Abstract
The composition and physicochemical characteristics of polysaccharides extracted from spent liquors of catalytic and noncatalytic delignification of larch wood in the acetic acid-hydrogen peroxide medium were studied. The following were used as catalysts: (NH4)6Mo7O24, MnSO4, TiO2, and ZnSO4. The structure of polysaccharides was identified by FTIR and NMR spectroscopy. Their composition was established using gas chromatography, and the physicochemical characteristics were studied using gel permeation chromatography, scanning electron microscopy, thermogravimetric analysis, gas adsorption, and elemental analysis. It was shown that in the process of oxidative delignification, a significant amount of hemicelluloses and arabinogalactan passes into the spent liquor. The yield of polysaccharides was up to 16.9 wt%. Polysaccharides had a weight-average molecular weight of up to 22.9 kDa and a narrow molecular weight distribution.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Hemicelluloses are polysaccharides in a plant cell wall that represent the most widespread, after cellulose, renewable plant polymers in the lignocellulosic materials. They are a type of heteropolysaccharides with a complex structure, which can contain glucose, xylose, mannose, galactose, arabinose, rhamnose, and glucuronic and galacturonic acids in different amounts, depending on a source. The dominant coniferous wood hemicelluloses are partially acetylated galactoglucomannans and glucomannans (up to 20‒25% of the wood weight) [1] and arabinoglucuronoxylans (5‒10% of the wood weight) [2]. Larch wood contains arabinogalactan in a fraction of 5‒25% of the wood weight, depending on larch species [3].
The recent investigations showed the benefits of polymeric hemicelluloses and their potential for use in various fields. According to the data on the modification of hemicelluloses, these polymers can be used in production of new industrial materials, e.g., biomedical hydrogels [4] and biodegradable food wrap [5]. The unique colloidal properties of the galactoglucomannan extracted from spruce wood can be used in the development of functional nano- or microstructures separately or in combination with other components [6]. The spruce hemicelluloses have prospects for application such as partial substituents of synthetic polylactide in 3D printing [7] and for the treatment of chronic prostatic inflammation [8]. Deloule et al. [9] demonstrated the importance of softwood hemicelluloses as a potential source of prebiotics. Gautam et al. [10] used the hemicellulose extracted from pine needles as an effective adsorbent for removal of malachite green from wastewater. Mikkonen et al. [11] reported on new promising engineering emulsion systems for alkyd paints stabilized by the hemicelluloses extracted from birch and spruce.
In recent years, the extraction of hemicelluloses from wood raw materials has been studied intensively. Hemicelluloses were isolated by the hot-water extraction [12, 13], hot pressurized water pretreatment [14], alkaline hydrogen peroxide pretreatment [15], microwave-assisted extraction under atmospheric pressure [16, 17], and steam explosion extraction [18].
Currently, new methods of cellulose production are being developed, which is based on the peroxide delignification of wood in organic solvents, e.g., acetic and formic acids, and in the presence of various catalysts [19,20,21]. The treatment of wood with organic solvents as delignification media allows the lignocellulosic materials to be fractionated to produce lignin, cellulose, and hemicelluloses, thereby providing an excellent approach in terms of biomass use. The spent liquors consisting of soluble hemicelluloses and lignin depolymerization products do not contain toxic chlorine- and sulfur-containing compounds. Easy solvent recovery and environmental friendliness are important features of such processes [22].
A mixture of acetic acid and hydrogen peroxide is considered to be very effective in cleaving the aromatic rings of lignin and is also an environmentally friendly solvent [23]. Pretreatment with acetic acid with hydrogen peroxide is an effective approach to selectively remove lignin from biomass while retaining most of the carbohydrates [24, 25]. In addition, the presence of catalysts based on variable valence metals intensifies the delignification process due to the formation of peroxo complexes, which are strong oxidants and carry out the destruction of lignin [26, 27].
Studies [20, 21] were devoted to the oxidative catalytic delignification of various timber species in the hydrogen peroxide-acetic acid–water medium in the presence of the solid TiO2 catalyst and the soluble MnSO4 one. The study [19] compared the catalytic properties of the soluble (CuSO4, ZnSO4, MnSO4, FeSO4, and (NH4)6Mo7O24) and solid (TiO2, KN-30, and C-FeZSM-5 zeolites) catalysts for delignification of wood including the same solvent. The main attention in these works was paid to the yield and composition of solid cellulosic products.
This study aimed to examine the composition, structure, and physicochemical characteristics of the polysaccharides extracted from the spent liquor of catalytic and noncatalytic acetic acid-peroxide delignification of larch wood.
2 Experimental
2.1 Raw materials
The raw material used was sawdust (a fraction of 2.0–5.0 mm) of the Siberian larch (Larix Sibirica) grown in the Krasnoyarsk Territory. The sawdust chemical composition was determined using conventional wood chemistry analytical methods [28]. The main larch wood components (wt%) were cellulose (41.2 ± 0.8), lignin (28.1 ± 0.6), polysaccharides, hydrolysable with 2% hydrochloric acid (26.4 ± 0.5), extractive substances (2.8 ± 0.0), and ash (0.8 ± 0.0).
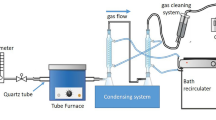
A scheme of the oxidative catalytic fractionation of larch wood in the acetic acid-hydrogen peroxide medium with the formation of cellulose and polysaccharides is shown in Fig. 1.
2.2 Larch wood delignification
The shredded larch wood was delignified in a 250-mL glass reactor equipped with a stirrer and a reflux condenser. The delignification solution consisted of glacial acetic acid (30 wt%), hydrogen peroxide (6 wt%), and distilled water. The liquid wood ratio (LWR) was 15. In this study, we used four catalysts ((NH4)6Mo7O24, MnSO4, TiO2, and ZnSO4), which demonstrate the high delignification efficiency, in a fraction of 1% of the wood weight. The delignification was performed for 4 h at temperatures of 90 °C and 100 °C under constant stirring. After completion of the delignification process, a solid residue (the cellulosic product) was separated from the spent liquor by filtration with a Buchner funnel, washing until neutral pH, and drying to the air-dry state.
2.3 Extraction of polysaccharides
The spent liquor (liquor 1 in Fig. 1) was concentrated on a rotary evaporator. Then, hot (about 60 °C) water was added at a volumetric ratio of 1:1 and driven off in a rotary evaporator. This procedure was performed twice to remove acetic acid. Polysaccharides were precipitated with a fivefold volume of ethanol (96% wt.) under slow stirring and then held for 12 h at a temperature of 4 °C. The obtained white curdled precipitate of polysaccharides was separated from the lignin depolymerization products (liquor 2 in Fig. 1) by filtration with a Buchner funnel, washed with ethanol, frozen, and dried in an Iney-6 freeze dryer. The yield of polysaccharides was determined in relation to the initial wood weight. The measurements were carried out in triplicate. Polysaccharides, obtained at a delignification temperature of 100 °C, were analyzed by Fourier-transform infrared (FTIR) and nuclear magnetic resonance (NMR) spectroscopy, gas chromatography (GC), gel permeation chromatography (GPC), scanning electron microscopy (SEM), and thermogravimetric analysis (TGA). The elemental composition and textural characteristics were examined.
2.4 Monosaccharide composition of polysaccharides
To determine the monosaccharide composition, the polysaccharides were hydrolyzed by the HCl solution (2%) for 3 h. The individual composition and monosaccharide content in the hydrolysates were determined on a VARIAN-450 GC gas chromatograph equipped with a flame ionization detector on a VF-624 ms capillary column with a length of 30 m and an inner diameter of 0.32 mm. A hydrolysate sample was pre-derivatized by the technique described in Ruiz-Matute et al. [29] with the formation of trimethylsilyl derivatives. The silylating reagent was a mixture of trimethylchlorosilane and hexamethyldisilazane in pyridine and sorbitol was used as an internal standard (IS). Standards for the analysis of the hydrolysates were glucose, arabinose, galactose, sorbitol, mannose, and xylose (Panreac, Germany).
2.5 Gel permeation chromatography
The weight-average molecular weight (Mw), number-average molecular weight (Mn), and polydispersity (PDI) of the polysaccharide samples were determined by GPC using an Agilent 1260 Infinity II Multi-Detector GPC/SEC System chromatograph with a refractive detector. The separation was made on two Agilent PL aquagel-OH columns using the solution of 0.2MNaNO3 + 0.01 M NaH2 PO4 in water (a pH of 7) as a mobile phase. The column was calibrated using the polyethylene glycol standards (Agilent, US). The eluent flow rate was 1 mL/min and the sample volume was 100 μL. Before the analysis, the samples were dissolved in the mobile phase (~ 5 mg/mL) and filtered through a 0.45-μm Agilent PES membrane filter (Millipore). The data collection and processing were performed using the Agilent GPC/SEC MDS software.
2.6 FTIR and NMR analysis of polysaccharides
The IR spectra were recorded on a Shimadzu IRTracer-100 FTIR spectrometer (Japan). Specimens for recording the IR absorption spectra were pressed in tablets containing 3 mg of the sample in a potassium bromide matrix. The NMR analysis (heteronuclear single quantum coherence (HSQC) spectroscopy) of polysaccharides was made on a Bruker Avance III 600 spectrometer (Germany). Before the analysis, the sample was completely dissolved in the DMSO-d6 solvent and placed into a 5-mm NMR tube at room temperature.
2.7 Elemental analysis
The elemental analysis of the polysaccharides isolated from the spent liquors of the catalytic and noncatalytic larch wood delignification was made on a Vario El Cube ELEMENTAR CHNSO analyzer (Germany).
2.8 Thermogravimetric analysis
The thermogravimetric analysis was made on a NETZSCH STA 449 F1 Jupiter simultaneous thermal analysis instrument (Germany). The polysaccharide samples were analyzed in argon at a heating rate of 10 °C · min‒1, temperatures from 30 to 900 °C, and protective and blowout gas flow rates of 20 and 50 mL ∙ min‒1, respectively. The Al2O3 cylindrical crucible with a perforated cover was used and a reference was the empty corundum crucible with a cover. The instrument was calibrated according to the specification using reference substances supplied with the instrument. The mass of the samples for analysis was determined on a Sartorius BP121S analytical lab scale digital balance. The measurement results were processed using the NETZSCH. Proteus Thermal Analysis.5.1.0 software supplied with the instrument.
2.9 Analysis of the pore volume and surface area
The textural characteristics of polysaccharides were obtained using the nitrogen adsorption and desorption isotherms measured on a Micromeritics ASAP 2020 automatic adsorption analyzer (US) at 77 K under relative pressures of P/P0 = 5 ∙ 10–6‒0.998. Before the adsorption measurements, the samples were degassed in vacuum for 12 h at 90 °C. The parameters used to identify the porous structure of the samples were specific surface area SBET and average pore size Dpore [30]. The total pore volume Vtot calculated by a single-point technique from the volume of nitrogen adsorbed under a relative pressure of P/P0 ≥ 0.98. The micropore volume Vmicro was calculated by the t-plot method [31].
2.10 Scanning electron microscopy
The morphology of the polysaccharide samples was identified on a Hitachi S5500 scanning electron microscope (Japan) with a cold tungsten cathode equipped with a Hitachi BF/DF DUO-STEM STEM detector at a variable electron collection angle, an accelerating voltage of 30 kV, and a spatial resolution of 0.4 nm in secondary-electron imaging.
3 Results and discussion
3.1 Delignification and yield of polysaccharides
The polysaccharides were extracted from the spent liquors of the catalytic and noncatalytic larch wood delignification after the cellulose isolation. The conditions for delignification were chosen on the basis of earlier study [19], where it was shown that, at a temperature of 100 °C, a hydrogen peroxide content of 6 wt%, an acetic acid of 30 wt%, and a LWR of 15, the deepest delignification of wood occurs, which ensures a residual lignin content below 1% in the cellulose product. In this case, water-soluble low-molecular-weight lignin degradation products, as well as polysaccharides, pass into the solution. The dissolved polysaccharides were isolated from the spent liquor by ethanol precipitation. The yields of polysaccharides extracted from the spent liquors of the oxidative catalytic and noncatalytic larch wood delignification are given in Table 1.
As shown in Table 1, the polysaccharide yield at a process temperature of 90 °C is lower than that at 100 °C. This is due to the fact that at 100 °C the process of wood delignification is more complete [19, 20] and, therefore, a greater amount of structural polysaccharides passes into liquor 1. The use of the (NH4)6Mo7O24 and MnSO4 catalysts ensures a fairly high yield of polysaccharides already at 90 °C. The TiO2 catalyst works much more efficiently at 100 °C than at 90 °C. When the ZnSO4 catalyst is used, the polysaccharide yield is at the lowest, which is apparently related to the strong destructive effect of this catalyst on polysaccharides.
3.2 Carbohydrate composition of polysaccharide mixture
The carbohydrate composition of the polysaccharides obtained by the oxidative catalytic and noncatalytic delignification of larch wood is illustrated in Fig. 2.
According to the literature data, the mannose:galactose:glucose ratio in galactoglucomannan of conifers is 3:1:1 and the galactose:arabinose ratio in arabinogalactan of larch wood is 6:1 [2, 3]. The high galactose and mannose contents in the samples show that galactoglucomannan and arabinogalactan are the predominant polysaccharides isolated from spent liquor. The presence of xylose is indicative of the presence of small amounts of arabinoglucuronoxylan, which is also a typical representative of softwood hemicelluloses. We see that the polysaccharides we have isolated are a mixture of structural wood polysaccharides (hemicelluloses) and arabinogalactan, which is an extractive polysaccharide. The lower galactose and arabinose contents in the polysaccharide mixture obtained with ZnSO4 suggest that this catalyst apparently degrades arabinogalactan stronger.
3.3 FTIR and NMR spectroscopy of polysaccharides
The FTIR spectra of the samples contain all bands characteristic of heteropolysaccharides (Fig. 3), which are described in detail in [18, 32,33,34,35]. The absorption bands at 3433 and 2924 cm‒1 refer to the O‒H and C‒H stretching, respectively. The bands at 1377 and 1245 cm‒1 correspond to the O‒H and C‒O bending in polysaccharides. The band at 893 cm‒1 corresponds to the vibrations of the C1 atom and four surrounding atoms, which is typical of the β-glycosidic bonds between sugar molecules. It can be seen that all the polysaccharide samples are acetylated, which is reflected in the three acetyl ether bands at 1736 cm‒1 (ester C = O), 1377 cm−1 (–C–CH3), and 1245 cm–1(–C–O–, the stretching band). The band at 1635 cm‒1 corresponds to the absorbed water bending. The band at 1075 cm‒1 corresponds to stretching of the C‒O bond in the HC‒OH group of the pyranose ring. The polysaccharide samples are free of residual lignin since the spectra do not include the lignin absorption band at 1520 cm‒1.
The HSQC NMR study allowed us to identify and compare the polysaccharide structures (Fig. 4). The HSQC spectra contain typical light circles expected for polysaccharide fragments; the cross-peak signals were assigned according to reliable works [36,37,38]. The spectral region δC/δH 55–82/2.9–3.9 contains the signals characteristic of C2, C3, and C4 mannose, galactose, glucose, xylose, and arabinose atoms. The spectral region δC/δH 61–63/3.15–3.9 contains the signals from C5 and C6 carbohydrate atoms. The H1/C1 cross peaks assigned to the mannose, glucose, galactose, and xylose molecules were observed at 4.75/99.0, 4.55/101.0, 4.29/103.0, and 4.16/103.1 ppm, respectively.
The two peaks at δC/ δH 71.3/5.38 and 73.0/4.75 ppm in the spectra indicate that the hydroxyl groups on carbon 2 and 3 of mannose links are partially acetylated in all the samples. Such an acetylation type is typical of native hemicelluloses of coniferous wood. The spectrum of the sample obtained with the MnSO4 catalyst includes no signals of carbon atoms associated with the acetyl groups and C1 carbohydrate atoms. This is apparently due to the fact that manganese forms complexes with polysaccharides, which leads to the paramagnetic broadening of the NMR signals and complicates their observation. Such complexes were described for arabinogalactan in Mercê et al. [39].
3.4 Molecular weight distribution
To illustrate the degree of depolymerization of the polysaccharides at the catalytic and noncatalytic delignification of larch wood, the molecular weights were determined by GPC using a refractive detector. The obtained weight- and number-average molecular weights of the polysaccharide samples and the polydispersity indices are given in Table 2.
According to the literature data, the weight-average molar weight Mw of native acetylated galactoglucomannan can lie between 20 and 50 kDa [1, 3] and for arabinogalactan, between 20 and 100 kDa [3]. Chadni et al. [16] studied the hemicelluloses isolated from spruce sawdust in a neutral medium at 100 °C for 1 h with presoaking and reported an Mn value of 20.730 kDa, an Mw value of 37.870 kDa, and a PDI of 1.82 for them. In the study [18], the same research team examined the effect of steam explosion conditions on the extraction of hemicelluloses from spruce sawdust,the weight-average molecular weight Mw of hemicelluloses obtained in a neutral medium was found to be 9‒27 kDa and the PDI value was 2.3‒2.6. The work [40] studied the high-temperature extraction of galactoglucomannan from pine wood. Mw at optimum extraction parameters of 7 kDa at 170° and an extraction time of 20 min.
Treatment with an organic acid in an oxidizing environment in the presence of various catalysts results in leads to partial destruction of the macromolecular structure of polysaccharides, which is associated with the rupture of glycosidic bonds between polysaccharide molecules. This is especially the case for the polysaccharides obtained with the soluble catalysts (NH4)6Mo7O24, MnSO4, and ZnSO4. The number-average molecular weights Mn of the obtained polysaccharides range from 11.985 to 14.150 kDa and the weight-average molecular weights Mw from 1.584 to 22.946 kDa. The low (1.31‒1.65) polydispersity indices indicate that the isolated polysaccharides are uniform in the molecular weight. Molecular weight distribution curves of larch wood polysaccharides obtained by catalytic and noncatalytic delignification are shown in Fig. 5.
3.5 Elemental composition
The elemental composition of the polysaccharides isolated from the spent liquors of the catalytic and noncatalytic delignification of larch wood is given in Table 3. The polysaccharides isolated from the spent liquors of the noncatalytic delignification and in the presence of the solid TiO2 catalyst have the high H/C and O/C atomic ratios (Table 3). This points out that the destruction of the polysaccharide macromolecular structure occurs mainly via detaching the side chains with the acetylation of the end groups [41]. When the soluble (NH4)6Mo7O24, MnSO4, and ZnSO4 catalysts are used, the glycosidic bonds between the main polysaccharides chain links appear to be depolymerized, which is consistent with their lower molecular weights (Table 2).
3.6 Thermal analysis of polysaccharides
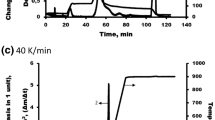
The thermal properties of the polysaccharide samples were studied by TGA. Figure 6 presents the derivative thermogravimetric (DTG) and thermogravimetric (TG) curves for the polysaccharides isolated from the spent liquors.
The polysaccharides extracted from larch wood are resistant to the heat up to ~ 200 °C and, upon further heating, begin destructing at an increasing rate. The thermal decomposition of the polysaccharide samples can be divided into three phases. In the first phase, the weight loss observed up to 100 °C is caused by evaporation of adsorbed water. In the second phase, the intense decomposition of the polysaccharides occurs, which begins at ~ 200 °C for all the samples. As the temperature increases above 200 °C, the thermal destruction reactions with hemolytic opening of the glycosidic and C‒C bonds in the monosaccharide links already occur [42]. In the investigated polysaccharide samples, the most rapid weight loss was observed at 280‒313 °C. The intense decomposition ends at a temperature of ~ 390 °C for the four samples and 420 °C for the sample obtained with ZnSO4. In the last phase, when the temperature exceeds 390 °C, the weight loss is insignificant and the removal of volatiles occurs until the temperature reaches 899.8 °C. Table 4 gives the data of the main stages of decomposition of the polysaccharides extracted from the spent liquor of the catalytic and noncatalytic larch wood delignification.
The presented results show that polysaccharides obtained with TiO2 and without catalyst have higher thermal stability. The lowest thermal stability is observed in the samples obtained with (NH4)6Mo7O24 and ZnSO4.
3.7 Pore volume and surface area
Specific surface area SBET, pore size distribution, and total pore volume are important indicators for further modification of polysaccharides and creation of composites.
The data given in Table 5 show that the textural characteristics of the polysaccharides extracted from the spent liquors of the catalytic and noncatalytic delignification of larch wood are very close. All samples contain a small amount of micropores. The lowest surface area is observed for the PSZn sample, which is consistent with the analysis of the monosaccharide composition of this sample (see 3.2). The analysis showed that the PSZn sample contained less arabinogalactan, which is a more branched polysaccharide than galactoglucomannan.
3.8 Scanning electron microscopy
Polysaccharides extracted from spent liquors of oxidative catalytic and noncatalytic larch wood delignification were also studied by the SEM method. SEM images (Fig. S2) show irregular coral formations that are hierarchical and indicate a highly branched amorphous polysaccharide structure.
The obtained polysaccharides can be used for the synthesis of sulfated derivatives that have anticoagulant properties and other types of biological activity [43]. Glucomannans can be candidates for use as potential therapeutic agents for the treatment of a number of physiological disorders [44], and they are widely used in the food industry [45]. It is also noted that heteropolysaccharides consisting of glucose, galactose, mannose, arabinose, rhamnose, xylose, and other monosaccharides have anticancer activity [46, 47].
4 Conclusions
Polysaccharides isolated from the spent liquors of catalytic and noncatalytic larch wood delignification are a mixture of structural polysaccharides and arabinogalactan. The polysaccharide yield ranges from 12.6 to 16.9 wt%, depending on the process temperature and the catalyst used. The catalyst used affects the molecular weight, elemental and monosaccharide composition, and textural and thermal properties of polysaccharides.
PSMn has the highest content of galactose and arabinose and the lowest content of mannose; PSZn has the highest content of mannose and glucose and the lowest content of galactose. The structure of polysaccharides was identified by FTIR and NMR spectroscopy. FTIR spectroscopy showed the absence of absorption bands of phenylpropane units, which indicates that the polysaccharide samples do not contain residual lignin. DTG analysis shows that PSTi and PSno cat have higher thermal stability, while PSMo and PSZn show lower thermal stability. PSTi and PSno cat have a weight-average molecular weight Mw of 18.80 and 22.9 kDa and a polydispersity index of 1.33 and 1.65, respectively. PSMo, PSMn, and PSZn have lower molecular weights Mw 16.5–17.5 kDa and polydispersity index 1.31–1.38. The specific surface area SBET for PSno cat, PSMo, PSMn, and PSTi was 20.3–32.0 m2/g, and for PSZn − 4.1 m2/g, which is associated with the lowest content of arabinogalactan.
These polysaccharides can be used to create bioactive composites, multicomponent coatings, and fillers for the food and pharmaceutical industries.
References
Willför S, Sundberg K, Tenkanen M, Holmbom B (2008) Spruce-derived mannans – a potential raw material for hydrocolloids and novel advanced natural materials. Carbohyd Polym 72:197–210
Bajpai P (2018) Chapter 2 - Wood and fiber fundamentals. In: Bajpai P (ed) Biermann’s handbook of pulp and paper, 3rd edn. Elsevier, pp 19–74
Timell TE (1967) Recent progress in the chemistry of wood hemicelluloses. Wood Sci Technol 1:45–70
Markstedt K, Xu W, Liu J, Xu C, Gatenholm P (2017) Synthesis of tunable hydrogels based on O-acetyl-galactoglucomannans from spruce. Carbohyd Polym 157:1349–1357
Mendes FRS, Bastos MSR, Mendes LG, Silva ARA, Sousa FD, Monteiro-Moreira ACO, Cheng HN, Biswas A, Moreira RA (2017) Preparation and evaluation of hemicellulose films and their blends. Food Hydrocolloids 70:181–190
Bhattarai M, Valoppi F, Hirvonen S-P, Hietala S, Kilpeläinen P, Aseyev V, Mikkonen KS (2020) Time-dependent self-association of spruce galactoglucomannans depends on pH and mechanical shearing. Food Hydrocolloids 102:105607
Xu W, Pranovich A, Uppstu P, Wang X, Kronlund D, Hemming J, Öblom H, Moritz N, Preis M, Sandler N, Willför S, Xu C (2018) Novel biorenewable composite of wood polysaccharide and polylactic acid for three dimensional printing. Carbohyd Polym 187:51–58
Konkol Y, Vuorikoski H, Tuomela J, Holmbom B, Bernoulli J (2017) Galactoglucomannan-rich hemicellulose extract from Norway spruce (Picea abies) exerts beneficial effects on chronic prostatic inflammation and lower urinary tract symptoms in vivo. Int J Biol Macromol 101:222–229
Deloule V, Boisset C, Hannani D, Suau A, Le Gouellec A, Chroboczek J, Botté C, Yamaryo-Botté Y, Chirat C, Toussaint B (2020) Prebiotic role of softwood hemicellulose in healthy mice model. J Funct Foods 64:103688
Gautam D, Kumari S, Ram B, Chauhan GS, Chauhan K (2018) A new hemicellulose-based adsorbent for malachite green. J Environ Chem Eng 6:3889–3897
Mikkonen KS, Kirjoranta S, Xu C, Hemming J, Pranovich A, Bhattarai M, Peltonen L, Kilpeläinen P, Maina N, Tenkanen M, Lehtonen M, Willför S (2019) Environmentally-compatible alkyd paints stabilized by wood hemicelluloses. Ind Crops Prod 133:212–220
Krogell J, Korotkova E, Eränen K, Pranovich A, Salmi T, Murzin D, Willför S (2013) Intensification of hemicellulose hot-water extraction from spruce wood in a batch extractor – effects of wood particle size. Biores Technol 143:212–220
Song T, Pranovich A, Holmbom B (2011) Effects of pH control with phthalate buffers on hot-water extraction of hemicelluloses from spruce wood. Biores Technol 102:10518–10523
Gallina G, Cabeza Á, Grénman H, Biasi P, García-Serna J, Salmi T (2018) Hemicellulose extraction by hot pressurized water pretreatment at 160℃ for 10 different woods: yield and molecular weight. J Supercrit Fluids 133:716–725
Alvarez-Vasco C, Zhang X (2017) Alkaline hydrogen peroxide (AHP) pretreatment of softwood: enhanced enzymatic hydrolysability at low peroxide loadings. Biomass Bioenerg 96:96–102
Chadni M, Bals O, Ziegler-Devin I, Brosse N, Grimi N (2019a) Microwave-assisted extraction of high-molecular-weight hemicelluloses from spruce wood. C R Chim 22:574–584
Gulbrandsen TA, Johnsen IA, Opedal M, Toven K, Øyaas K, Pranovich A, Mikkola JP, Hoff B (2015) Extracting hemicelluloses from softwood and bagasse as oligosaccharides using pure water and microwave heating. Cellul Chem Technol 49:117–126
Chadni M, Grimi N, Bals O, Ziegler-Devin I, Brosse N (2019b) Steam explosion process for the selective extraction of hemicelluloses polymers from spruce sawdust. Ind Crops Prod 141:111–757
Kuznetsov BN, Sudakova IG, Garyntseva NV, Tarabanko VE, Yatsenkova OV, Djakovitch L, Rataboul F (2021) Processes of catalytic oxidation for the production of chemicals from softwood biomass. Catal Today 375:132–144. https://doi.org/10.1016/j.cattod.2020.05.044
Sudakova IG, Garyntseva NV, Chudina AI, Kuznetsov BN (2020) Experimental and mathematical optimization of the peroxide delignification of larch wood in the presence of MnSO4 catalyst. Catal Ind 12:265–272
Kuznetsov BN, Sudakova IG, Yatsenkova OV, Garyntseva NV, Rataboul F, Djakovitch L (2018) Optimizing single-stage processes of microcrystalline cellulose production via the peroxide delignification of wood in the presence of a Titania Catalyst. Catal Ind 10:360–367
Vila C, Santos V, Parajó JC (2003) Simulation of an organosolv pulping process: generalized material balances and design calculations. Ind Eng Chem Res 42:349–356
Khama L, Bigot YL, Delmas M, Avignon G (2005) Delignification of wheat straw using a mixture of carboxylic acid and peroxoacids. Ind Crops Prod 21:9–15
Bragatto J, Segato F, Squina FM (2013) Production of xylooligosaccharides (XOS) from delignified sugarcane bagasse by peroxide-HAc process using recombinatant xylanase from Bacillus subtilis. Ind Crop Prod 51:123–129
Wen P, Zhang T, Wang J, Lian Z, Zhang J (2019) Production of xylooligosaccharides and monosaccharides from poplar by a two-step acetic acid and peroxide/acetic acid pretreatment. Biotechnol Biofuels 12:87
Suchy M, Argyropoulos DS (2002) Catalysis and activation of oxygen and peroxide delignification of chemical pulps: a review. Tappi J 1:16
Ma R, Xu Y, Zhang X (2015) Catalytic oxidation of biorefinery lignin to value-added chemicals to support sustainable biofuel production. Chemsuschem 8:24–51
Sjöström E, Alén R (1999) Analytical methods of wood chemistry, pulping, and papermaking. Springer, Berlin, Heidelberg
Ruiz-Matute AI, Hernández-Hernández O, Rodríguez-Sánchez S, Sanz ML, Martínez-Castro I (2011) Derivatization of carbohydrates for GC and GC–MS analyses. J Chromatogr B 879:1226–1240
Rouquerol J, Avnir D, Fairbridge CW, Everett DH, Haynes JH, Pernicone N, Ramsay JDF, Sing KSW, Unger KK (1994) Recommendations for the characterization of porous solids [IUPAC Recommendations 1994]. Pure Appl Chem 66:1739–1758. https://doi.org/10.1351/pac199466081739
Scherdel C, Reichenauer G, Wiener M (2010) Relationship between pore volumes and surface areas derived from the evaluation of N2-sorption data by DR-, BET- and t-plot. Microporous Mesoporous Mater 132:572–575
Kac̆uráková M, Capek P, Sasinková V, Wellner N, Ebringerová A (2000) FT-IR study of plant cell wall model compounds: pectic polysaccharides and hemicelluloses. Carbohyd Polym 43:195–203
Muthu S, Renuga S (2014) Vibrational spectra and normal coordinate analysis of 2-hydroxy-3-(2-methoxyphenoxy) propyl carbamate. Spectrochim Acta Part A Mol Biomol Spectrosc 132:313–325
Oinonen P, Krawczyk H, Ek M, Henriksson G, Moriana R (2016) Bioinspired composites from cross-linked galactoglucomannan and microfibrillated cellulose: thermal, mechanical and oxygen barrier properties. Carbohyd Polym 136:146–153
Peng F, Ren J-L, Xu F, Bian J, Peng P, Sun R-C (2009) Comparative study of hemicelluloses obtained by graded ethanol precipitation from sugarcane bagasse. J Agric Food Chem 57:6305–6317
Capek P, Alföldi J, Lišková D (2002) An acetylated galactoglucomannan from Picea abies L. Karst. Carbohydr Res 337:1033–1037
Giummarella N, Lawoko M (2017) Structural insights on recalcitrance during hydrothermal hemicellulose extraction from wood. ACS Sustain Chem Eng 5:5156–5165
Tang S, Jiang M, Huang C, Lai C, Fan Y, Yong Q (2018) Characterization of arabinogalactans from Larix principis-rupprechtii and their effects on NO production by macrophages. Carbohyd Polym 200:408–415
Mercê ALR, Landaluze JS, Mangrich AS, Szpoganicz B, Sierakowski MR (2001) Complexes of arabinogalactan of Pereskia aculeata and Co2+, Cu2+, Mn2+, and Ni2+. Biores Technol 76:29–37
Benouadah N, Pranovich A, Aliouche D, Labidi J, Willför S (2021) Optimization of the extraction of galactoglucomannans from Pinus halepensis. Holzforschung 75:563–573
Xu F, Liu CF, Geng ZC, Sun JX, Sun RC, Hei BH, Lin L, Wu SB, Je J (2006) Characterisation of degraded organosolv hemicelluloses from wheat straw. Polym Degrad Stab 91:1880–1886
Yang H, Yan R, Chen H, Lee DH, Zheng C (2007) Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 86:1781–1788
Kazachenko AS, Malyar YN, Vasilyeva NY, Fetisova OY, Chudina AI, Sudakova IG, Antonov AV, Borovkova VS, Kuznetsova SA (2021) Isolation and sulfation of galactoglucomannan from larch wood (Larix sibirica). Wood Sci Technol 55:1091–1107
Tester RF, Al-Ghazzewi FH (2013) Mannans and health, with a special focus on glucomannans. Food Res Int 50:384–391
Singh S, Singh G, Arya SK (2018) Mannans: an overview of properties and application in food products. Int J Biol Macromol 119:79–95
Carlotto J, de Almeida Veiga A, de Souza LM, Cipriani TR (2020) Polysaccharide fractions from Handroanthus heptaphyllus and Handroanthus albus barks: structural characterization and cytotoxic activity. Int J Biol Macromol 165:849–856
Li N, Wang C, Georgiev MI, Bajpai VK, Tundis R, Simal-Gandara J, Lu X, Xiao J, Tang X, Qiao X (2021) Advances in dietary polysaccharides as anticancer agents: structure-activity relationship. Trends Food Sci Technol 111:360–377
Acknowledgements
FTIR and NMR analysis, gel permeation chromatography, elemental and thermogravimetric analysis, and SEM observations were done using the equipment of Krasnoyarsk Regional Research Equipment Centre of SB RAS.
Funding
The reported study was funded by RFBR, project number 20–33-70256. This work was conducted within the framework of the budget project # 0287–2021-0012 for Institute of Chemistry and Chemical Technology SB RAS.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chudina, A.I., Malyar, Y.N., Sudakova, I.G. et al. Physicochemical characteristics of polysaccharides from catalytic and noncatalytic acetic acid-peroxide delignification of larch wood. Biomass Conv. Bioref. 13, 9765–9774 (2023). https://doi.org/10.1007/s13399-021-01833-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-021-01833-y