Abstract
In this present study, it aimed to characterize biomass and obtain charcoal by means of pyrolysis of Cocos nucifera L. from a fractional planning 24-1 to be used in the removal of methylene blue. The investigated effects were temperature of 500 and 550 °C, heating rate 10 and 20 °C/ min, gas flow 1 and 4 mL/ min, and time of 30 and 60 min. The biomass composition showed values for cellulose 36%, hemicellosis 11.2%, lignin 32.1%, calorific value of 17.5 MJKg-1, density 0.158 g / mL, carbon 45.1%, hydrogen 6.8%, oxygen 0.4%, and nitrogen 47.7%. The results indicate that among the investigated effects, only the temperature influenced the charcoal yield. The best yield was at 500 °C with a specific surface area of 30.3801m2/ g, a maximum methylene blue adsorption capacity of 67.7%, and better fit to the Langmuir isotherm model with a correlation coefficient R2 = 0.9804. Regarding thermal analysis, it was observed that above 250 °C lignocellulosic thermal decomposition begins. The charcoal presented uniform size and pores, which influenced the adsorptive process of removing methylene blue.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The thermogravimetric treatment of residues of cocos nucifera L. using the rapid pyrolysis technique for the production of charcoal is justified in the provisions of Law 12.305 / 2010, which institutes the National Policy for Solid Waste (PNRS) in Brazil. This law provides for the non-generation, reduction, reuse, recycling, and treatment of solid waste [1, 2].
The use of solid urban waste in the thermal process contributes to the diversification of the Brazilian energy plan. These are presented as raw material in the thermogravimetric treatment to obtain biofuels, such as charcoal [3]. The use of the Cocos nucifera L. residue resulting from industrialization reduces the inadequate disposition and adds value to the production chain.
Pyrolysis is a thermal degradation treatment of lignocellulosic biomass carried out in the total or partial absence of oxygen under an inert atmosphere that consists of producing charcoal, bio oil, and gas. The final yield for each product depends on the investigated effects [4].
The residues of Cocos nucifera L. for the production of charcoal is justified by its low cost, availability in the country and by the need to know its characteristics and behavior in terms of heat treatment at different temperatures, as well as obtaining information related to the removal of contaminants through charcoal [5, 6].
The pyrolysis temperatures 500 and 550 °C were chosen in order to verify the charcoal yield in a small temperature variation and to generate information for future research. The stability of the produced charcoal depends on the composition of the biomass and the effects investigated in the process. Pyrolysis when carried out at high temperatures, the other factors do not influence it. The high rate of heating increases the combustion of volatiles without interfering with charcoal yields [[7, 8]].
Residues of Cocos nucifera L. have already been studied in the pyrolysis process at different temperatures (350, 450, and 550 °C), and it is reported that higher levels of fixed carbon can be obtained at higher temperatures [9].
The methylene blue cationic dye in aqueous solution serves as a model compound for adsorption of organic contaminants. The Cocos nucifera L. charcoal was produced, characterized in terms of its properties and adsorption capacity in order to produce knowledge for future research.
In this study, the objective was to characterize the biomass, to obtain charcoal by means of pyrolysis of Cocos nucifera L. from a fractional planning 24-1 to be used in the removal of methylene blue.
2 Materials and methods
2.1 Waste collection, preparation, and characterization of biomass and charcoal
The material under study is the Cocos nucifera L. residue, consisting of the epidermis, fibrous mesocarp, endocarp, and albumen. Cocos nucifera L. waste was collected from a park in the urban sector of Palmas in Tocantins, Brazil. Figure 1 presents the methodological evolution of the procedures performed in the research.
All procedures employed in biomass characterization are presented in Table 1. Thermogravimetric characterization-TGA was performed with heating rates of 10 and 30 °C / min. Thermogravimetric analyzer (Shimadzu Brand and Model TGA-50).
2.2 Pyrolysis unit
The conversion was performed in a fixed pyrolysis reactor that has a 100 °C stainless steel bipartite furnace, where a 100-cm long quartz tube and 10 cm external diameter is coupled. The reactor is of the FLYEVER brand of model FE50RPN, line 05/50 with micro controller.
The quartz tube is the region where the thermogravimetric process occurs. The carrier gas traverses the entire dimension by dragging the liquid and gaseous fractions of the reaction bed.
The pyrolysis process was performed in a fixed bed reactor with a split reclining oven heated at temperatures of 500 and 550 oC. The total residence time of the biomass in the reactor was 2 h and the residence time of the vapors was considered the period of occurrence of the carrier gas flow. Figure 2 shows the pyrolytic treatment unit used for thermogravimetric conversion of biomass to solid fraction.
For mass balance purposes, the solid product was collected directly from the reactor, and the pyrolytic liquids were recovered in the vapor condensation system and had their yields calculated.
2.3 Experimental procedure
The experimental procedure in this study was divided into three steps whose operating conditions are found in the Table 2.
For the determination of charcoal and liquid through fast pyrolysis, a fractional experimental multivariate planning in the order of 24-1 was used, in which a total of 11 processes were performed. The investigated effects were temperature (°C), heating rate (°C/min), gas flow (mL/min), and reaction time (min). The domains of the parameter ranges studied in this research have already been studied by other researchers [8, 9].
The number of factors whose effects are to be verified is directly proportional to the factorial order of the planning and thus to the number of interactions. With the factorial design in the order of 2k, it also increases the number of interactions.
The fractional planning allows only obtaining information of main effects in the process. The choice for fractional planning was due to its advantages such as the reduction of the number of experiments and consequently the reduction of analysis costs and optimization of time without compromising the process. The experiments were performed according to Table 3.
3 Results and discussion
3.1 Biomass Characterization
Table 4 informs a value related to the characterization of the residual biomass of Cocos nucifera L.
Table 4 shows the average density of the Cocos nucifera L. fiber approximately 0.16 g/mL. The low value suggests a porous structure, with empty spaces filled with air.
The elementary analysis allows the identification of the parts percentage: carbon, nitrogen, and hydrogen, which are important parts on the production of the desirable final solid product, which is charcoal, and, therefore, related to the presence of mineral substances, such as calcium, potassium, phosphorous, magnesium, iron, and sodium present in the soil or in fertilization.
The humidity is the weight loss suffered by the product when heated in conditions where water is removed. And under these conditions, some volatile substances that make up the biomass are also removed.
It is important to understand that low humidity values are interesting for several reasons, as they interfere with the calorific value of the solid product, which is the main criterion for fuel quality. The higher the humidity content of the wood, the lower its combustion power, due to the humidity evaporation process, which absorbs the combustion energy. According to Vale et al. [10], the high humidity content can contribute to the spread of the fungus, degradation of matter and even increase transportation costs.
The residual biomass of the Cocos nucifera L. presented an average of humidity value of 7.6%, slightly lower when compared with the values presented by Tsai, Lee, and Chang [11] that obtained humidity of approximately 11.26%.
The quantity of volatile components determines the ignition potential of any given material. When quantified the material expresses the potential of reactivity on the presence of heat. The average value of volatile materials identified on the studied biomass was 81.5%, a higher value than the one identified by Cortez et al. [12], which was 73.30%.
The level of fixed carbon (FC) found was of 8.92% and represents the more stable compounds that form the structure of the studied biomass. The fixed carbon contributes to the formation of charcoal and bio-oil when submitted to the process of pyrolysis.
On the elementary analysis of the Cocos nucifera L. fiber, the identified values are informed on Table 4 and are similar to the ones found by Cortez et al. [12] and De Andrade et al. [9], especially the level of carbon, which was respectively of 42.11 and 48.23% for the carbon. According to Cortez et al. [12], the values obtained on the elementary analysis can vary because of the variety analyzed, as well as the maturation level.
The energetic potential of plant biomass is related to the calorific value and a higher concentration of carbon, while the presence of sulfur and nitrogen indicates a potential polluter to the environment, since after combustion they are liable to form compounds such as nitrogen oxides (NOx) and sulfur (SOx).
The decomposition of the chemical compounds occurs in different temperature ranges, while the hemicellulose and the cellulose are decomposed on temperature ranges of 220–315 °C e 315–400 °C, respectively. The lignin will decompose on a much broad temperature range of 160–900 °C.
The determination of lignocellulosic material levels is very important, as they are similar to the values obtained from the products on the end of the pyrolysis. The percentual of lignocellulosic materials of Cocos nucifera L. fiber found was of 36.0% of cellulose, 11.2% of hemicellulose and 32.1% of lignin. It is worth to note that the difference obtained for the value of 100% is related to the level of ashes and extractives that are not evaluated here.
For Demirbas [13], the level of lignin is directly related to the calorific power of the vegetal biomass. According to Demirbas [13], the higher the level of carbon is, the higher will be the thermal stability, which is a indicative of quality and production of vegetal charcoal.
The superior calorific power found was of 17.5 MJKg-1, relatively equal to the value obtained by Cortez et al. [12], who obtained 17.41, and lower than the values of 20.51 and 22.83 found by Tsamba, Yang, and Blasiak [14] and Tsai, Lee, and Chang [11], respectively.
On the analysis of the X-ray fluorescence spectroscopy of the Cocos nucifera L. fiber, the five chemical elements that presented the highest concentration were K, Ca, Cl, Si, and P. According to Antonkiewicz et al. [15], the elements K, Ca, and P are macronutrients essential to the development of the plants, as they are easily absorbed in the soil.
3.1.1 Thermogravimetric analysis (TG) e derivative thermogravimetric
Figure 3 shows the thermogravimetric curves (TG) and the derivative thermogravimetric (DTB). The curves were obtained with the application of heating rates of 10 and 30 °C/min.
Through the ATG/ DTG curves, it is possible to observe the events that occurred during the decomposition process of the Cocos nucifera L. fiber. The ATG curve presents a decline on the range of 25–150 °C, which corresponds to a mass volume loss of 10.84%. This loss is related to the elimination of water present on the fiber.
On the temperature range of 150–650 °C, observed the second event of the fiber decomposition is observed, which corresponds to a mass volume loss of 57.04%. Therefore, this is the most intense event of the matter decomposition with a significative mass volume loss, which corresponds to the thermal degradation of the lignocellulosic components of the Cocos nucifera L. fiber.
The second event occurs on a large temperature scale, and this happens because of the degradation of substances which possesses on its composition groups of oxygen on the surface suggesting that these are respectively the hemicellulose and cellulose.
When observing the DTG curve, it is possible to confirm that the highest variation on the thermal degradation process occurs on the second event, most precisely on the temperature range of 278.56–327.99 °C on a heating rate of 2.51 min/°C.
After the temperature reaches 650 °C, there are no more relevant events, and a stability process is observed, possibly connected to carbolic groups that are more stable than the oxygenic groups previously described, which possibly happens because of the thermal degradation of the lignin.
Albinante [16] informs that the natural fibers are composed by cellulose, hemicellulose, pectin, lignin, and extractives (fats, proteins, and inorganic salts), with the cellulose being responsible for its resistance, because of its higher level of polymerization and molecular orientation.
3.1.2 Spectroscopy of Infrared With Altered Fourrier –FTIR
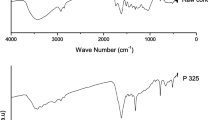
Figure 4 informs the infrared spectrum of the Cocos nucifera L. biomass obtained through the FTIR technique. The interpretation was based on the tables of Silverstein et al. [17] and Pedroza et al. [18], and the results are demonstrated on Table 5.
3.1.3 Scanning Electron Microscope- SEM
The determination of the structure of a surface was obtained through a scanning electron microscope. Figures 5 and 6 show a micro image of the fiber Cocos nucifera L. with a porous structure in evidence.
It is possible to observe a rough surface, with the presence of cracks, as well as the presence of microcrystals, which can be deposits of silicates, inorganic substances impregnated in the fiber as shown in Fig. 6. The silicates, which are compounds formed by silicon and oxygen atoms (SixOy) are minerals found on rocks, so their presence shows the influence of the soil on the composition of the Cocos nucifera L. fiber. It is possible to infer according to the image that this is not a compact structure, which suggests a matter with the presence of empty spaces. These empty spaces can contribute with the transfer of heat and the thermogravimetric degradation.
3.2 Results of the pyrolysis products on the experimental planning24-1
A mass balance with the goal of obtaining the results of the pyrolysis for solid and liquid fractions was performed. The results of the products of the Cocos nucifera L. fiber pyrolysis obtained with the experimental planning 24-1 on a fixed pyrolysis reactor are presented in Table 6.
The maximum results of pyrolytic liquid product (bio oil + acid extract) was 37.43% at a temperature of 500 °C in an inert gas outflow rate of 1 mL/ min, heating rate of 20 °C/min, and 60 min pyrolysis time, while the minimum result was 28.39% at a temperature of 500 °C in an inert gas outflow rate of 1 mL/ min, heating rate of 10 °C/min, and 30 min pyrolysis time.
The maximum results of Cocos nucifera L. fiber charcoal obtained were 35.57% at a temperature of 500 °C in an inert gas outflow rate of 1 mL/min, heating rate of 20 °C/min, and 60 min pyrolysis time, while the minimum result was 28.39% at a temperature of 500 °C in a 4 mL/ min inert gas flow, a heating rate of 10 °C/min and a pyrolysis time of 60 min and the minimum yield was 20.18% at 550 °C in an inert gas outflow rate of 1 mL/ min, heating rate of 20 °C/min, and pyrolysis time of 30 min.
In the study of pyrolysis results in the central point presented in Table 6, it was possible to verify that the average charcoal result was over to 35% in which it resembles tests 1 and 5 which also has the highest charcoal results according to the experimental design studied.
To verify the influence of the studied parameters on the charcoal production, it was used on Statistica Software version 10 to evaluate the effects shown in Fig. 7.
It is possible to observe that none of the studied factors has influence on the pyrolysis process for charcoal and bio oil production, since the values of the parameters used in the experimental design were not statistically significant, which may possibly attributed to the losses resulting from the pyrolysis process in the reactor used as shown in Fig. 7. Thus, among the parameters evaluated for Cocos nucifera L. biomass, one can choose the one with the lowest energy cost and time.
3.3 Charcoal and liquid results obtained by coconut fiber pyrolysis
Experimental data obtained on Cocos nucifera L. fiber pyrolysis with regard to temperature are presented in Tables 7 and 8 for charcoal and liquid production, respectively.
In the analysis of charcoal variances, considering a 95% confidence level, it was observed that the value of T found is less than the value of t-critical, which means null or zero effect because it is very close to the error standard. Such observation is repeated for both the one-tailed and bi caudal hypothesis test.
According to Pedroza et al. [18], the reactions that occur during the pyrolysis process are fully correlated with the temperature at which biomass is subjected. The temperature is a parameter that influences the results in charcoal, liquids, and non-condensable gases, and the higher the temperature, the higher the degree of thermal decomposition of the biomass, with consequent increase in the amount of pyroligneous liquids and gases and the lower yield in charcoal. To verify the existence or not of significant difference, the t test was applied simulating equivalent variances for the charcoal and bio oil, and the results are shown in Tables 7 and 8.
Observing Table 9, which refers to the t test evaluating the variances of obtaining the pyroligneous liquid, within a 95% confidence level, it was also possible to perceive behavior similar to that of charcoal. The value of T found is less than the value of t-critical, which means null or equals to zero because it is very close to standard error. This observation is repeated for both the one-tailed and two-tailed hypothesis testing.
The values observed in Tables 8 and 10 indicate that there are no significant differences in average charcoal and liquid production in the Cocos nucifera L. fiber pyrolysis process in the two treatments employed (temperatures of 500 and 550 oC), at a 95% confidence level. This indicates that the pyrolysis reactor can be operated at a lower temperature, thus representing a process energy saving.
3.4 Charcoal Characterization
3.4.1 Elemental analysis—CNH
Table 11 reports the chemical composition obtained in the analysis of Cocos nucifera L. charcoal as well as the results of other authors.
Analyzing Table 11, the values obtained in the elementary analysis are very close to the values found by other authors. The carbon value obtained is similar to that of Tsai, Lee, and Chang [11].
3.4.2 Determination of total organic carbon (TOC)
In the elemental analysis, the determined carbon content includes values of organic carbon and inorganic carbon, therefore it is important to verify the percentage of organic carbon present in charcoal, since it is understood that it is directly related to the quality of charcoal and its calorific value, in the process of evaluating the energy potential of matter.
Although it is expected that virtually all carbon available in charcoal is from an organic source, it is observed that there is a significant inorganic percentage that must be related to the CO2 of the carbon cycle that was absorbed by the plant to produce glucose and carbonates and bicarbonates of the soil where the cultivation occurred of the species.
The average of the organic carbon values found in the analysis of the studied charcoal, which was performed in triplicate as well as the relationship between the total carbon percentage of the elemental analysis to find the percentage of inorganic carbon contained in the Cocos nucifera L. charcoal found were 47.03% total organic carbon, 69.32% total carbon, and 22.29% inorganic carbon.
3.4.3 Scanning electron microscopy (SEM)
The scanning electron microscopy was employed in order to obtain information on the structure and pore distribution of the surface of the charcoals formed in the pyrolysis process.
According to Mohan et al. [19] the pyrolysis process gives charcoal a microporous structure to charcoal formed with potential for filtration and adsorption of organic and inorganic pollutants. It is important to note that the properties of products formed from pyrolysis are significantly influenced according to the temperature assigned to the process [7]. Figure 8 shows images obtained with magnitudes 500 and 1.0 k times the sample size.
By observing the images shown in Fig. 8, it is possible to identify a vast amount of spherically shaped pores that are arranged in size and evenly. Therefore, it is accepted that the high number of pores may contribute to a larger surface area, but it is not a determining factor.
The size of the pores can be classified according to the International Union of Applied Pure Chemistry - IUPAC in micro pores having molecular dimensions less than 2 nm (0 ̴ 20 Å), meso pores with dimensions between 2 and 50 nm (20–500 Å), macro pores which have dimensions between 50 and 7500 nm (0.05–7.5 μm), and mega pores those with dimensions greater than 7500 nm (˃ 7.5 μm). Figure 9 provides information on the pore size observed in Cocos nucifera L. fiber charcoal obtained in the pyrolysis process. These images are micro image with magnitude 1.5 k times the sample size.
Regarding the type, the pores can be classified as open (a, b, c) or closed (d) according to their availability to an external fluid. Closed pores influence mechanical properties while open pores are active in the flow of gases and liquids [20].
Figure 10 divides the pore types according to Ferreira et al. [20] into (b) bottle neck, (c) cylindrical, and (d) closed and (e) represents the surface roughness
Classification of pore types. Source: Ferreira et al. [20]
From Fig.10 it is possible to suggest the nature of the pore size in Fig. 9, indicating that it is a type C and D pore with homogeneous surface, elongated and oval cavities in the opening path.
3.4.4 Surface area analysis (BET/N2)
In the pore surface area analysis, a result of 30. 3801 m2/g was obtained, characterizing the low surface area charcoal for adsorption. Relating this surface area value to information obtained in SEM (pore type, pore size, and homogeneity) allows us to suggest that the surface area should be compromised with the presence of amorphous carbons, highlighting the need to employ an activation method for charcoal produced as a way to eliminate them. According to Moreno [21], the metals present in charcoal, (Si, P, Ca, Fe, K) may have contributed to the reduction of the adsorption potential of the material, with the consequent reduction of porosity.
3.4.5 Methylene blue index
The determination of the methylene blue index is based on verifying the absorptive capacity of the charcoal produced in the Cocos nucifera L. fiber pyrolysis process. The charcoal on which the tests were carried out in accordance with JIS 1474 had a maximum methylene blue adsorption value of 67.7%.
Adsorption is understood as the event that occurs at the interface between two substances, which can be between a solid and a liquid (under studying), a solid and a gas, or a liquid and a gas. According to Soleimani and Kaghazchi [22], the adsorption is influenced by several factors among them the nature of the adsorbent, adsorbate, and the adsorption conditions (temperature and pressure and pH). In addition, the adsorption capacity is understood to be determined by its specific surface area, such as the presence, pore size and distribution, ash content, density and the nature of functional groups present on its surface.
According to Moreno [21], by eliminating the ashes, charcoal has increased hydrophobic character as well as adsorption capacity. The ash content is due to the presence of inorganic substances including inorganic oxides, phosphates, sulfates, chlorides, carbonates, and silicates of K, Na, Ca, Mg, Fe, and Al, soluble in polar substances such as water.
The specific surface area is determined through the analysis of BET (method proposed by Brunauner, Emmet and Teller). In this work, nitrogen gas was used to obtain information about the distribution of the size of the porous and the total surface area.
For Cabal et al. [23] the composition of biomass also emits information about the nature of the pores. High lignin values provide meso pore structure, while high cellulose values may suggest the formation of micro-pore rich structures.
From the adsorption of methylene blue, it is possible to evaluate how charcoal interacts with the methylene blue solution, so that, understanding the nature of the interaction, it is possible to make the best use of carbon (adsorbent). By relating the concentration of methylene blue (adsorbate) and the adsorbent mass used in the adsorption process, it is possible to obtain the maximum amount of adsorption under the conditions in which it was submitted.
Through the equations, results obtained in the adsorption of methylene blue were shown in Fig. 11 and Fig. 12 that present linear graphs to obtain allusive parameters to the Freundlich and Langmuir models. Figure 11 shows the equilibrium analysis according to the adsorption isotherms of the Freundlich model.
The equation of the line obtained by linearization is as follows: y = 0. 0009x + 0.3775 (R2 = 0.9772). Thus, considering that the linearization of the Freundlich isotherm the log K represents the linear coefficient of the line, corresponding experimentally to - 0.3775.
As for the inverse of n (1/ n), corresponding to the angular coefficient of the line, the value found was 0.0009. Thus, n is equal to 1.11 and corresponds to the inverse of the adsorbent mass used (1/ m). Only values of the exponent 1/n in the range of 0.1 < 1 n < 1 are known to indicate favorable conditions so, as the value of n = 1.11, it indicates that the adsorption does not fit the model since unfavorable conditions are present. A value of n > 1 may mean that there was some significant loss of adsorbate, or even in the presence of impurities, there may have been mass loss of the adsorbent during the experiment.
Figure 12 shows the equilibrium analysis according to the Langmuir model adsorption isotherms.
From the analysis of the curve of the straight line in Langmuir model, it was noticed that the correlation coefficient (R2) is 0.9804, higher than (R2) found in Freundlich isotherm, thus suggesting a better fit to this model because it presents the correlation coefficient is very close to 1. This result still predicts the adsorption has a chemical interaction between the adsorbent Cocos nucifera L. charcoal and the methylene blue adsorbate.
4 Conclusions
According to the experimental ones of this research, the following conclusions are presented:
Despite the interest in verifying the sensitivity of the reactor used, the small temperature variation 500 and 550 °C in the pyrolysis process under study did not present significant significance in the yields. In other words, the reactor was not sensitive to admit variations of this magnitude.
Under pyrolysis operating conditions at temperature 500 °C, heating rate 10 °C / min, gas flow 4 mL/min and residence time of 60 min, the Cocos nucifera L. charcoal showed the highest yield, 69.32% carbon, 2.79% hydrogen, 1.14% nitrogen, 47.03% total organic carbon, and 30.3801m2/g specific surface area. The SEM of the charcoal presented uniform size and pores, which influenced the adsorptive process of removing methylene blue. The maximum adsorption capacity of methylene blue was 67.7%, and the Freundlich and Langmuir isotherms and their straight line equations demonstrate that the model that best fit was for Langmuir with a correlation coefficient R2 = 0.9804.
References
Fonseca MC, Ritter EC, André LB (2019) Aplicação de solução semianalítica para modelagem de ensaios de sorção e difusão pura com lixiviado. Engenharia Sanitaria e Ambiental, 24(5), 965-973 Epub 24(November 25):965–973. https://doi.org/10.1590/s1413-41522019183126
Oliveira TB, Galvão J, Alceu C (2016) Planejamento municipal na gestão dos resíduos sólidos urbanos e na organização da coleta seletiva. Engenharia Sanitaria e Ambiental 21(1):55–64. https://doi.org/10.1590/S1413-41520201600100155929
Pedroza MM, Vieira GEG, Sousa JF, Pickler AC, Leal ERM, Milhomen CC (2010) Produção e tratamento de lodo de esgoto–uma revisão. Rev Libr 11:89–188. https://doi.org/10.31514/rliberato.2010v11n16.p149
Zaman CZ, Pal K, Yehye WA, Sagadevan S, Shah ST, Adebisi GA, Marliana E, Rafique RF, Johan RB (2017) Pyrolysis: A Sustainable Way to Generate Energy from Waste. Pyrolysis. https://doi.org/10.5772/intechopen.69036
Johari K, Alias AS, Saman N, Song ST, Mat H (2015) Desempenho de remoção de mercúrio elementar por adsorventes de baixo custo preparados por métodos fáceis de carbonização e ativação de casca de coco. Waste Manag Res 33(1):81–88. https://doi.org/10.1177/0734242X14562660
Johari K, Saman N, Song ST, Chin CS, Kong H, Mat H (2016) Aprimoramento de adsorção de mercúrio elementar por várias cascas de coco com superfície modificada como adsorventes de baixo custo ecologicamente corretos. Int Biodeterior Biodegradation 109:45–52. https://doi.org/10.1016/j.ibiod.2016.01.004
Leng L, Huang H (2018) An overview of the effect of pyrolysis process parameters on biochar stability. Bioresour Technol 270:627–642. https://doi.org/10.1016/j.biortech.2018.09.030
Hu W, Feng Z, Yang J, Gao Q, Ni L, Hou Y, Liu Z (2021) Combustion behaviors of molded bamboo charcoal: Influence of pyrolysis temperatures. Energy 226:120253. https://doi.org/10.1016/j.energy.2021.120253
De Andrade Machado A, de Assis Passos R, Marques da Costa Marques LG, Basto Oliveira L, Vidaurre GB, das Dores de Sá Rocha J (2004) Pirólise de resíduos do coco-da-baía (Cocos nucifera Linn) e análise do carvão vegetal. Rev Árvore 28(5):707–714. https://doi.org/10.1590/S0100-67622004000500010
Vale ATD, Mendes RM, Amorim MRS, Dantas VFDS (2011) Energy potential of biomass and charcoal of Jatropha curcas peel and pie. Cerne 17(2):267–273. https://doi.org/10.1590/S0104-77602011000200015
Tsai WT, Lee MK, Chang YM (2006) Fast pyrolysis of rice straw, sugarcane bagasse and coconut shell in an induction-heating reactor. J Anal Appl Pyrolysis 76:230–237. https://doi.org/10.1016/j.jaap.2005.11.007
Cortez LAB, Perez JMM, Rocha JD, Jordan RA, Mesa HRM (2009) Processamento de casca e fibra de coco verde por carbonização para agregação de valor/processing of coconut shell and fiber for adding value. Revista Brasileira de Engenharia de Biossistemas 3(1):21–30. https://doi.org/10.18011/bioeng2009v3n1p21-30
Demirbaş A (2001) Relationships between lignin contents and heating values of biomass. Energy Convers Manag 42(2):183–188. https://doi.org/10.1016/S0196-8904(00)00050-9
Tsamba AJ, Yang W, Blasiak W (2006) Pyrolysis characteristics and global kinetics of coconut and cashew nut shells. Fuel Process Technol 87(6):523–530. https://doi.org/10.1016/j.fuproc.2005.12.002
Antonkiewicz J, Popławska A, Kołodziej B, Ciarkowska K, Gambuś F, Bryk M, Babula J (2020) Application of ash and municipal sewage sludge as macronutrient sources in sustainable plant biomass production. J Environ Manag 264:110450. https://doi.org/10.1016/j.jenvman.2020.110450
Albinante SR, Pacheco ÉBAV, Visconte LLY (2013) A review on chemical treatment of natural fiber for mixing with polyolefins. Quim Nova 36(1):114–122. https://doi.org/10.1590/S0100-40422013000100021
Silverstein RM, Webster FX, Kiemle DJ (2006) Identificação espectrométrica de compostos orgânicos. LTC, Rio de Janeiro, pp 70–104 ISBN: 9788521615217
Pedroza MM, Sousa JF, Vieira GEG, Bezerra MBD (2014) Characterization of the products from the pyrolysis of sewage sludge in 1 kg/h rotating cylinder reactor. J Anal Appl Pyrolysis 105:108–115. https://doi.org/10.1016/j.jaap.2013.10.009
Mohan D, Pittman CU Jr, Steele PH (2006) Pyrolysis of wood/biomass for bio-oil: a critical review. Energy Fuel 20(3):848–889. https://doi.org/10.1021/ef0502397
Ferreira OP, Alves OL, Macedo JDS, Gimenez IDF, Barreto LS (2007) Ecomaterials: development and application of functional porous materials for environmental protection. Química Nova 30(2):464–467. https://doi.org/10.1590/s0100-40422007000200039
Moreno CC (2004) Adsorption of organic molecules from aqueous solutions on carbon materials. Carbon 42(1):83–94. https://doi.org/10.1016/j.carbon.2003.09.022
Soleimani M, Kaghazchi T (2008) Adsorption of gold ions from industrial wastewater using activated carbon derived from hard shell of apricot stones–An agricultural waste. Bioresour Technol 99(13):5374–5383. https://doi.org/10.1016/j.biortech.2007.11.021
Cabal B, Budinova T, Ania CO, Tsyntsarski B, Parra JB, Petrova B (2009) Adsorption of naphthalene from aqueous solution on activated carbons obtained from bean pods. J Hazard Mater 161(2-3):1150–1156. https://doi.org/10.1016/j.jhazmat.2008.04.108
Acknowledgment
The authors to thank the Conselho Nacional de Desenvolvimento Cientifico e Tecnológico (CNPq), CAPES.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Paz, E.C.S., Paschoalato, C.F., Arruda, M.G. et al. Production and characterization of the solid product of coconut pyrolysis. Biomass Conv. Bioref. 13, 6317–6329 (2023). https://doi.org/10.1007/s13399-021-01561-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-021-01561-3