Abstract
Solid-state fermentation (SSF) is an efficient technology to produce compounds of economic interest that allow the reduction of production costs when lignocellulosic residues, such as pineapple leaf waste (PLW), are used both as a carbon source and as support for productive microorganisms. In this work, three strains of Trametes hirsuta were evaluated for PLW bioconversion into fermentable sugars and the production of laccases by SSF technology. SSF experiments were performed at 27 and 35 °C for 21 days. The highest level of laccase activity was obtained at 27 °C in SSF with T. hirsuta AHB-6 (6513.8 U/mL). However, the greater PLW delignification rate (28%) was observed in biomass SSF with T. hirsuta RT-1 at 35 °C. Structural changes allowed sugar yields until 90.8% from cellulose. Enzymatic hydrolysis achieved a reducing sugar concentration of 580 mg/g PLW biomass, while in assisted enzymatic hydrolysis (with an enzymatic crude extract from SSFs), up to 2.5 times higher reducing sugar concentration than control. The characterization of the recovered fermentable sugars showed that hemicellulolytic enzymes are present in the crude enzymatic extracts of T. hirsuta and that in synergism with commercial cellulases allowed the 100% saccharification of the holocellulose. Since PLW showed suitable characteristics as a substrate for T. hirsuta, the addition of another nutrient was not necessary to obtain functional lignocellulolytic enzymes, as well as possible antioxidants and proteins. These results can lead to a low-cost and efficient process under the biorefinery concept.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Waste disposal is one of the main problems that most agro-food industries face. Accumulation in the field or burning of vegetable waste has a negative environmental impact; on the other hand, its use as animal feed is non-viable due to its low digestibility and the presence of anti-nutritional factors. Moreover, these management strategies are insufficient given the large amount of wastes generated during the harvesting and processing of agricultural products, which generally represent 50% of the crops, which include: stems, empty fruits, bunches, and leaves [1, 2]. Valorization of these residues would contribute both to facilitating their disposal and to producing high-value biotechnological products, such as enzymes, alcohols, hydrogen, bio-pesticides, and organic acids [3, 4].
In the pineapple industry, residues from its harvest represent 80% of the total mass of the plant since only the fruits are readily consumed. In Mexico, 875,830 tons of pineapple fruit are produced, which roughly represent 4 million tons of leaves. Commonly, these are incinerated or left to decompose in open fields, generating serious environmental problems [5]. Recent studies have determined that these wastes present a high holocellulose content (62.17% w/w) [6], hence making them a promising raw material for fermentable sugars and second-generation bioethanol production [7]. A common bottleneck for the use of harvest residues is its heterogeneous composition, which includes the presence of lignin, so an adequate pretreatment to break or reduce recalcitrance with low energy consumption is necessary to allow access to the carbohydrates of interest. In this sense, recent developments in bioprocessing have identified solid-state fermentation (SSF) as a suitable process that has a low environmental impact and represents low costs, so agro-industrial by-products can be utilized for enzyme production [1, 8].
SSF involves the use of solids as starting materials that are almost devoid of free water but must contain enough moisture to support the growth and metabolism of the microorganisms [9]. Both yield and speed in obtaining the final product depend on the organism and the substrate used [10]. During the solid-state fermentation process with lignocellulosic material as the substrate, compounds of economic value can be produced, such as enzymes, lignin derivatives (vanillic acid), and other proteins [11], since microorganisms, such as fungi, can naturally synthesize enzymes that break down the plant cell wall and thereby hydrolyzing and mobilizing compounds towards the extraction solvent [12].
The use of lignocellulosic wastes as substrate offers the possibility to reduce production costs of lignocellulolytic enzymes, as the cost of raw materials accounts for one third of the total cost of this process [13]. Lignocellulolytic enzymes represent more than 20% of worldwide sales of commercially available enzymes [14] and have numerous applications in textile, food, animal feed, paper, biofuel, and pharmaceutical industries [1, 15]. Microorganisms have the ability to employ multiple substrates in order to face the challenge of utilizing lignins of heterogeneous nature and then produce extracellular oxidative enzymes [16]. T. hirsuta laccases have a great potential of being applied in bioremediation processes, and also, some of their isoenzymes and their synergistic action have been studied for textile effluent decolorization. This fact allows visualization of the scope and future development of delignification processes with isoenzyme mixtures. This is in order to improve fermentable sugar yields from lignocellulosic biomass [17].
Therefore, the aim of this work was to obtain fermentable sugars and enzymes from raw pineapple leaf residues by solid-state fermentation technology using native T. hirsuta strains. In addition, the synergistic effect of enzyme raw extract from SSF in the improvement of enzymatic hydrolysis of PLW was evaluated.
2 Materials and methods
2.1 Raw material and microorganism
Pineapple leaf waste (PLW) was obtained from Ananas comosus var. “cabezona” plants, from the municipality of Cardenas, Tabasco, Mexico. Samples were collected postharvest and dried at 90 °C for 3 days in a convection oven (Binder, Fed model 115®, Tuttlingen, Germany). Dry biomass was milled and sieved for particle size distribution [6].
Strains of T. hirsuta Bm-2 (GQ280373.1), T. hirsuta AHB-6 (GQ280372.1), and T. hirsuta RT-1 came from the strain bank of Renewable Energy Unit of Yucatan Center for Scientific Research and were maintained by subculturing in potato dextrose-agar (PDA) plates at 32 °C [18].
2.2 Laccase activity test
Plates of ME medium (2% w/v malt extract and 2% w/v agar) with 5 mM 2,2’-azinobis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS; Sigma Aldrich, St. Luis, MO, USA) were inoculated with a 1-cm diameter disk of 4-day-old mycelium and incubated for 4 days at 35 °C. Extracellular laccase activity was defined as positive when the fungus produced a dark green–blue halo due to ABTS oxidation [18].
2.3 Solid-state fermentation (SSF) conditions
Strains of T. hirsuta were grown in PLW-agar (2% w/v) for 4 days at 32 °C. A 1-cm diameter disk of each fungus in PLW-agar was placed on the substrate surface center. SSF was performed using an Erlenmeyer flask (250 mL) containing 5 g PLW, distilled water was added until humidity reached 80%, and then autoclaved at 121 °C and 1 Kgf/cm2 for 20 min. SSF was carried out for 21 days in the dark at 27 or 35 °C in triplicates. Samples were unmounted at 0, 7, 14, and 21 days. Each sample was added 50 mL of sterile water and shaken at 130 rpm for 30 min at 30 °C. Liquid fractions were recovered by filtration and analyze as enzymatic crude extracts (laccase activity and phenols content), whereas the solid fractions were dehydrated and characterized (cellulose composition, SEM, and FTIR) for the saccharification phase [19,20,21].
2.3.1 Determination of total phenols content
The concentration of total phenols in the crude enzymatic extract was determined by reduction of the Folin–Ciocalteau reagent (Merck KGaA, Darmstadt, Germany). The determination of total phenols was carried out in triplicates. Gallic acid was used as a standard, and the results obtained were expressed as milligrams of gallic acid/mL [22].
2.3.2 Assay for laccase activity
Laccase activity of crude extracts was measured at 40 °C using ABTS in triplicate. The assay mixture contained 1 M sodium acetate buffer (pH 4.5) and 5 mM ABTS in a total volume of 1 mL. Oxidation of ABTS was measured by the increase in absorbance at 420 nm as described in a previous study [23]. One enzyme unit (U) was defined as the amount of enzyme required to oxidize 1 µmol of ABTS per min under the assay’s conditions. The amount of the enzyme production was expressed as U/mL.
2.3.3 Delignification efficiency process on biomass
Residual lignin content in solids after SSF (27 °C and 35 °C) was determined by gravimetry with a method established by National Renewable Energy Laboratory (NERL). Delignification percentage was calculated by the difference between initial and residual lignin contents using Eq. 1 [24]:
2.4 Characterization of biomass
Characterization was performed only on the solid fraction from those SSF samples with the best delignification percentage and raw PLW.
2.4.1 Composition analysis
Cellulose, hemicellulose, and lignin contents were determined with methods defined by NREL [25]. Carbohydrate polymers were estimated by quantifying monomeric sugars by HPLC in an Agilent 1260 Infinity II chromatograph, with a MetaCarb 87H column (300 mm × 7.8 mm, Agilent) at 60 °C and 0.7 mL/min sulfuric acid (0.5 mM) as mobile phase, and klason lignin was determined by a gravimetric method [26].
2.4.2 Scanning electron microscopy (SEM)
Structural changes after SSF were observed in a JEOL scanning electron microscope (model JSM-6360LV; JEOL, Tokyo, Japan). Untreated PLW samples and SSF treated samples with the different T. hirsuta strains were mounted on carbon with adhesive tape on a metal barrel, metalized with a 15-nm gold layer, and observed at 20 kV [27].
2.4.3 Fourier transform infrared spectroscopy (FT-IR)
FTIR spectrometry analysis was employed to determine modifications in functional groups in the biomass after SSF. Spectral data were obtained in the region 4000–600 cm−1 using Bruker Tensor II spectrometer (Bruker, Ontario, ON, Canada) with an attenuated total reflection (ATR) accessory [13].
2.5 Enzymatic hydrolysis of pineapple leaf waste
Enzymatic hydrolysis of raw PLW, and pretreated biomass, was performed in Erlenmeyer flasks (50 mL) with 1% (w/v) solid fraction in 25 mL sodium citrate buffer (50 mM, pH 4.8) at 50 °C and 150 rpm in a shaking incubator and an enzymatic ratio of 15 FPU Cellic® Ctec3 (40 mg, Novozymes A/S, Bagsværd, Denmark) per gram of cellulose contained in the solid fraction [28]. Enzymatic hydrolysis was monitored at 6, 12, 24, 48, and 72 h. The experiments were performed in triplicates.
2.5.1 Enzymatic hydrolysis assisted
Enzymatic hydrolysis assisted was performed for raw PLW and pretreated biomass. The experiments were carried out by adding 1 mL of their corresponding enzymatic crude extract in addition to Cellic® Ctec3 under the conditions described previously.
2.5.2 Determination of reducing sugars
In order to determine the concentration of released reducing sugars by enzymatic hydrolysis, the Miller colorimetric method was used and oxidation of 3,5-dinitrosalicylic acid was monitored by changes in absorbance at 550 nm. Results obtained were expressed as the concentration of glucose (g/g biomass). Hydrolysis yield (%) was calculated with Eq. 2 using data from glucose concentration (g/L), load biomass, conversion factor of cellulose to glucose equivalents (1.111), and cellulose fraction of dry biomass (f) [29].
Sugar composition was measured in a high-performance liquid chromatography (HPLC) system using a carbohydrate analysis column (4.6 × 250 nm, 5 microns), performed at 40 °C at a flow rate of 1.5 mL/min and acetonitrile:water (75:25) as mobile phase.
2.6 Statistical analysis
Data represent the average of results from three replicates and were presented as the mean ± SD. One-way variance analysis (ANOVA) with Tukey’s pairwise comparisons was used to determine significance among treatment means. Significance was set at p < 0.05.
3 Results and discussion
3.1 Laccase activity test
T. hirsuta laccase activity in plate test can be seen in Fig. 1. ABTS oxidation, indicative of the presence of extracellular laccases, is observed as a blue–green halo around mycelial growth. Test plates showed that T. hirsuta strains AHB-6 and RT-1 presented higher rates of ABTS oxidation compared to T. hirsuta Bm-2. This variation in laccase activity levels could be attributed to the different isolation sites of the strains [18].
T. hirsuta has a wide range of laccase isoenzymes with high redox potential. Vasina et al. [30] identified five laccase genes in T. hirsuta, which may indicate its potential use in a wide range of biotechnological processes. Two of these laccase genes were also identified in T. hirsuta Bm-2 by Pereira et al. [31], which when induced with grapefruit peel, their expression increased up to 90 times compared to levels observed when grown in the basal medium [32]. Since the visualization of higher level oxidation with T. hirsuta AHB-6 and RT1 than Bm-2, which was more studied, this can be ascribed to genetic variability among T. hirsuta strains, which in turn would influence their enzymatic performance in the substrate cleavage.
3.2 Solid-state fermentation of pineapple leaf waste
Extracellular laccase enzyme production from T. hirsuta strains in solid-state fermentation (SSF), using PLW as substrate, was carried out at 27 °C and 35 °C and is depicted in Fig. 2a, b, respectively. T. hirsuta Bm-2 in SSF at 35 °C secreted 2083.7 U/mL on day 7 and reached a maximum activity of 3003.2 U/mL on day 21 (Fig. 2b), as reported by Zapata-Castillo et al. [33], who obtained an enzymatic extract with 2496 U/mL in submerged fermentation in basal medium with wheat bran (2% w/v). When the bran load was increased to 3% (w/v), laccase activity reached 4081.2 U/mL [34]. Ancona et al. [34] mentioned that enzyme dynamics had a close relationship to that of phenolic compounds release (from 2.18 to 3.46 mg/mL), i.e., when wheat bran lignin depolarization occurs, phenols concentration in the medium increased as well as other 3–4 carbon-free radical compounds that may act as enzyme mediator.
In the case of T. hirsuta Bm-2 in SSF at 35 °C, phenols concentration increased from 0.44 to 1.71 mg/mL, showing similar behavior to observed at this temperature in previous studies. In contrast, when using the same strain at 27 °C (Fig. 2a), phenols levels decreased to 0.18 mg/mL, suggesting that phenols that were recognized as substrates or inducers were oxidized, causing diminished laccase activity from day 14, with a maximum activity of 1809.2 U/mL. This is a similar result to that reported by Tapia et al. [35], who obtained 1777.7 U/mL of laccase activity by submerged fermentation with vinasse at the same temperature. This first SSF report and previous ones with submerged fermentation demonstrate that temperature represents an important factor for increasing laccase activity using the T. hirsuta Bm-2 strain.
The enzymatic activity levels obtained with T. hirsuta RT-1 strain were lower than those with T. hirsuta Bm-2; however, maximal laccase activity was similar at both temperatures: 1915.2 U/mL at 35 °C and 1752.7 U/mL at 27 °C after 21 days of incubation. Phenols concentration decreased to 0.09 mg/mL at 27 °C and increased to 1.69 mg/mL when the incubation temperature was 35 °C. In this sense, in this first study with T. hirsuta RT-1 strain and PLW in SSF, there is enzymatic production stability with respect to temperature.
On the other hand, T. hirsuta AHB-6 showed the highest laccase activity levels among the three studied strains at both temperatures, reaching 3268 U/mL at 35 °C and doubled it at 27 °C (6513.8 U/mL). Regarding phenols concentration, the same behavior as with the other strains was observed, with values of 0.08 and 1.79 mg/mL at 27 °C and 35 °C, respectively. It has been shown that in some microorganisms, the release of phenols can be influenced by temperature, and the structure and composition of monolignols can vary according to the enzymes secreted in the employed temperatures [36]; therefore, phenols at specific concentrations can be recognized as inducers that trigger variability in enzyme activity. Ferulic acid, vanillic acid, and guaiacol have been reported to induce laccase genes in T. hirsuta Bm-2, [35]; however, in PLW, only p-coumaric has been identified [37], which served as an inducer to stimulate high levels of enzymatic activity. It has been shown that it is possible to stimulate mycelial growth and laccase production in SSF by adding Cu2+, Ag2+, Cd2+, Mn2+ ions, among others [38]; in this study, mycelial growth and enzyme production occurred naturally since PLW contains traces of Cu2+ and Mn2+ ions [39].
Furthermore, substrate recognition by microorganisms is highly influenced by the nature of the original habitat. In the case of T. hirsuta Bm-2, it came from rotting wood, while T. hirsuta AHB-6 was obtained from residual henequen fibers that contained high concentrations of organic acids [18], and T. hirsuta RT-1 was found under saprophytic conditions in Acalypha gaumeri, which is an endemic species of the state of Yucatán (México) studied for its high flavonoids content, terpenes, and amides with antagonistic potential to phytopathogens.
3.2.1 Delignification efficiency process on biomass
Evaluated T. hirsuta strains showed different capacities for PLW delignification after 21 days of incubation (Table 1). The highest percentage of delignification obtained was 28.45 ± 0.43% at 35 °C with T. hirsuta RT-1. The delignification percentages of PLW by SSF with T. hirsuta Bm-2 at 27 °C and 35 °C were statistically similar, which implies that temperature did not influence this process with this strain; while with T. hirsuta AHB-6 and RT-1 strains, delignification percentages obtained by SSF at 35 °C were slightly more than double than those reached at 27 °C. Our results confirm the report of Isroi et al. [40], whose report points out that T. hirsuta can hydrolyze lignin at temperatures higher than 30 °C, unlike laccases from other microorganisms, whose ideal reaction temperatures are between 20 and 28 °C.
There are no available reports on PLW delignification by SSF and only a few studies with enzymatic pretreatments; for instance, Banerjee et al. [6] reported a delignification percentage of 81.12%, employing PLW for submerged fermentation with a solid load of 21.5% (w/v) and laccases produced by Lentinus squarrosulus (3500 U/g of the substrate) with biomass with 14% initial lignin content. These authors affirm that the solid load influenced the optimization of PLW enzymatic delignification, as efficiency tends to increase when workloads are relatively low due to better oxygen homogenization and, thus, more accessibility of enzymes to the substrate [24]. Hence, it is to be expected that delignification by SSF could generally be less efficient compared to submerged fermentation. Vasco-Correa et al. [41] suggested a gradual adaptation mechanism through subcultures for biomass degradation with high lignin content. By utilizing Ceriporiopsis subvermispora, they achieved delignification percentages similar to the ones obtained by SSF with T. hirsuta RT-1 in this work, without any subcultures in PLW (30% lignin content).
Laccases are the most important delignifying enzymes in T. hirsuta, but they are considered nonspecific since they are able to recognize a wide range of substrates; however, the redox potentials of enzymes may be different in concordance with their microbial origin [42]. Lignin is a heteropolymer, which comprises approximately 70% of non-phenolic compounds and whose structural conformation varies for each plant species [43]; therefore, laccases require the concerted action of auxiliary enzymes or the presence of mediators to fully degrade this macromolecule [44]. No external mediators were added to SSF of PLW, so it is assumed that non-phenolic compound degradation or modification occurred through recognition of free radicals or redox mediators generated in the first oxidation stages of PLW since it has been reported that these eleven recovered compounds can perform this same function in in vitro systems, although this interference has not yet been demonstrated in spontaneous delignification [44].
Biological delignification is a slow process, although it can be more effective, economical, ecofriendly, and less health hazardous when compared to physicochemical or chemical procedures. Laccase is capable of depolymerizing phenolic compounds that are normally toxic or recalcitrant to conventional biomass decomposition [42] and allows to recover compounds of added value, such as antioxidants and antimicrobial products of interest for the food, cosmetic, and pharmaceutical industries [37].
3.2.2 Compositional and structural characterization of pretreated PLW
Composition of hemicellulose contained in raw PLW (Table 2) was similar to that reported: cellulose, 42.29% (w/w) [24] and hemicellulose, 21.02% (w/w) [6]. In contrast to these data, lignin content was higher (30%). Differences in lignin content in crops of the same species are the result of climatic and soil conditions since this biopolymer gives support and protection to plants.
T. hirsuta strains evaluated in this study significantly decreased lignin content in PLW through SSF at 35 °C; hence, only the recovered biomass from experiments at this temperature was characterized. A different behavior could be observed among the three strains regarding holocellulose degradation. T. hirsuta is a ligninolytic microorganism belonging to the WRF group, which has the ability to degrade both simultaneously and/or selectively lignocellulosic biomass. In the first case, it involves the gradual degradation of the three biopolymers in a single process, while in selective degradation type, depolymerization occurs gradually, starting with lignin, followed by hemicellulose, and most of the time keeps cellulose intact [45].
T. hirsuta RT-1 strain showed more selectivity towards lignin depolymerization and allowed a proportional increase in hemicellulose from an initial 18.52% (w/w) to 29.45% (w/w) after SSF, while T. hirsuta Bm-2 and T. hirsuta AHB-6 strains reduced hemicellulose up to 12.36 and 15.73% (w/w), respectively. Regarding cellulose contents, all strains led to increases of 49.97% (w/w) with T. hirsuta RT-1, 62.53% (w/w) with T. hirsuta Bm-2, and 63.01% (w/w) with T. hirsuta AHB-6. Although the obtained holocellulose values were lower (75–80% w/w) than those reported by Moya et al. [46], who reached up to 90–92% w/w through PLW biopulping with Pleorotus ostreatus and Trametes versicolor in 6 weeks, our results were obtained in SSF of PLW without contact surface improvement and in half the time.
Functional group changes corresponding to the modification of biopolymers (cellulose, hemicellulose, and lignin) in raw PLW and SSF recovered biomass are shown in Fig. 3. The peak at absorbance between 3600 and 3100 cm−1 represents H–O stretching vibration, while that between 2950 and 2800 cm−1, the C–H stretching vibration [47]. Signals of C = O stretching vibration of acetyl groups in the spectrum were present at 1739 cm−1, corresponding to hemicellulose, and at 1680 cm−1 for pectin wax [6, 24]. The first peak was conserved in SSF with T. hirsuta RT-1 biomass (Fig. 3d) by degradation specificity (no hemicellulose degradation), while the second one was modified by fungal biomass present in all SSF biomass. Some signals in the range of 1454–1050 cm−1 indicated structural modifications of carbohydrates in pretreated SSF biomass. A slight increase in the 1424-cm−1 signal from SSF T. hirsuta Bm-2 biomass (Fig. 3b), corresponding at C–O–C asymmetric stretching from cellulose and hemicellulose, was exposed after pretreatment in comparison to raw PLW (Fig. 3a). Variations in intensity or the absence of signals could be ascribed to the different behavior of the batteries of enzymes in each strain; therefore, distinct structural modifications occurred, i.e., in SSF with T. hirsuta AHB-6 biomass (Fig. 3c) showed a diminished wavenumber at 1370 cm−1and an increased one at 1336 cm−1, which corresponded to crystalline cellulose type II and C–H2 stretching, respectively [41, 48], due to a better cellulose exposure (Fig. 4). For the other two SSF biomasses, the level of signals was similar to that in the control. Syringyl ring breathing of lignin and C–O stretching of xylan (hemicellulose) are recognized at 1251 cm−1, while in wavelengths of 665–619 cm−1 range, signals correspond to aliphatic sections and C–H stretching of the aromatic compounds of lignin [24]. In the SSF biomasses (as biopolymers decrease), the first signals appeared as lower peaks than in raw PLW, while the second peak showed distinct alterations due to structural modification by delignification, especially with T. hirsuta RT-1 (Fig. 3d).
Structural changes were observed in micrographs obtained by SEM. In Fig. 4a, the raw PLW sample can be seen as complex fibers with a rough surface and some lignin accumulation in spheres (yellow circle). Few thin filaments (mycelial growth) were observed in SSF-treated biomass with T. hirsuta Bm-2 (Fig. 4b, yellow circle), and some of these were in direct contact with lignin (Fig. 4b', yellow arrow) due to selective delignification and utilization of lignin as a carbon source [41]. Cellulose tubes exposed a perfectly linear order (Fig. 4c), whose diameters were approximately 10–15 µm (Fig. 4c', yellow arrow) and a smooth surface due to the presence of waxes and oils observed in SSF pretreated biomass with T. hirsuta AHB-6, in the same way to PLW cellulose described by Abraham et al. [49] and Cherian et al. [50], who used aggressive extractions methods. In SSF with T. hirsuta RT-1 biomass, although a complete cellulose exposure could not be seen since hemicellulose was conserved, porous surfaces (Fig. 4d) and holes (Fig. 4d', yellow arrows) could be observed, similar to those reported by enzymatic action [41, 51]. Characterization of SSF biomass confirmed variability in the behavior among strains of the same species and, therefore, structural modifications influence the process for obtaining the final products.
3.3 Enzymatic hydrolysis
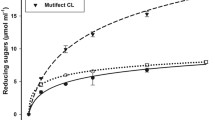
Enzymatic hydrolysis of SSF biomasses and raw PLW are observed in Fig. 5. Saccharification yield for raw PLW was 71.3%, while structural and compositional modifications that were generated during SSF influenced the hydrolysis reaction rate, making cellulose more susceptible to enzymatic attack, and enabling yields up to 90.8%, 83.3%, and 78.1% with T. hirsuta RT-1, T. hirsuta Bm-2, and T. hirsuta AHB-6 strains, respectively. These results support the notion that delignification improves the yield of reducing sugars from PLW as reported by Baneerje et al. [6].
Reducing sugar concentration obtained from raw PLW was 0.33 g/g biomass, while in the case of SSF pretreated biomass, this content practically doubled (Table 3). SSF bioconversion by T. hirsuta Bm-2, with maximum sugar productivity of the three strains in the first 12 h (0.35 g·L−1·h), resulted in 580 mg/g hydrolyzed biomass, which was higher than that reported by SSF of sweet sorghum bagasse with Corolius versicolor [51]. According to Moya et al. [46], obtained increments in sugars were attributed to the selectivity of the enzymatic battery of WRF, which through delignification allowed greater cellulose proportions in the resulting biomass. With this approach, sugar concentrations obtained by saccharification of SSF T. hirsuta RT-1 biomass were similar to those obtained by Banerjee et al. [6], reaching 502 mg/g PLW with a previous delignification up to 80%. This delignification percentage was higher than that achieved in SSF (28%), which means that structural modifications in the SSF biomass had a greater influence on the performance of enzymatic hydrolysis than delignification itself.
With the premise that enzymatic battery from T. hirsuta produced during the SSF process generates suitable biomass that allows an increased concentration of reducing sugars, an assisted enzymatic hydrolysis experiment of raw PLW was performed by adding the enzymatic raw extracts obtained from SSF to the reaction. The addition of enzymatic raw extracts would be conducive to the following scenarios (Fig. 6): enzymatic raw extracts did not inhibit commercial cellulases action, a possible acceleration of the reaction may occur, and reducing sugar yields could be improved. Yields obtained by the addition of the enzymatic raw extracts were only 10% higher than in the control; nevertheless, reaction conditions, such as temperature and pH, could be optimized for laccase activity [24]. It is important to point out that though yield values were similar to the control, they were obtained in shorter periods of reaction time, i.e., the main 24 h for T. hirsuta Bm-2 enzymatic raw extract to reach reducing sugar levels similar and with the other two enzymatic extracts in 48 h.
Hence, SSF enzymatic raw extracts were added to the enzymatic hydrolysis reaction of the pretreated biomass from which it was obtained. Reducing sugar yields obtained after 72 h under the enzymatic hydrolysis-assisted reaction are enlisted in Table 3. The amount of reducing sugars increased after the addition of enzymatic raw extracts, and up to 2.5 times when biomass and enzymatic raw extract from T. hirsuta AHB-6 were used compared to the ones obtained from raw PLW.
Although in assisted enzymatic hydrolysis, the sugar productivities at 12 h were similar to that in conventional enzymatic hydrolysis (0.37, 0.32, and 0.22 g·L−1·h with T. hirsuta Bm-2, T. hirsuta AHB-6, and T. hirsuta RT-1, respectively) due to synergistic effect between commercial enzyme (cellulases) and enzymatic crude extract; the maximum theoretical yields of saccharification were reached after 48 h with the biomass SSF T. hirsuta Bm-2 and SSF T. hirsuta AHB-6 (Fig. 7). Variations in kinetic behavior can be associated with an improved exposure of cellulose as mentioned previously (Sect. 3.2.2.). In contrast, particularly in the case of the assisted enzymatic hydrolysis of SSF T. hirsuta RT-1 biomass, an atypical behavior was determined since cellulose was less exposed as detected by the proportional increase of hemicellulose compared to the other SSF biomasses and that enzymes contained in the raw extract acted synergistically with cellulases, so a reduction in the reaction rate between 24 and 48 h occurred.
After 72 h, the maximum possible theoretical yields of reducing sugars were obtained using the liquid fractions obtained from the assisted enzymatic hydrolysis and then the carbohydrate profiles were determined (Table 4). In the assisted hydrolysis of SSF T. hirsuta AHB-6 biomass, the highest glucose content was obtained (79%), even to that reported by Chintagunta et al. [52] despite their usage of pure xylanase–cellulase enzymes. They also got 39% of cellobiose remnants (without hydrolyzing), an amount greater than those obtained in this study with enzymatic raw extracts. Particularly, with assisted enzymatic hydrolysis from SSF T. hirsuta RT1 biomass produced high percentages of reducing sugars: xylose (38.6%) and glucose (61.3%), along with an almost null presence of cellobiose, which signifies the obtained reducing sugar concentration (0.72 g) corresponding to complete saccharification of the biomass that originally contained 49.9% cellulose and 29.5% hemicellulose. In this sense, owing to the obtained percentages for each carbohydrate correlated to the initial composition of each SSF biomass, it is possible to suggest that enzymatic raw extracts contained hemicellulolytic enzymes.
Some species of WRF have the ability to excrete enzymatic complexes that allow total degradation of lignocellulosic biomass [42], and the production of xylanase enzymes has been reported in the case of T. hirsuta [53]. Since T. hirsuta does not provide a wide enzymatic lignocellulolytic complex, it was not possible to quantify reducing sugars in the liquid fraction recovered from SSF, as enzymatic activation depends on the contact surface and substrate composition. For raw PLW biomass-assisted saccharification reaction, enzymes needed for lignin attack (first surface barrier) were activated, causing a slight improvement in cellulose susceptibility, while in the case of SSF biomasses, they allowed the activation of hemicellulolytic enzymes due to modifications on the contact surface and, hence, a total recovery of reducing sugars from hemicellulose and cellulose. Indeed, under the biorefinery concept, SSF is one of the most important technologies for obtaining products that will allow satisfying future needs [54], such as enzymes, secondary metabolites, antioxidants, and proteins. So this approach, the implementation of SSF technology with T. hirsuta on agro-industrial waste, such as PLW, allows inexpensive production of ligninolytic enzymes with important roles in processes such as toxic compound elimination and of hemicellulolytic enzymes that allow complete saccharification of holocellulose in combination with commercial cellulases.
4 Conclusion
The native strains used were able to recognize PLW as a substrate to produce ligninocellulytic enzymes, which delignified and modified biomass. Pretreatment made it possible to reach yields up to 90% in the production of fermentable sugars from cellulose. Similarly, assisted enzymatic hydrolysis with enzymatic extracts from SSF decreased saccharification time and allowed total hydrolysis of holocellulose, hence, it is concluded that in addition to laccase enzymes, accessory enzymes were produced that allowed this process. The SSF strategy allows valorization of PLW and reduces simultaneously the contamination that is generated with the current management that these residues receive. The methodology proposed in this study gives an approach at integral management of the pineapple crop in order to achieve the development of a zero-waste process.
On the other hand, this study arises opportunities for further studies, such as identification, characterization, and purification of the lignocellulosic enzymes obtained from SSF's, as well as identification of phenolic compounds recovered (possible antioxidants) to be necessary to achieve biorefinery targets and, on the other hand, optimizing the condition adaptation of the fungus into substrate would imply a major reduction in process costs.
References
Leite P, Sousa D, Fernandes H et al (2021) Recent advances in production of lignocellulolytic enzymes by solid-state fermentation of agro-industrial wastes. Curr Opin Green Sustain Chem 27:100407. https://doi.org/10.1016/j.cogsc.2020.100407
Olguin-Maciel E, Singh A, Chable-Villacis R et al (2020) Consolidated bioprocessing, an innovative strategy towards sustainability for biofuels production from crop residues: an overview. Agronomy 10:1834. https://doi.org/10.3390/agronomy10111834
Melnichuk N, Braia MJ, Anselmi PA et al (2020) Valorization of two agroindustrial wastes to produce alpha-amylase enzyme from Aspergillus oryzae by solid-state fermentation. Waste Manag 106:155–161. https://doi.org/10.1016/j.wasman.2020.03.025
Conesa C, Seguí L, Fito P (2018) Hydrolytic performance of Aspergillus niger and Trichoderma reesei cellulases on lignocellulosic industrial pineapple waste intended for bioethanol production. Waste and Biomass Valorization 9:1359–1368. https://doi.org/10.1007/s12649-017-9887-z
Yusof Y, Yahya SA, Adam A (2015) Novel technology for sustainable pineapple leaf fibers productions. Procedia CIRP 26:756–760. https://doi.org/10.1016/j.procir.2014.07.160
Banerjee R, Chintagunta AD, Ray S (2017) A cleaner and eco-friendly bioprocess for enhancing reducing sugar production from pineapple leaf waste. J Clean Prod 149:387–395
Aruna TE (2019) Production of value-added product from pineapple peels using solid state fermentation. Innov Food Sci Emerg Technol 57:102193. https://doi.org/10.1016/j.ifset.2019.102193
dos Santos Costa R, de Almeida SS, Cavalcanti EDAC, Freire DMG, Moura-Nunes N, Monteiro M, & Perrone D. (2021). Enzymes produced by solid state fermentation of agro-industrial by-products release ferulic acid in bioprocessed whole-wheat breads. Food Research International 140:109843
Fang W, Zhang P, Zhang X et al (2018) White rot fungi pretreatment to advance volatile fatty acid production from solid-state fermentation of solid digestate : efficiency and mechanisms. Energy 162:534–541. https://doi.org/10.1016/j.energy.2018.08.082
Tosuner, ZV, Taylan G G, Özmıhçı S (2019) Effects of rice husk particle size on biohydrogen production under solid state fermentation. Int J Hydrog Energy 44(34):18785–18791
Soccol CR, da Costa ESF, Letti LAJ et al (2017) Recent developments and innovations in solid state fermentation. Biotechnol Res Innov 1:52–71. https://doi.org/10.1016/j.biori.2017.01.002
Olukomaiya OO, Fernando WC, Mereddy R et al (2020) Solid-state fermentation of canola meal with Aspergillus sojae, Aspergillus ficuum and their co-cultures: effects on physicochemical, microbiological and functional properties. Lwt 127:109362. https://doi.org/10.1016/j.lwt.2020.109362
Olguin-Maciel E, Larqué-Saavedra A, Lappe-Oliveras PE et al (2019) Consolidated bioprocess for bioethanol production from raw flour of Brosimum alicastrum Seeds using the native strain of Trametes hirsuta Bm-2. Microorganisms 7:483
Ruiz HA, Rodríguez-Jasso RM, Fernandes BD et al (2013) Hydrothermal processing, as an alternative for upgrading agriculture residues and marine biomass according to the biorefinery concept: a review. Renew Sustain Energy Rev 21:35–51. https://doi.org/10.1016/j.rser.2012.11.069
Hasunuma T, Okazaki F, Okai N et al (2013) Bioresource technology a review of enzymes and microbes for lignocellulosic biorefinery and the possibility of their application to consolidated bioprocessing technology. Bioresour Technol 135:513–522. https://doi.org/10.1016/j.biortech.2012.10.047
Avanthi A, Banerjee R (2016) A strategic laccase mediated lignin degradation of lignocellulosic feedstocks for ethanol production. Ind Crop Prod 92:174–185. https://doi.org/10.1016/j.indcrop.2016.08.009
Zapata-Castillo P, Villalonga-Santana L, Islas-Flores I et al (2015) Synergistic action of laccases from Trametes hirsuta Bm2 improves decolourization of indigo carmine. Lett Appl Microbiol 61:252–258. https://doi.org/10.1111/lam.12451
Tapia-tussell R, Pérez-brito D, Rojas-herrera R et al (2011) New laccase-producing fungi isolates with biotechnological potential in dye decolorization. Afr J Bitechnol 10:10134–10142. https://doi.org/10.5897/AJB11.331
Parenti A, Muguerza E, RedinIroz A et al (2013) Induction of laccase activity in the white rot fungus Pleurotus ostreatus using water polluted with wheat straw extracts. Bioresour Technol 133:142–149. https://doi.org/10.1016/j.biortech.2013.01.072
Rana S, Tiwari R, Arora A et al (2013) Biocatalysis and agricultural biotechnology prospecting Parthenium sp. pretreated with Trametes hirsuta, as a potential bioethanol feedstock. Biocatal Agric Biotechnol 2:152–158. https://doi.org/10.1016/j.bcab.2013.02.002
Aydinoǧlu T, Sargin S (2013) Production of laccase from Trametes versicolor by solid-state fermentation using olive leaves as a phenolic substrate. Bioprocess Biosyst Eng 36:215–222. https://doi.org/10.1007/s00449-012-0777-2
Machu L, Misurcova L, Ambrozova JV et al (2015) Phenolic content and antioxidant capacity in algal food products. Molecules 20:1118–1133. https://doi.org/10.3390/molecules20011118
Johannes C, Majcherczyk A (2000) Natural mediators in the oxidation of polycyclic aromatic hydrocarbons by laccase mediator systems. Appl Environ Microbiol 66:524–528
Banerjee R, Chintagunta AD, Ray S (2019) Laccase mediated delignification of pineapple leaf waste: an ecofriendly sustainable attempt towards valorization. BMC Chem 1–11. https://doi.org/10.1186/s13065-019-0576-9
Sluiter A, Hames B, Ruiz R et al (2008) Determination of structural carbohydrates and lignin in biomass: laboratory analytical procedure (LAP); Issue Date: April 2008; Revision Date: July 2011 (Version 07–08–2011) - 42618.pdf. Tech Rep NREL/ TP -510 -42618 1–15
Ruiz HA, Cerqueira MA, Silva HD et al (2013) Biorefinery valorization of autohydrolysis wheat straw hemicellulose to be applied in a polymer-blend film. Carbohydr Polym 92:2154–2162. https://doi.org/10.1016/j.carbpol.2012.11.054
Olguin-Maciel E, Larqué-Saavedra A, Pérez-Brito D et al (2017) Brosimum alicastrum as a novel starch source for bioethanol production. Energies 10. https://doi.org/10.3390/en10101574
Sun FF, Hong J, Hu J et al (2015) Accessory enzymes influence cellulase hydrolysis of the model substrate and the realistic lignocellulosic biomass. Enzyme Microb Technol 79–80:42–48. https://doi.org/10.1016/j.enzmictec.2015.06.020
Gonçalves FA, Ruiz HA, Nogueira CDC et al (2014) Comparison of delignified coconuts waste and cactus for fuel-ethanol production by the simultaneous and semi-simultaneous saccharification and fermentation strategies. Fuel 131:66–76. https://doi.org/10.1016/j.fuel.2014.04.021
Vasina DV, Mustafaev ON, Moiseenko KV et al (2015) The Trametes hirsuta 072 laccase multigene family: genes identification and transcriptional analysis under copper ions induction. Biochimie 116:154–164
Pereira-Patrón A, Solis-Pereira S, Lizama-Uc G et al (2019) Molecular characterization of laccase genes from the basidiomycete Trametes hirsuta Bm-2 and analysis of the 5′ untranslated region (5′UTR). 3 Biotech 9. https://doi.org/10.1007/s13205-019-1691-y
Tapia-Tussell R, Pereira-Patrón A, Alzate-Gaviria L et al (2020) Decolorization of textile effluent by Trametes hirsuta Bm-2 and lac-T as possible main laccase-contributing gene. Curr Microbiol 77:3953–3961. https://doi.org/10.1007/s00284-020-02188-9
Zapata-Castillo P (2012) Purification and characterization of laccase from Trametes hirsuta Bm-2 and its contribution to dye and effluent decolorization. African J Biotechnol 11:3603–3611. https://doi.org/10.5897/ajb11.2050
Ancona-Escalante W, Tapia-Tussell R, Pool-Yam L et al (2018) Laccase-mediator system produced by Trametes hirsuta Bm- 2 on lignocellulosic substrate improves dye decolorization. 3 Biotech 8(7):1–8
Tapia-Tussell R, Pérez-Brito D, Torres-Calzada C et al (2015) Laccase gene expression and vinasse biodegradation by Trametes hirsuta strain Bm-2. Molecules 20:15147–15157. https://doi.org/10.3390/molecules200815147
José Carlos DLM, Leonardo S, Jesús MC et al (2020) Solid-state fermentation with aspergillus niger gh1 to enhance polyphenolic content and antioxidative activity of castilla rose (purshia plicata). Plants 9(11):1518
Rodrigues CI, da Costa DM, Santos ACV, et al (2020) Assessment of in vitro anthelmintic activity and bio-guided chemical analysis of BRS Boyrá pineapple leaf extracts. Vet Parasitol 285. https://doi.org/10.1016/j.vetpar.2020.109219
Tychanowicz GK, De Souza DF, Souza CGM et al (2006) Copper improves the production of laccase by the white-rot fungus Pleurotus pulmonarius in solid state fermentation. Braz Arch Biol Technol 49:699–704. https://doi.org/10.1590/S1516-89132006000600002
Ch’ng HY, Ahmed OH, Kassim S, et al (2013) Co-composting of pineapple leaves and chicken manure slurry. Int J Recycl Org Waste Agric 2(1):1–8
Isroi Millati R, Syamsiah S et al (2011) Biological pretreatment of lignocelluloses with white-rot fungi and its applications: a review. BioResources 6:5224–5259. https://doi.org/10.15376/biores.6.4.5224-5259
Vasco-Correa J, Luo X, Li Y, Shah A (2019) Comparative study of changes in composition and structure during sequential fungal pretreatment of non-sterile lignocellulosic feedstocks. Ind Crops Prod 133:383–394. https://doi.org/10.1016/j.indcrop.2019.03.043
Janusz G, Pawlik A, Sulej J et al (2017) Lignin degradation: microorganisms, enzymes involved, genomes analysis and evolution. FEMS Microbiol Rev 41:941–962
Ralph J, Lapierre C, Boerjan W (2019) Lignin structure and its engineering. Curr Opin Biotechnol 56:240–249. https://doi.org/10.1016/j.copbio.2019.02.019
Janusz G, Pawlik A, Świderska-Burek U et al (2020) Laccase properties, physiological functions, and evolution. Int J Mol Sci 21:1–25
Yoon LW, Ang TN, Ngoh GC, Chua ASM (2014) Fungal solid-state fermentation and various methods of enhancement in cellulase production. Biomass Bioenerg 67:319–338. https://doi.org/10.1016/j.biombioe.2014.05.013
Moya R, Berrocal A, Rodríguez-Zúñiga A et al (2016) Biopulp from pineapple leaf fiber produced by colonization with two white-rot fungi: Trametes versicolor and Pleurotus ostreatus. BioResources 11:8756–8776. https://doi.org/10.15376/biores.11.4.8756-8776
Ilyas RA, Sapuan SM, Ishak MR, Zainudin ES (2017) Effect of delignification on the physical, thermal, chemical, and structural properties of sugar palm fibre. BioResources 12:8734–8754. https://doi.org/10.15376/biores.12.4.8734-8754
Cassellis MER, Pardo MES, López MR, Escobedo RM (2014) Structural, physicochemical and functional properties of industrial residues of pineapple (Ananas comosus). Cellul Chem Technol 48:633–641
Abraham E, Deepa B, Pothan LA et al (2011) Extraction of nanocellulose fibrils from lignocellulosic fibres: a novel approach. Carbohydr Polym 86:1468–1475. https://doi.org/10.1016/j.carbpol.2011.06.034
Cherian BM, Leão AL, de Souza SF et al (2010) Isolation of nanocellulose from pineapple leaf fibres by steam explosion. Carbohydr Polym 81:720–725. https://doi.org/10.1016/j.carbpol.2010.03.046
Mishra V, Jana AK (2019) Sweet sorghum bagasse pretreatment by Coriolus versicolor in mesh tray bioreactor for selective delignification and improved saccharification. Waste and Biomass Valorization 10:2689–2702. https://doi.org/10.1007/s12649-018-0276-z
Chintagunta AD, Ray S, Banerjee R (2017) An integrated bioprocess for bioethanol and biomanure production from pineapple leaf waste. J Clean Prod 165:1508–1516
Carrillo-Nieves D, Saldarriaga-Hernandez S, Gutiérrez-Soto G et al (2020) Biotransformation of agro-industrial waste to produce lignocellulolytic enzymes and bioethanol with a zero waste. Biomass Convers Biorefinery. https://doi.org/10.1007/s13399-020-00738-6
Costa JAV, Treichel H, Kumar V, Pandey A (2018) Advances in solid-state fermentation. In Current developments in biotechnology and bioengineering. Elsevier, pp 1–17
Acknowledgements
The authors wish to acknowledge Edwin J. Chan-González, R.E. and Jorge A. Dominguez-Maldonado, M.Sc., for the technical assistance. This research was funded by the Consejo Nacional de Ciencia y Tecnología (CONACYT) of Mexico through grant 297333, awarded for doctoral studies, CONACYT-SENER Energy Sustainability project no. 254667, and CONACYT Infrastructure project no. 253986.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chablé-Villacis, R., Olguin-Maciel, E., Toledano-Thompson, T. et al. Enzymatic hydrolysis assisted with ligninocellulolytic enzymes from Trametes hirsuta produced by pineapple leaf waste bioconversion in solid-state fermentation. Biomass Conv. Bioref. 13, 9095–9106 (2023). https://doi.org/10.1007/s13399-021-01851-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-021-01851-w