 2018) have been also used for polygalacturonase production. Few yeast such as Wickerhanomyces anomalus (Maidana et al. 2019), Yamadazyma. sp. (Daskaya-Dikmen et al. 2018) and Geotrichum candidum (Ejaz et al. 2018) have also been reported recently for polygalacturonase production. The phylogenetic tree was constructed on the aligned datasets using the Maximum Likelihood implemented in the program MEGA5 (Supplementary Fig. 1). The tree was constructed in silico using evolutionary model based on nucleotide substitutions.
Screening of solid substrate and SEM analysis
Tropical agro-industrial crops and residues have mostly been chosen as suitable substrates for polygalacturonase production since they are abundantly available in developing countries like India.
In the present study, out of eight substrates (viz. wheat bran, rice bran, corn cob, sugarcane bagasse, orange peel, groundnut shell and papaya peel), papaya peel maximally yielded polygalacturonase (48.66 ± 1.4 U/gds) by A.niger AN07 at 80% moisture level and 30ºC. Orange peel was found to be the second prime substrate for polygalacturonase production by A. niger AN07 with 12.91 ± 0.91 U/gds at 30°C. However, sugarcane bagasse and wheat bran exhibited polygalacturonase activity 9.38 ± 0.7 U and 6.74 ± 0.5 U/gds, respectively. A very low polygalacturonase activity 2.45 ± 0.5 U, 1.55 ± 0.4 U and 1.29 ± 0.3 U/gds was shown when rice bran, corncob and groundnut shell, respectively were used as solid substrate.
Papaya peel is an enriched source of all nutrients required for fungal growth and provides suitable environment for polygalacturonase production due to high percentage of pectin, reducing sugars and protein content (Table 3). The dried papaya peel includes high percentage of pectin (25.11%) that is generally essential for polygalacturonase production. The penetrative hyphae of A. niger AN07 may have utilized the high carbohydrate and protein of papaya peel for proper growth and production of pectinolytic enzymes.
The negligible amount of pectin content in wheat bran (1.68%), groundnut shell (1.0%), rice bran (0%), corncob (0%) and sugarcane bagasse (0%) may decrease the growth of A. niger AN07 and therefore, lesser amount of polygalacturonase is produced in the medium (Sabry 1993; Paulchamy 2007; Alonso Pippo et al. 2011). Earlier reports have also demonstrated poor polygalacturonase production using rice bran, corncob and groundnut shell (Pagnonceli et al. 2019). However, wheat bran has been used earlier for polygalacturonase production by bacteria B. pumilus (Kaur et al. 2019).
Various fruit and agro-industrial wastes viz. banana peel (Zehra et al. 2020), passion fruit peel (Pagnonceli et al. 2019; de Carvalho Silva et al. 2019), Nephrolepis biserrata leaves (Pagarra et al. 2019), orange peel (Nighojkar et al. 2006; Marzo et al. 2018; Adedeji et al. 2019), sweet lime (Mehmood et al. 2018), mango peel (Kumar et al. 2012b), mixture of grape pomace and orange peel (Diaz et al. 2012), lemon peel (Maller et al. 2011) and sunflower head (Patil and Dayanand 2006) have been used in SSF or SmF for polygalacturonase or pectinase production due to their high pectin and other nutrient values.
Papaya peel treated with 0.1 N HCl and 0.1 N NaOH exhibited low polygalacturonase production as compared to untreated papaya peel (Table 4). The SEM analysis indicates that untreated papaya peel may have preserved large amounts of pectin which remains intact in the solid structure (Fig. 1). The untreated solid substrate could stimulate the polygalacturonase production (48.66 U/gds) compared to acid–alkali pretreated substrate, where hemicellulose layer in the papaya peel might have been lost along with the pectin structure, resulting in lesser enzyme production. The treatment time with acid–alkali also affects the enzyme production in SSF. Similar effect has been reported upon drying of pretreated lignocelluloses that can cause a collapse in substrate pore structure, resulting in decreased enzymatic hydrolysis (Gervais and Molin 2003). Moreover, the reduction in concentration of carbohydrates such as glucose, fructose and sucrose by acid–alkali pretreatment, which is due to their solubility in acid or alkali may affect the fungal growth and enzyme production (Acuna-Arguelles et al. 1994). Additionally, chemical treatment of substrate also reduces protein content, which may affect the growth of fungi and thereby lowers the polygalacturonase synthesis (Adedeji and Ezekiel 2019; Amin et al. 2020). Therefore, untreated papaya peel was further used for polygalacturonase production.
SEM micrographic analysis of papaya peel: (a) untreated papaya peel; (b) papaya peel treated with 0.1 N HCl^; (c) papaya peel treated with 0.1 N NaOH^; (d) untreated papaya peel degraded by Aspergillus niger #. ^Treatment time and temperature: 10 min at 25°C. #Aspergillus niger growth conditions: temperature 30°C, time 120 h, moisture content 80%
Statistical optimization
Screening of important factors using PBD
Environmental factors have always been of great interest to researchers for low cost production system (Uzuner and Cekmecelioglu 2015; Tari et al. 2007). PBD was employed to evaluate the factors which significantly affect the polygalacturonase production by A. niger AN07. Variation in two factors i.e. moisture content and fermentation time was found to be most significant in affecting polygalacturonase production (Fig. 2). Based on the results from PBD, moisture content and fermentation time study in SSF was carried out by RSM using RCCD. Maidana et al. (2019) also tried PBD to determine significant factors and reported pectin, calcium and yeast extract as significant factors for maximum polygalacturonase production. Out of 10 factors, Kavuthodi and Sebastian (2018) screened three factors namely yeast extract, calcium chloride and inoculum size to optimize maximum pectinase production and further studied using CCD. PBD is routinely used to determine significant fermentation factors. However, Ejaz et al. (2018) showed insignificant effect of PBD on polygalacturonase production by immobilized yeast G. candidum. This indicates that PBD may be insufficient to determine significant factors. Therefore, further study for optimization of parameters is desired. However, Vibha and Negi (2018) preferred One Factor at a Time (OFAT) approach to screen significant factors for pectinase production and their concentration was further optimized using Evolutionary Optimization factorial design (EVOP).
Optimization of significant variable using RCCD
RSM is most popular method based on mathematical and statistical modeling. It is used to optimize process parameters to enhance the yield without affecting cost of the product (Bas and Boyacl 2007). The moisture content and fermentation time, screened statistically as most significant variables for polygalacturonase production by A. niger AN07 under SSF were optimized using RCCD. Experimental design of the RCCD and results of the experiments are shown in Table 2. Result of ANOVA (Table 5) was used for analysis of regression coefficient. The second order polynomial Eq. (1) was used to fill the coded values of independent variables, moisture content (A) and fermentation time (B). The polygalacturonase activity (Y) in each trial was calculated as the average of triplicates. The second order polynomial obtained was represented as follows:
The F-value of 367.93 indicates that the model is significant. In this case, both moisture content and fermentation time are significant model terms. Kavuthodi and Sebastian (2018) also used three level factorial design for optimization of yeast extract, calcium chloride and inoculum size for maximum pectinase production. However, Pagarra et al. (2019) used two level fractional factorial design with 38 experimental runs and six centre points for optimization of exo-polygalacturonase production. On the basis of P-value, incubation time, moisture content, pectin concentration and temperature were reported as significant factors.
The "Lack of Fit F-value" of 483.85 implies that the Lack of Fit is significant. There is only a 0.01% chance that this large "Lack of Fit F-value" could occur due to noise. The "Predicted R-Squared" of 0.97 is in reasonable agreement with the "Adjusted R-Squared" of 0.99; i.e. the difference is less than 0.2.
Moreover, closer the R2 value to 1.0, the model is authentic and exhibits better predicted response (Handa et al.2016). The results are in good agreement with other reports. Recently, Thite et al. (2020) predicted and adjusted R2 values above 0.77 and 0.92, during the optimization of wheat bran and citrus peel respectively, for enzyme production using CCD.
The Coefficient of Variation (CV) is a measure of residual variation of the data relative to the size of the mean. Usually, higher the value of CV, lower the reliability of experiment (Reddy and Saritha 2016). In the present case, a low value of CV (2.72%) indicated a greater reliability of the experiment. This is in accordance with earlier reports (Raol et al. 2015; Patidar et al. 2016). However, reasonably high CV value (> 4%) is reported by Handa et al. (2016) in CCD model used to optimize pectinase production by Rhizopus sp. C4.
The maximum predictable response for polygalacturonase production based on regression equation was found to be 258.30 U/gds. The optimum value for moisture content and fermentation time was found to be 90% and 144 h, respectively. The R2 value of 99.60% indicates the appropriate prediction of moisture content and fermentation time for maximum polygalacturonase production. OFAT approach used to determine the optimum value doesn’t give idea about the interaction between different factors. Besides, OFAT approach is time consuming and some times give pseudo results (Gupta et al. 2008).
The effect of moisture content and fermentation time on polygalacturonase production as in Fig. 3(a, b) shows increased polygalacturonase yield with increasing moisture content of up to 90% and fermentation time 144 h. For the production of metabolites using SSF, moisture is a crucial factor. Higher moisture content (90%) decreases the polygalacturonase yield possibly due to reduced hyphal growth. Most fungi show optimum growth in the range of 40 to 120% moisture (Blandino et al. 2002; Castilho et al. 2000; Demir and Tari 2014; Heerd et al. 2012; Ruiz et al. 2012). The growth also depends on water absorption capacity of the substrate (Orzua et al. 2009). The effect of moisture content on enzyme production is well established. The low moisture content leads to decrease in enzyme production due to less availability of nutrients and slow rate of gas exchange during fermentation (Gervais and Molin 2003).
Fermentation time significantly influences product formation. Polygalacturonase yield initially increases as the fermentation time increases, but decreases beyond 144 h. The reported optimum time for polygalacturonase production varies between 4 and 7 days (Botella et al. 2005; Demir and Tari 2014; Heerd et al. 2012). Handa et al. (2016) used CCD to optimize fermentation time of 7 days for maximum pectinase production by Rhizopus sp.
The validation results show that the maximum polygalacturonase yield (264.20 ± 9.32 U/gds) corresponds to the value predicted by the model (258.3 U/gds). The RCCD model significantly affected the enzyme production and a 5.4-fold increase in yield of polygalacturonase production was obtained. Tari et al. (2007) reported 74% increase in yield of polygalacturonase enzyme by A. sojae (ATCC 20235) in submerged fermentation. Similarly, Uzuner and Cekmecelioglu (2015) used RSM and reported 2.7-fold increase in pectinase production by B. subtilis. Vibha and Negi (2018) reported EVOP to optimize substrate, pH and temperature for maximum pectinase and laccase production, and reported 247 U/gds and 250 U/gds of enzyme production, respectively.
Papaya peel as growth medium for Aspergillus sp.
The 7.9 WAI of papaya peel indicated higher water absorption capacity. This is favourable for fungal growth and exhibited high growth rate. In present study, A. niger AN07 exhibited highest growth rate of 0.55 mm/h (Table 6) with a short lag phase and extended logarithmic phase when cultivated on papaya peel at 90% moisture content and 30°C (Fig. 4). Similar pattern of growth was shown by A. tubingensis MP30 with 0.52 mm/h growth rate. However, the extended lag phase of about 40 h was exhibited by A. fumigatus M1 and A. sydowii under similar conditions with growth rate 0.39 and 0.25 mm/h, respectively. Orzua et al. (2009) reported 0.44 mm/h growth rate of A. niger Aa-20 on orange peel containing 25 g/l glucose at 70% moisture content. The polygalacturonase production by A. niger AN07, A.tubingensis MP30, A. fumigatus M1 and A. sydowii corresponded to their growth rate and exhibited 264.20, 237.31, 155.90 and 132.63 U/gds polygalacturonase production, respectively (Table 6).
In present study, dried papaya peel alone is used as solid substrate in SSF which exhibited high nutritional value in proximate analysis (Table 3). High pectin content 25.11% of papaya peel promotes the polygalacturonase production by Aspergillus sp. The pH of the hydrolyzed papaya peel was found to be 5.4, which is favourable for most polygalacturonase producing fungi (Jayani et al. 2005). In SSF, fungi grow well on complex and porous solid substrates in near absence of free water in fermentation medium. Most of the fruit and food waste such as dried lemon peel, orange peel, coconut husk etc. exhibit high water absorption capacity and require high moisture content in SSF; whereas corncob, sugarcane bagasse, groundnut shell etc. require low moisture content in SSF due to low WAI.
The percentage of moisture content varies from 40 to 120% due to different water absorption capacity of various solid substrates (Demir and Tari 2014; Heerd et al. 2012; Ruiz et al. 2012). Hence, 90% of moisture content used in this study provides water free condition and desired humidity in the process, and therefore, good growth rate of Aspergillus sp. is observed. The considerable growth rate of Aspergillus sp. in papaya peel indicated the suitability of optimized fermentation medium for industrial enzyme production.
Native PAGE and Zymogram study
Native PAGE analysis and zymogram studies revealed that the enzyme was purified to near homogeneity (Supplementary Fig. 2A). Polygalacturonase hydrolyzed polygalacturonic acids present in the gel produced a zone of clearance when overlaid with CTAB (Supplementary Fig. 2B). A single transparent zone in gel exhibited single form of polygalacturonase enzyme produced by A. niger AN07. However, it has been reported that isoforms of extracellular enzyme depends on the fermentation conditions (Silva et al. 2007). Purified polygalacturonase was used further for pomegranate juice clarification.
Application of enzyme
In present study, pomegranate juice was treated with different concentrations of purified polygalacturonase enzyme (0, 10, 20, 40, 80 U) produced by A. niger AN07 in SSF. The increase in polygalacturonase concentration from 0 to 80 U enhanced the pomegranate fruit juice clarification from 6.8% to 24.6% at 30°C. The pomegranate juice contains 1.4% pectin and it is responsible for viscosity and turbidity of fruit juice even in low concentration (El-Nemr et al. 1990; Löfgren and Hermansson 2007). The enzymatic treatment reduced the turbidity of the juice due to hydrolysis of low methoxyl pectin by polygalacturonase enzyme. The reduction in turbidity is because of the hydrolysis of pectin which leads to flocculation of the pectin–protein complex (Lee et al. 2006). Haze formation in treated juice was evaluated by addition of 0.3% gelatin. The initial turbidity of the juice was 1.6 TU which insignificantly increased up to 1.7 TU after incubation with polygalacturonase. In untreated juice, the turbidity was drastically increased up to 57 TU. These results are in accordance with those reported by Cerreti et al. (2016). There are several reasons of haze formation, but interaction of proteins and polysaccharides present in juice is most effective and prominent (Siebert 2009). According to Cerreti et al. (2016), haze formation in pomegranate juice is due to formation of pectin envelope outside the protein molecules. Moreover, enzymatic treatment decreases the pectin content of the juice, which leads to low juice turbidity. Due to numerous health properties such as anticancer, antimutagenic, antioxidant etc., pomegranate juice is consumed worldwide and therefore, need of time is to improve its quality (Zarfeshany et al. 2014; Putnik et al. 2019). Earlier, several reports have been published on enzymatic treatment of pomegranate juice (Rinaldi et al. 2013; Cerreti et al. 2016). The enzymatic treatment leads to increase in soluble sugars, soluble dry matters and organic acids which are beneficial to human health (Rinaldi et al. 2013).
In present study, discarded papaya peel has been exploited for polygalacturonase production from indigenous isolate A.niger AN07. Papaya peel has been reported for the first time for production of polygalacturonase enzyme. The optimized process is cost effective and high amount of enzyme is obtained. No other organic and inorganic chemicals have been used in the process. Partially purified polygalacturonase has been successfully used for pomegranate juice clarification.
Conclusion
Enhanced production of polygalacturonase (5.4-fold; 264.20 U/gds) by A. niger AN07 was obtained after statistical optimization using untreated papaya peel as solid substrate in SSF without the addition of any other chemicals. The optimized fermentation medium and conditions were found for other Aspergillus sp. too. The present study also depicts the efficiency of using the agro-industrial waste of papaya processing units for production of commercially viable industrial enzymes and disposal of organic wastes. The results obtained are encouraging for utilization of polygalacturonase production for industrial purposes. The results of purified A. niger AN07 polygalacturonase for pomegranate juice clarification were promising for application in other food industries, for efficient and economic production of clear fruit juices.
References
Abd El-Rahim WM, Moawad H, Hashem MM, Gebreil GMM, Zakaria M (2020) Highly efficient fungal pectinase and laccase producers among isolates from flax retting liquor. Biocatal Agric Biotechnol 25:101570
Acuna-Arguelles ME, Gutierrez-Rojas M, Viniegra-Gonzalez G, Favela-Torres E (1994) Effect of water activity on exo-pectinase production by A. niger CH4 in solid state fermentation. Biotechnol Lett 16:23–28
Adedeji OE, Ezekiel OO (2019) Pretreatment of selected peels for polygalacturonase production by Aspergillus awamori CICC 2040: purification and application in mango juice extraction. Bioresour Technol Rep 7:100306
Aggarwal R, Dutta T, Sheikh J (2020) Extraction of pectinase from Candida isolated from textile mill effluent and its application in bio-scouring of cotton. Sustain Chem Pharm 17:100291
Ali Z, Hussain M (2014) Saccharification of corn cobs an agro-industrial waste by sulphuric acid for the production of monomeric sugars. Int J Biosci 5:204–213
Alonso Pippo W, Luengo CA, Alberteris LAM, Garzone P, Cornacchia G (2011) Energy recovery from sugarcane-trash in the light of 2nd generation biofuels. part 1: current situation and environmental aspects. Waste Biomass Valor 2:1–16
Amin F, Bhatti HN, Bilal M (2019) Recent advances in the production strategies of microbial pectinases: a review. Int J Biol Macromol 122:1017–1026
Amin F, Mohsin A, Bhatti HN, Bilal M (2020) Production, thermodynamic characterization, and fruit juice quality improvement characteristics of an Exo-polygalacturonase from Penicillium janczewskii. BBA Protein Proteom 1868:140379
AOAC (1970) Official methods of analysis, 11th edn. Association of Official Analytical Chemists, Washington DC
Bas D, Boyacı IH (2007) Modeling and optimization I: Usability of response surface methodology. J Food Eng 78:836–845
Blandino A, Iqbalsyah T, Pandiella SS, Cantero D, Webb C (2002) Polygalacturonase production by Aspergillus awamori on wheat in solid-state fermentation. Appl Microbiol Biotechnol 58:164–169
Botella C, de Ory I, Webb C, Cantero D, Blandino A (2005) Hydrolytic enzyme production by Aspergillus awamori on grape pomace. Biochem Eng J 26:100–106
Caffall KH, Mohnen D (2009) The structure, function, and biosynthesis of plant cell wall pectic polysaccharides. Carbohydr Res 344:1879–1900
Castilho LR, Medronho RA, Alves TLM (2000) Production and extraction of pectinases obtained by solid-state fermentation of agroindustrial residues with Aspergillus niger. Bioresour Technol 71:45–50
Cerreti M, Liburdi K, Benucci I, Esti M (2016) The effect of pectinase and protease treatment on turbidity and on haze active molecules in pomegranate juice. LWT Food Sci Technol 73:326–333
Chaiwut P, Pintathong P, Rawdkuen S (2010) Extraction and three-phase partitioning behavior of proteases from papaya peels. Process Biochem 45:1172–1175
Combo AMM, Aguedo M, Goffin D, Wathelet B, Paquot M (2012) Enzymatic production of pectic oligosaccharides from polygalacturonic acid with commercial pectinase preparations. Food Bioprod Process 90:588–596
Daskaya-Dikmen C, Karbancioglu-Guler F, Ozcelik B (2018) Cold active pectinase, amylase and protease production by yeast isolates obtained from environmental samples. Extremophile. https://doi.org/10.1007/s00792-018-1020-0
de Carvalho SJ, de Franca PRL, de Melo AHF, Neves-Petersen MT, Converti A, Porto TS (2019) Optimized production of Aspergillus aculeatus URM4953 polygalacturonases for pectin hydrolysis in hog plum (Spondias mombin L.) juice. Process Biochem 79:18–27
Demir H, Tari C (2014) Valorization of wheat bran for the production of polygalacturonase in SSF of Aspergillus sojae. Ind Crop Prod 54:302–309
Diaz AB, Alvarado O, de Ory I, Caro I, Blandino A (2012) Enhance hydrolytic enzymes production by Aspergillus awamori on supplemented grape pomace. Food Bioprod Process 90:72–78
Dongare ML, Buchade PB, Awatade MN, Shaligram AD (2013) On-line turbidity measurement of clear juice. J Opt 42(2):92–95
Ejaz U, Ahmed A, Sohail M (2018) Statistical optimization of immobilization of yeast cells on corncob for pectinase production. Biocatal Agric Biotechnol 14:450–456
El-Nemr SE, Ismail IA, Ragab M (1990) Chemical composition of juice and seeds of pomegranate fruit. Die Nahrung 34:60–606
Gervais P, Molin P (2003) The role of water in solid state fermentation. Biochem Eng J 13:85–101
Gupta S, Kapoor M, Sharma KK, Nair LM, Kuhad RC (2008) Production and recovery of an alkaline exo-polygalacturonase from Bacillus subtilis RCK under solid-state fermentation using statistical approach. Bioresour Technol 99:937–945
Handa S, Sharma N, Pathania S (2016) Multiple parameter optimization for maximization of pectinase production by Rhizopus sp C4 under solid state fermentation. Fermentation 2:10. https://doi.org/10.3390/fermentation2020010
Heerd D, Yegin S, Tari C, Fernandez-Lahore M (2012) Pectinase enzyme-complex production by Aspergillus spp. in solid-state fermentation: a comparative study. Food Bioprod Process 90:102–110
Hernandez N, Rodriguez-Alegría ME, Gonzalez F, Lopez-Munguia A (2000) Enzymatic treatment of rice bran to improve processing. J Am Oil Chem Soc. https://doi.org/10.1007/s11746-000-0028-2
Huang D, Song Y, Liu Y, Qin Y (2019) A new strain of Aspergillus tubingensis for high-activity pectinase production. Braz J Microbiol. https://doi.org/10.1007/s42770-018-0032-3
IHD (Indian horticulture Database) Ministry of Agriculture Government of India. 2013. Indian Horticulture Production at a Glance, pp 100–105.
Jayani RS, Saxena S, Gupta R (2005) Microbial pectinolytic enzymes: a review. Process Biochem 40:2931–2944
Kaur A, Varghese LM, Mahajan R (2019) Simultaneous production of industrially important alkaline xylanase-pectinase enzymes by a bacterium at low cost under solid-state fermentation conditions. Biotechnol Appl Biochem. https://doi.org/10.1002/bab.1757
Kavuthodi B, Sebastian D (2018) Response surface methodological approach to optimize the critical medium components for augmented pectinase production by Bacillus subtilis BKDS1. J Appl Pure Microbiol. 1:1. https://doi.org/10.22207/JPAM.12.2.62
Koubala BB, Christiaens G, Kansci S, Loey AMV, Hendrickx ME (2014) Isolation and structural characterization of papaya peel pectin. Food Res Int 55:215–221
Kumar A, Garg S, Garg N (2012) Biochemical tests principle and protocols. Viva Books Pvt, Ltd, New Delhi
Kumar YS, Kumar PV, Reddy OVS (2012) Pectinase production from mango peel using Aspergillus foetidus and its application in processing of mango juice. Food Biotechnol 26:107–123
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Lee WC, Yusof S, Hamid NSA, Baharin BS (2006) Optimizing conditions for enzymatic clarification of banana juice using response surface methodology (RSM). J Food Eng 73:55–63
Li Q, Ray CS, Callow NV, Loman AA, Islam SMM, Ju L-K (2020) Aspergillus niger production of pectinase and α-galactosidase for enzymatic soy processing. Enzym Microb Technol 134:109476
Lofgren C, Hermansson A (2007) Synergistic rheological behaviour of mixed HM/LM pectin gels. Food Hydrocolloid 21:480–486
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with Folin-phenol reagent. J Biol Chem 193:265–275
Maidanaa SA, Butiuka AP, Zubreskia ER, Hoursb RA, Brumovskya LA, Martosa MA (2019) Production of an endopolygalacturonase from Wickerhanomyces anomalus with disintegration activity on plant tissues. Biocatal Agric Biotechnol 18:101042
Maller A, Damasio ARL, da Silva TM, Jorge JA, Terenzi HF, de Moraes Polizeli MLT (2011) Biotechnological potential of agroindustrial wastes as a carbon source to thermostable polygalacturonase production in Aspergillus niveus. Enzym Res. https://doi.org/10.4061/2011/289206
Maran JP, Prakash KA (2015) Process variables influence on microwave assisted extraction of pectin from waste Carcia papaya L. peel. Int J Biol Macromol 73:202–206
Marzo C, Díaz AB, Caro I, Blandino X (2018) Valorization of agro-industrial wastes to produce hydrolytic enzymes by fungal solid-state fermentation. Waste Manag Res. https://doi.org/10.1177/0734242X18798699
Mehmood T, Saman T, Irfan M, Anwar F, Ikram MS, Tabassam Q (2018) Pectinase production from schizophyllum commune through central composite design using citrus waste and its immobilization for industrial exploitation. Waste Biomass Valor 10:2527–2536
Mahmoodi M, Najafpour GD, Mohammadi M (2019) Bioconversion of agroindustrial wastes to pectinases enzyme via solid state fermentation in trays and rotating drum bioreactors. Biocatal Agric Biotechnol 21:101280
Nakkeeran E, Umesh-Kumar S, Subramanian R (2011) Aspergillus carbonarius polygalacturonases purified by integrated membrane process and affinity precipitation for apple juice production. Bioresour Technol 102:3293–3297
Nelson N (1944) A photometric adaptation of the Somogyi method for the determination of glucose. J Biol Chem 153:375–380
Nighojkar A, Patidar MK, Nighojkar S (2019) Pectinases: Production and applications for fruit juice beverages. In: Grumezescu AM, Holban AM (eds) Processing and Sustainability of Beverages. Woodhead Publishing, pp 235–273.
Nighojkar S, Phanse Y, Sinha D, Nighojkar A, Kumar A (2006) Production of polygalacturonase by immobilized cells of Aspergillus niger using orange peel as inducer. Process Biochem 41:1136–1140
Oduguwa OO, Edema MO, Ayeni AO (2008) Physico-chemical and microbiological analyses of fermented corn cob, rice bran and cowpea husk for use in composite rabbit feed. Bioresour Technol 99:1816–1820
OECD (2005) The organisation for economic co-operation and development annual report: 2005. Consensus document on the biology of papaya (Carica papaya) series on harmonisation of regulatory oversight in biotechnology No. 33. https://www.oecd.org/env/ehs/biotrack/46815818.pdf
Orzua MC, Mussatto SI, Contreras-Esquivel JC, Rodriguez R, de la Garza H, Teixeira JA, Aguilar CN (2009) Exploitation of agro industrial wastes as immobilization carrier for solid-state fermentation. Ind Crop Prod 30:24–27
Pagarraa H, Rahmanb RA, Rachmawatya AM (2019) Screening of factors influencing exo-polygalacturonase production by Aspergillus niger ATCC 120120 using two-level fractional factorial design. J Teknol 81:73–80
Pagnonceli J, Rasbold LM, Rocha GB, Silva JLC, Kadowaki MK, Simao RCG, Maller A (2019) Biotechnological potential of an exo-polygalacturonase of the new strain Penicillium janthinellum VI2R3M: biochemical characterization and clarification of fruit juices. J Appl Microbiol. https://doi.org/10.1111/jam.14426
Pandey A, Soccol CR, Nigam P, Soccol VT (2000) Biotechnological potential of agro-industrial residues. I: sugar cane bagasse. Bioresour Technol 74:69–80
Parniakov O, Barba FB, Grimi N, Lebovka N, Vorobiev E (2014) Impact of pulsed electric fields and high voltage electrical discharges on extraction of high-added value compounds from papaya peels. Food Res Int 65:337–343
Patidar MK, Nighojkar S, Kumar A, Nighojkar A (2018) Pectinolytic enzymes-solid state production, assay methods and applications in fruit juice industries: a Review : 3Biotech 8:199.
Patidar MK, Nighojkar S, Kumar A, Nighojkar A (2016) Papaya peel valorization for production of acidic pectinmethylesterase by Aspergillus tubingensis and its application for fruit juice clarification. Biocataly Agric Biotechnol 6:58–67
Patil SR, Dayanand A (2006) Production of polygalacturonase from deseeded sunflower head by Aspergillus niger in submerged and solid-state conditions. Bioresour Technol 97:2054–2058
Paulchamy C (2007) Solid-state cultivation of Aspergillus niger NCIM 548 for glucoamylase production on groundnut shell. Internet J Microbiol 5
Plackett RL, Burman JP (1946) The design of optimum multi-factorial experiments. Biometrika 33:305–325
Putnika P, Kresoja Z, Bosiljkov T, Jambrak AR, Barba FJ, Lorenzo JM, Roohinejad S, Granato D, Žuntar I, Kovačević DB (2019) Comparing the effects of thermal and non-thermal technologies on pomegranate juice quality: a review. Food Chem 279:150–161
Raol GG, Raol BV, Prajapati VS, Bhavsar NV (2015) Utilization of agro-industrial waste for b-galactosidase production under solid state fermentation using halotolerant Aspergillus tubingensis GR1 isolate. 3 Biotech 5:411–421.
Reddy MPC, Saritha KV (2016) Effects of the culture media optimization on pectinase production by Enterobacter sp. PSTB-1. 3 Biotech 6:207.
Rinaldi M, Caligiani A, Borgese R, Palla G, Barbanti D, Massini R (2013) The effect of fruit processing and enzymatic treatments on pomegranate juice composition, antioxidant activity and polyphenols content. LWT Food Sci Technol 53:355–359
Ruiz HA, Rodríguez-Jasso RM, Rodríguez R, Contreras-Esquivel JC, Aguilar CN (2012) Pectinase production from lemon peel pomace as support and carbon source in solid-state fermentation column-tray bioreactor. Biochem Eng J 65:90–95
Sabry SA (1993) Protein-enrichment of wheat bran using Aspergillus terreus. Microbiol Sem 9:125–133
Samadi S, Mohammadi M, Najafpour GD (2016) Production of single cell protein from sugarcane bagasse by Saccharomyces cerevisiae in tray bioreactor. Int J Eng 29:1029–1036
Sareena C, Sreejith MP, Ramesan MT, Purushothaman E (2014) Biodegradation behaviour of natural rubber composites reinforced with natural resource fillers: monitoring by soil burial test. J Reinfor Plast Compos 33:412–429
Siebert KJ (2009) Haze in beverages. Adv Food Nutr Res. https://doi.org/10.1016/S1043-4526(09)57002-7
Silva D, Martins ES, Ribeiro Leite RS, Da Silva R, Ferreira V, Gomes E (2007) Purification and characterization of an exo-polygalacturonase produced by Penicillium viridicatum RFC3 in solid-state fermentation. Process Biochem 42:1237–1243
Singh S, Mandal SK (2012) Optimization of processing parameters for production of pectinolytic enzymes from fermented pineapple residue of mixed Aspergillus species. Jordan J Biol Sci 5:307–314
Somogyi M (1952) Notes on sugar determination. J Biol Chem 195:19–25
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5 molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance and maximum parsimony methods. Mol Biol Evol 28:2731–2739
Tari C, Gogus N, Tokatli F (2007) Optimization of biomass, pellet size and polygalacturonase production by Aspergillus sojae ATCC 20235 using response surface methodology. Enzyme Microb Technol 40:1108–1116
Thite VS, Nerurkar AS, Baxi NN (2020) Optimization of concurrent production of xylanolytic and pectinolytic enzymes by Bacillus safensis M35 and Bacillus altitudinis J208 using agro-industrial biomass through response surface methodology. Sci Rep 10:3824
Uzuner S, Cekmecelioglu D (2015) Enhanced pectinase production by optimizing fermentation conditions of Bacillus subtilis growing on hazelnut shell hydrolyzate. J Mol Catal B: Enzym 113:62–67
Vibha K, Negi S (2018) Enhanced production of laccase and pectinase using co-culture of Trametes hirsuta and Phanerochaete sp. through EVOP-factorial design technique. 3 Biotech 8:490.
Viniegra-González G, Favela-Torres E, Aguilar CN, Rómero-Gomez SJ, Dıaz-Godınez Tariq G, Augur C (2003) Advantages of fungal enzyme production in solid state over liquid fermentation systems. Biochem Eng J 13:157–167
Zarfeshany A, Asgary S, Haghjoo Javanmard S (2014) Potent health effects of pomegranate. Adv Biomed Res. https://doi.org/10.4103/2277-9175.129371
Zehra M, Syed MN, Sohail M (2020) Banana Peels: a promising substrate for the coproduction of pectinase and xylanase from Aspergillus fumigatus MS16. Polish J Microbiol 69:19–26
Acknowledgements
The authors acknowledge the facilities provided by the Department of Biotechnology, Ministry of Science and Technology, Government of India, New Delhi (DBT) in the School of Biotechnology, Devi Ahilya University, Indore under M.Sc. Biotechnology Program and Bioinformatics sub center. The authors acknowledge Dr. V. R. Reddy and Dr. D. M. Phase, UGC-DAE Consortium for Scientific Research, Indore for helping in Scanning Electron Microscopic analysis. The authors also acknowledge facilities of Department of Biosciences, Maharaja Ranjit Singh College of Professional Sciences, Indore.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest involved.
Ethical statement
The study does not involve any work on animals or human hence does not require any ethical clearance.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Patidar, M.K., Nighojkar, S., Kumar, A. et al. Production of polygalacturonase using Carica papaya peel biowaste and its application for pomegranate juice clarification. Environmental Sustainability 3, 509–520 (2020). https://doi.org/10.1007/s42398-020-00138-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42398-020-00138-6