Abstract
The present study explores biodiesel production from the single-cell oil (SCO) synthesized by Yarrowia lipolytica MTCC 9520, utilizing slaughterhouse lipid waste, goat tallow, as the carbon substrate. Various parameters, including cultivation time (96 h), pH(6), substrate concentration (1.5%, v/v), inoculum size (5%, v/v), and C/N ratio (100), were optimized to attain the maximum biomass and lipid yield and lipid content of 3.8 g/L, 2.6 g/L, and 69.3% (g/g dry weight), respectively. The presence of intracellular lipid bodies in Y. lipolytica was confirmed by observing the Nile red-stained cells by fluorescence microscopy. Besides, the fluorescence intensities of the intracellular lipid bodies were determined by the flow cytometer. The extracted SCO from Y. lipolytica MTCC 9520 was analyzed by Fourier transform infrared (FT-IR) spectroscopy. Gas chromatography-mass spectrometry (GC-MS) analysis of the transesterified SCO presented the fatty acid methyl ester (FAME) profile, including palmitic acid (42.9%), stearic acid (21.5%), myristic acid (18.3%), and oleic acid (7.0%). The obtained FAME composition was further used to predict the properties of the biodiesel. The revealed characteristics of the transesterified FAMEs signify the candidature of the synthesized SCO as an alternate feedstock for biodiesel production.

Graphical abstract
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
In recent years, the use of fossil fuels has raised serious environmental concerns. Furthermore, petroleum-based resource availability has plummeted remarkably with an upsurge in worldwide exigency for energy [1]. Biofuels are customarily advocated as a cost-effective and environmentally benign alternative to petroleum and other fossil fuels. Biodegradability and descended toxicity of biodiesel ascertain it as a superior substitute for petroleum-based fuels [2]. Biodiesel can be utilized in ternary fuel blends, meaning a mixture of diesel, biodiesel, and bioethanol, in diesel engines [3]. The primary feedstock derived from plant sources like rapeseed oil, soybean oil, palm oil, etc. is used for commercial biodiesel production. More than 95% of feedstock comes from edible oils since they are produced in many regions. The properties of biodiesel produced from these oils are appropriate as a substitute for diesel [4]. However, agricultural biomass as a substrate is not economical, leading to food shortage, insecurity, and consecutively high prices [5]. Additionally, the consumption of an enormous amount of these expensive raw materials may lead to a deficiency of edible oils and, thereby, a massive increase in food prices [6, 7]. Further, the use of agricultural wastes, wastes from food industries, municipal wastes, and waste cooking oils could not sustain the whole demand for biodiesel, and the production process for employing these wastes needs to be standardized [8]. Although lipid-rich wastes, i.e., fat, oil, grease (FOG), would be the ideal material for biodiesel production, the high production cost of converting these lipidic wastes directly into biodiesel obstructs its feasibility in replacing the conventional diesel [9]. Thus, utilizing lipid-rich feedstock to synthesize microbial oils and biodiesel conversion resolves the drawbacks of the direct conversion of waste into biodiesel. Biodiesel production using microbial oils has recently gained significant attention because of their high productivity, less labor, easy scaling up, and short life cycle. Microbial oils or single-cell oils (SCO) are promising options and offer many advantages over vegetable oils. They are devoid of seasonal or climatic requirements, easy maintenance, and can be easily modified to generate higher yields in small areas [5]. This paves the way for developing green and sustainable biodiesel production process without obstructing the food supply chain [10, 11].
SCOs are intracellular lipids comprising triacylglycerols (TAGs) produced by oleaginous yeasts, potential sources for lipid production. They exhibit a fast growth rate, and their cultures are more easily scaled up [12, 13]. Yarrowia lipolytica is one of the specific oleaginous yeasts that can convert hydrophobic substrates into lipids through the ex novo synthesis pathway. The ex novo synthesis of lipids is the biomodification of fats and oils by Y. lipolytica [14, 15]. Y. lipolytica, a dimorphic yeast, can exist as single cells or as filamentous hyphae and is considered as GRAS (Generally Regarded as Safe) microbial strain [16]. Lipid accumulation in Y. lipolytica starts when nitrogen in the culturing medium is low, and the carbon source is excessive [17]. Y. lipolytica is known to have the proficiency in assimilating hydrophobic substrates like TAGs, fats, and oils and in producing single-cell oils, lipase, and organic acids [18,19,20]. In this context, the production of microbial lipids using low-cost raw materials like slaughterhouse lipid wastes would significantly reduce the lipid production cost. The produced lipids have characteristics similar to vegetable oils and can be used as an alternate feedstock to produce biodiesel [6, 21]. Hydrophobic sources like waste oils and waste fish oil are utilized as substrates to produce microbial lipids [22, 23]. The fatty acid composition of the hydrophobic substrates affected the lipid accumulation and fatty acid composition in the Y. lipolytica cells [19]. The ex novo synthesis of lipids in Y. lipolytica is a growth-coupled process in the presence of fat or hydrophobic materials as the carbon source to convert and accumulate them as SCO [24]. However, there are less reported studies on the utilization of industrial wastes, i.e., animal fats as a substrate for microbial lipids (or SCO) production and their conversion into biodiesel [15, 19,20,21]. Therefore, there is a need to explore the potential use of industrial wastes as substrates in the production of SCOs and their further conversion into biodiesel. Goat tallow is a slaughterhouse lipid waste, which serves as a substrate for the production of SCO. The high-lipid content of goat tallow can make it a suitable carbon source rather than the standard carbon sources like glucose or glycerol [25]. Hence, in the present study, the goat tallow was utilized as the substrate by Y. lipolytica MTCC 9520 for the synthesis of SCO, which was further converted to biodiesel through trans-esterification, a catalyzed chemical reaction involving SCO and methanol to yield fatty acid methyl esters (FAMEs), i.e., biodiesel. The effect of various parameters was optimized to achieve the maximum production of SCO. The accumulated lipid bodies were visualized by fluorescence microscopy, and its fluorescence intensity was measured with the flow cytometer. The extracted SCO from Y. lipolytica MTCC 9520 was analyzed by Fourier transform infrared (FT-IR) spectroscopy. The FAME profile of the trans-esterified SCO was determined using gas chromatography-mass spectrometry (GC-MS) analysis. Further, the FAME composition was used to determine the synthesized biodiesel properties and compared with standards stated by the American Society for Testing Materials (ASTM) and the European Nations (EN) to signify SCO’s aptitude as an alternative feedstock for biodiesel production.
2 Materials and methodology
2.1 Materials
The goat tallow was obtained from the local slaughterhouse. The oleaginous yeast Yarrowia lipolytica MTCC 9520 was procured from Microbial Type Collection Centre (MTCC), Chandigarh, India. The strain was preserved in maltose, glucose, yeast extract, and peptone (MGYP) agar slant at 4 °C at a pH of 6.2 [5]. All chemicals and reagents entailed for the production and the extraction have been purchased from RANKEM RFCL Ltd. and Sisco Research Laboratories Pvt. Ltd. (SRL).
2.2 Characterization of the substrate
The goat tallow collected from the local slaughterhouse was heated up to 60 °C and filtered using Whatman filter paper No. 1, which was then stored at 4 °C until further use. The biochemical properties of the goat tallow, including the total organic carbon (TOC) content and total Kjeldahl nitrogen (TKN), were determined using the standard methods described by APHA (2005) [26]. The carbohydrate, protein, and fat contents were determined using the anthrone, Bradford, and sulfosalicylic acid assay, respectively [27, 28]. The goat tallow was converted to FAMEs through acid-catalyzed trans-esterification, as described by Dobrowolski et al. [11] with slight modifications. A total of 20 mg of goat tallow oil was added to 20 mL of 5% (v/v) H2SO4 in methanol and was transferred to a round bottom flask. The round bottom flask was immersed in a silicone oil bath placed on a heating mantle at 90 °C for 6 h. Finally, the sample was quenched by adding water, and FAMEs were extracted using hexane as a solvent. The solvent phase was evaporated using a rotary vacuum evaporator; the FAMEs were filtered using a 0.45-μm syringe filter and given for GC-MS analysis (Agilent Technologies, GC-7890B, and MS-5977A). The elution was performed with an HP-5MS 5% phenyl methyl siloxane column with dimensions 30 m × 250 mm × 0.25 mm. Helium was used as the carrier gas with a flow rate of 1.0 mL/min. The analysis was carried out at a temperature of 60 °C for 2 min, and then, the temperature was increased to 200 °C at a rate of 10 °C/min, and further to 240 °C at 5 °C/min, and was held at 240 °C for 10 min. The peaks obtained were identified with reference to the National Institute of Standards and Technology (NIST) standard library [5].
2.3 Preparation of growth media
The minimal salt media comprises yeast extract—0.5; KH2PO4—7; Na2HPO4—2.5; CaCl2·2H2O—0.15; (NH4)2SO4—1.0; MnSO4·H20—0.06; ZnSO4·7H2O—0.02; FeCl3·6H2O—0.15; and MgSO4·7H2O—1.5 in (g/L) [19]. The pH was adjusted to 7.0. A total of 1.5% of pre-processed goat tallow was added to the prepared growth media and sonicated at 20 kHz for 15 min using Bandelin Sonoplus ultrasonic homogenizers (BANDELIN electronic GmbH & Co. Germany). The growth medium was then inoculated with 5%, v/v of the 24-h grown culture of Y. lipolytica MTCC 9520, and was incubated at 37 °C for 96 h in an orbital shaker with a speed of 150 rpm. Simultaneously, samples were periodically removed from the fermentation broth, and optical density was measured at 600 nm.
2.4 Optimization of parameters
One factor at a time (OFAT) optimization was carried out in order to analyze the effect of various process parameters for lipid production such as cultivation time (24, 48, 74, 96, and 120), pH (4, 5, 6, 7, and 8), substrate concentration (0.5, 1, 1.5, 2.0, 2.5, and 3.0% (v/v)), inoculum size (2.5, 5, 7.5, 10, 12.5, and 15% (v/v)), and C/N ratio (25, 50, 75, 100, 125, and 150). The influence of these factors on lipid and biomass yield and lipid content were examined. All the measurements were analyzed by one-way ANOVA followed by Dunnett’s post hoc test.
2.5 Recovery and estimation of biomass and SCO
After 96 h, the growth media was centrifuged at 8000 rpm for 20 min at 4 °C, and the harvested cells were rinsed twice with distilled water. The biomass pellets were dried at 80 °C in a hot air oven until it attained a consistent dry weight, following which the biomass yield was quantified gravimetrically. The dried biomass was suspended in chloroform/methanol (2:1) and sonicated at 20 kHz for 15 min. The lipid (SCO) was further extracted using chloroform/methanol (2:1) solution and sonicated at 20 kHz for 20 min within an ice bath using Bandelin Sonoplus ultrasonic homogenizers (BANDELIN electronic GmbH & Co. Germany). Further, the solution was centrifuged at 8000 rpm for 20 min at 4 °C, and the supernatant was added with chloroform-methanol (2:1, v/v) solution again and incubated for an hour. The upper organic phase was removed after washing the solution with 0.9% (w/v) NaCl. The organic layer was retained and dried using a rotary vacuum evaporator. The lipid concentration (g/L), through gravimetric analysis, and the quantity of lipid obtained to the biomass quantity were expressed as a percentage of lipid content in the biomass (%, g/g) [29, 30].
2.6 Analytical techniques
2.6.1 Fluorescence microscopy
The accumulation of intracellular SCO was visualized through a fluorescence microscope. The biomass obtained from the centrifuged fermentation broth was washed with phosphate buffer saline (PBS) (1 mL). Nile red stock solution of 1 mg/mL in acetone was further added to the PBS cell suspension, followed by incubation in the dark for 10 min at room temperature [5]. The samples were then visualized under a Zeiss microscope using a 465–565-nm excitation filter, and Prog Res Capture Pro 2.7 software (Jenoptik optical systems, USA) was used for capturing the images.
2.6.2 Flow cytometry
Flow cytometry is a non-invasive technique for the detection and quantification of a population of cells or particles. Four samples of biomass (48 h, 72 h, 96 h, 120 h) after centrifugation were washed with phosphate buffer saline (PBS) (1 mL). Nile red stock solution of 1 mg/mL in acetone was further added to the PBS cell suspension, followed by incubation in the dark for 10 min at room temperature [31]. The sample cells were visualized by BD FACS Calibur flow cytometer. Non-stained cells were used as control during the data analysis of these cells.
2.6.3 FT-IR analysis
The FT-IR analysis method uses infrared light to scan test samples and observe chemical properties. The extracted SCO was investigated for functional groups using the Perkin-Elmer infrared spectrophotometer (Agilent Technologies, Cary 600 Series/a Perkin-Elmer infrared spectrophotometer) [5]. The samples with dimensions of about 10 mm diameter and 1 mm thickness were prepared with KBr (spectroscopic grade) pellet. The samples were scanned in the frequency range of 400 to 4000 cm−1 [32].
2.6.4 GC-MS analysis of extracted SCO
The extracted SCO was converted to FAMEs through acid-catalyzed trans-esterification; the analysis was carried out with specifications, as mentioned in section 2.2. The fatty acid profile of the SCO extracted from Y. lipolytica MTCC 9520 was studied to envisage its physical and chemical properties of biodiesel [33, 34]. Properties, including the degree of unsaturation (DU), cetane number (CN), iodine value (IV), oxidative stability (OS), long-chain saturated factor (LCSF), and cold filter plugging point (CFPP), were determined using correlation equations given by Wang et al. [35] and Guldhe et al. [36]. DU was calculated from the fatty acid profile using the equation:
The equations to calculate CN, IV, OS, LCSF, and CFPP were given as follows:
Moreover, other properties like density, kinematic viscosity, saturated fatty acids (SFA), cloud point (CP), pour point (PP), saponification value (SV), and high heating value (HHV) were determined using the software ‘Biodiesel Analyzer version 2.2’ [37]. The biodiesel properties derived from goat tallow and SCO of Y. lipolytica MTCC 9520 were compared with the international biodiesel standards (ASTM D6751 and EN 14214 standards) to assess its quality.
3 Results and discussion
3.1 Characterization of goat tallow
Firstly, the biochemical composition of the goat tallow was analyzed and presented in Table 1. The fat content in the substrate was obtained as 57 ± 0.05%. The carbohydrate and protein content of the substrate was found to be 4.3 ± 0.04% and 3.01 ± 0.009%, respectively. Predominantly, the fat content in the substrate was the highest, which reveals its hydrophobic nature. The ex novo synthesis of lipid in Y. lipolytica MTCC 9520 occurs in the presence of fat or hydrophobic materials as the carbon source to convert and amass them as SCO [24]. Hence, the high-fat content of goat tallow can make it a suitable carbon source for SCO production. Moreover, the total organic carbon (TOC) and the total Kjeldahl nitrogen (TKN) content in the goat tallow were 8.91 ± 0.21% and 0.00126 ± 0.07%, respectively. Furthermore, the goat tallow was subjected to acid transesterification, and the fatty acid composition of the trans-esterified goat tallow was identified from GC-MS analysis (Fig. S1). The various fatty acids were identified from their retention time, and their abundance was observed from the peak percentage areas. From the analysis, it was exemplified that palmitic acid (C16:0), stearic acid (C18:0), myristic acid (C14:0), and oleic acid (C18:1) were the major FAMEs with peak percentage areas of 40.7%, 24.9%, 8.3%, and 5.5%, respectively.
3.2 Growth profile of Y. lipolytica MTCC 9520
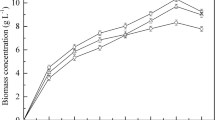
Periodically, the samples were taken from the fermented medium, and the optical density was measured at 600 nm. The growth curve (Fig. 1) represents the lag phase, log phase, and stationary phase. The log or exponential phase was observed to be begun approximately after 10–15 h of microbial growth. Cell doubling has been reported to occur during the exponential phase; thus, the mid-log stage, which was at 24 h, was considered for inoculum transfer. Figure 1 denotes that the log phase had been over by 40 h and by 45 h; it had already reached the stationary phase. The stationary phase is generally procured because of essential nutrient depletion and inhibition by specific metabolites. The specific growth rate (μ) of the Y. lipolytica MTCC 9520 was found to be 0.036 h−1.
3.3 Optimization of process parameters
3.3.1 Effect of incubation time on SCO production
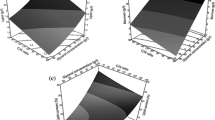
Cultivation time is an essential factor for the accumulation of SCO by the oleaginous yeast. The effect of time was determined by culturing Y. lipolytica MTCC 9520 in the media for various lengths of time (24–120 h). As time increased from 24 to 96 h, there was a sharp increase in the biomass yield and the lipid yield (Fig. 2a). Moreover, the lipid content was estimated and noted a gradual increase from 24 to 96 h. However, the lipid content and the lipid yield decreased rapidly from 96 to 120 h. The maximal biomass yield was achieved at 96 h when the cells enter into the stationary growth phase, and after that, it was gradually decreased. The decrease can be caused by the depletion of nutrients from the production media [38]. The highest biomass yield of 3.8 g/L, lipid yield of 2.6 g/L, and lipid content of 69.3% (g/g) was obtained at 96 h. Reports from various studies have indicated that the growth rate of Y. lipolytica MTCC 9520 is maximum between a range of 72–96 h. During this time, the organism has proficiency in utilizing the medium’s nutrients for its maximum growth. Similarly, in this study, the sharp upsurge from 24 to 96 h epitomizes the utilization of the nutrients by the yeast, Y. lipolytica MTCC 9520, to produce lipid intracellularly (SCO). The present study involving goat tallow as a carbon source by Y. lipolytica MTCC 9520 reported a higher lipid and lipid content at 96 h. Sestric et al. [39] reported lipid synthesis by Y. lipolytica on various substrates, where culture medium containing glycerol yielded 31% (g/g) lipid content and medium containing glycerol and dextrose produced 38% (g/g) lipid content. Besides, El Bialy et al. [40] reported lipid yield of 2.73 g/L and lipid content of 34.02% (g/g) for Y. lipolytica by utilizing meat product fat. These reports signposted that hydrophobic substrates could be considered as an appropriate substrate for the synthesis of SCO by Y. lipolytica compared with the hydrophilic substrates such as glucose/glycerol.
3.3.2 Effect of pH on SCO production
The pH is one of the vital attributes for SCO production. It is evidenced from the literature that studies have been conducted to determine the effect of pH on the SCO production by using the oleaginous strain Y. lipolytica MTCC 9520, as it is vitally essential for the assimilation of carbon. The variation in pH affects the cell membrane permeability and hence the carbon assimilation process [41]. The lipid and biomass yield were significantly influenced by the difference in the pH and thereby the lipid content. As the pH is increased from 4.0 to 7.0, the biomass and lipid yield, along with the lipid content, also increased (Fig. 2b). However, after obtaining a pH of 7, it decreased significantly. The highest biomass yield of 3.72 g/L, lipid yield of 2.33 g/L, and lipid content of 62.5% (g/g) were obtained at pH 7 at the optimum time of 96 h. The yields of biomass and lipid content attained in the present study corroborate with the previous study reported for the valorization of palm oil mill effluent into lipid by Y. lipolytica TISTR 5151, where the similar process conditions were maintained [15].
3.3.3 Effect of substrate concentration on SCO production
The substrate (goat tallow) concentration is the primary factor for SCO production. The oleaginous yeast utilizes the goat tallow provided in the medium as a growth substrate and accumulates the lipid components in the form of SCO intracellularly. The optimization of substrate concentration is essential to decelerate substrate inhibition. Therefore, in the present study, the substrate concentration was optimized by varying the concentrations from 0.5 to 3.0%. The yeast strain tends to proliferate rapidly under higher substrate concentration. Still, after some point, they stop utilizing the substrate due to the excessive substrate concentration, which further leads to the substrate inhibition and the decrease in the biomass yield as well as lipid content (Fig. 2c). The biomass yield, lipid yield, and the lipid content had a significant increase up to 1.5%, after which it decreased rapidly. At 1.5% of substrate concentration, the highest biomass yield of 3.69 g/L, lipid yield of 2.39 g/L, and the lipid content of 64.7% (g/g) were observed.
3.3.4 Effect of inoculum size on SCO production
The inoculum size has a considerable impact on the SCO accumulation by the yeast. The present study (Fig. 2d) reveals a maximum lipid yield of 2.35 g/L, maximum biomass yield of 3.5 g/L, and 67% (g/g) lipid content at 5% inoculum size. A steady increase was observed in the biomass yield, lipid yield, and lipid content until 5% of inoculum size; after that, a rapid decrease was observed in the biomass yield, lipid yield, and lipid content. It can be predicted that up to an inoculum size of 5%, all the yeast cells get enough nutrients to grow and accumulate single-cell oil. Beyond this inoculum size, the yeast cells are devoid of nutrients that prevent them from growing and accumulating lipid (Fig. 2d). Thus, the results suggested that 5% inoculums size is optimum for the better yield of biomass, lipid, and lipid content. Louhasakul et al. [15], Juanssilfero et al. [42], and Jiru et al. [43] also conducted studies on the effect of inoculum size on the proliferation and the lipid accumulation process in oleaginous yeasts, which revealed an optimum inoculum size ranging from 4 to 6%.
3.3.5 Effect of C/N ratio on SCO production
C/N ratio is the most crucial factor that regulates the SCO accumulation process. The C/N ratio was modified by altering the goat tallow, yeast extract, and (NH4)2SO4 concentrations, respectively, to obtain the desired value. On subjecting to different proportions of carbon and nitrogen, the biomass and lipid yields were observed to increase until the ratio of 100 (Fig. 2e). Maximum biomass yield of 3.5 g/L, lipid yield of 2.25 g/L, and lipid content of 64.28% (g/g) were observed at 100 C/N ratio. This can be explained due to the intensified production of intracellular lipids in the yeast due to the excess availability of carbon source in a limited nitrogen environment [20]. Nitrogen depletion causes the decline in nicotinamide adenine dinucleotide-isocitrate dehydrogenase content that finally results in the suppression of the TCA cycle and, thus, the inhibition of protein synthesis [34]. This allows the carbon to be channeled towards the SCO synthesis. After achieving a C/N ratio of 100, a gradual decline in the biomass yield, lipid yield, and lipid content were due to a distinguished nitrogen source reduction. A similar profile was discerned in the studies conducted by Dobrowolski et al. [11], Radha et al. [34], and Kuttiraja et al. [44], with an optimum C/N ratio of 100, in which lipid production by Y. lipolytica in the nitrogen-rich medium was lower than that observed in the low-nitrogen medium. The previously reported studies for Y. lipolytica using hydrophobic substrates such as sesame oil, rapeseed oil, borage oil, and industrial wastes like crude glycerol reported lower lipid content in a range of 25–51% (g/g) [8, 19, 44].
3.4 Fluorescence microscopy
The accumulated intracellular oil in Y. lipolytica MTCC 9520 was visualized for various C/N ratios using a lipophilic fluorescent dye such as Nile red. Nile red was observed to be intensely fluorescent in the lipid-rich environment of biomass, resulting in a robust golden-yellow emission, which was visualized under a fluorescence microscope (Fig. 3). Golden-yellow fluorescence was observed to be highest at 100 C/N ratio (Fig. 3d). As formerly explained, the excess availability of carbon source in a limited nitrogen environment intensified the production of SCO in Y. lipolytica [20]. Moreover, the highest biomass yield and lipid content were noted in the 100 C/N ratio, resulting in the visualization of more lipid bodies under a fluorescence microscope. In oleaginous yeasts, neutral lipids are stored in lipid bodies that are assembled in a specialized sub-domain of the endoplasmic reticulum where most biosynthetic enzymes and structural proteins are situated [5, 21]. The aggregated lipid bodies corroborate intracellular TAGs accumulated in Yarrowia biomass, which is termed as SCO. This was further extracted and converted to FAMEs for the synthesis of biodiesel.
3.5 Flow cytometry
The flow cytometry analysis gave a semi-quantitative approximate amount of lipid that has been accumulated in the cells. The mean fluorescence intensity (MFI) of the cells fermented for 48, 72, 96, and 120 h was detected, which have flown through the FL2 channel (Fig. S2). The highest MFI was observed at 96 h. The MFI value directly relates to the amount of lipid droplets in the cells detected via the FL2 channel, emitted from the Nile red upon binding with lipophilic compounds [31, 40]. The organism consumes time to convert the external lipid into SCO and store it intracellularly. With the shift of the M value towards the right side, it proves the presence of more lipids [34]. The highest values have been observed between 72 and 96 h. On inferring the M2 values of the cells at different time intervals, as represented in Fig. S2 (e), the highest MFI was observed at 96 h (768.7). After 96 h, there has been a decrease in the lipid content, which is correlated with the time optimization study. The results implied that the optimal time for the highest lipid accumulation was found to be 96 h.
3.6 FT-IR analysis
The functional groups of extracted SCO (lipid) were identified using FT-IR, and the obtained spectra were compared with a standard (cholesterol) to determine the functional groups involved (Fig. 4). The FT-IR spectral analysis from extracted SCO showed the transmittance spectra comparable with standard cholesterol (Fig. 4). The peak at 3428 cm−1 in the FT-IR spectra of SCO from Y. lipolytica MTCC 9520 indicated the presence of amine and hydroxyl groups. Infrared peaks at 2934.937 and 2869.227 cm−1 in the FT-IR spectra of cholesterol and extracted lipid, respectively, revealed the presence of aliphatic C–H stretching vibration, indicating a large amount of methyl and methylene groups [34]. The bands obtained in the region of 2830 and 2695 cm−1, respectively, denoted the asymmetrical and symmetrical C–H stretching [45]. The carbonyl group (C=O) stretching vibration present in extracted lipids with a peak value of 1626.559 cm−1 was found similar to the FT-IR spectrum in the study conducted by Booramurthy et al. [33]. Hence, the observed peaks confirmed the lipidic nature of the extracted SCO and justified its potential to be considered a suitable feedstock for its further conversion to biodiesel.
3.7 Analysis of the fatty acid composition of extracted SCO
The potential of Y. lipolytica MTCC 9520 lipid (SCO) to be transformed into biodiesel was explored by GC-MS analysis of the acid transesterified SCO. The fatty acid profile was obtained through GC-MS analysis (Fig. S3). The various fatty acids were identified from their retention time, and their abundance was observed from the peak percentage areas. From the analysis, it was deciphered that palmitic acid (C16:0), palmitoleic acid (C16:1), stearic acid (C18:0), myristic acid (C14:0), and oleic acid (C18:1) were the major FAMEs with peak percentage areas of 42.9%, 0.4%, 21.5%, 18.3%, and 7.0%, respectively. Besides, the biodiesel derived from direct conversion of goat tallow exhibited three primary fatty acids, including stearic acid (C18:0), myristic acid (C14:0), and oleic acid (C18:1). Comparing the goat tallow and synthesized SCO’s fatty acid composition, the goat tallow had exhibited a higher percentage of stearic acid and lower content of myristic acid and palmitic acid. Further, the FAME profile of the SCO was corroborated with the FAME composition of biodiesel produced from various lipid sources, as shown in Table 2, where palmitic, palmitoleic, oleic, and stearic acids were observed to be the major fatty acids in the transesterified lipid. Studies have shown that the utilization of various wastes such as industrial effluents, animal fats, wastewater, and crude glycerol as feedstock for lipid production, which reported a similar fatty acid composition of yeast lipids produced from Pichia kudriavzevii [5], Yarrowia lipolytica [15, 34], Cryptococcus psychrotolerans [45], Trichosporon cutaneum [46], and Meyerozyma caribbica [47]. Additionally, the SCO’s fatty acid composition was compared with several other plant oil-derived biodiesel, including palm oil, rapeseed oil, and soybean oil [48,49,50]. Apart from goat tallow, other animal fats like bovine tallow and chicken tallow have been explored as a lipid source for their direct conversion into biodiesel [50, 51].
The fatty acid profile of the fuel has a significant repercussion on biodiesel’s physical and chemical properties. A high degree of unsaturation is known to reduce viscosity and resist frost [52]. However, biodiesels with larger quantities of saturated fatty acids (SFA) bring out the advantage of higher thermal efficiency and emission of fewer oxides of nitrogen, which further makes them eco-friendly [53]. The properties of trans-esterified goat tallow and SCO have been compared with the international standard specifications stated by ASTM D6751 and EN 14214 (Table 3). The extracted SCO’s fatty acid composition was found to be majorly monounsaturated and saturated (high SFA), which ensures excellent flow properties and oxidative stability (OS) of the biodiesel. SCO-derived biodiesel achieved a density and kinematic viscosity of 0.86 g/cm3 and 3.7 mm2/s, respectively, which was observed to be within the range of EN standards. In contrast, the biodiesel from goat tallow manifested lower density and kinematic viscosity of 0.70 g/cm3 and 3.1 mm2/s, respectively. Cetane number (CN) is related to the ignition and combustion quality of the fuel, where high CN (above 50) guarantees good cold start properties. The IV reflects the stability of biodiesel to oxidation (i.e., OS), which depends on both the number and position of the double bonds in the FAMEs [54]. Hence, both goat tallow and SCO were found to have a moderate degree of unsaturation since the estimated CN, IV, and OS satisfied the limits imposed by EN 14214 standards. Concerning the CFPP, transesterified goat tallow depicted a considerable difference compared with trans-esterified SCO; thus, SCO-derived biodiesel is more suitable than biodiesel derived from goat tallow. Although SCO would not be advisable for cold climates due to its high CFPP value, which might be due to the high percentage of long-chain saturated fatty acids, such as palmitic acid (C16:0), stearic acid (C18:0), and other saturated fatty acids [36], EN 14214 does not specify limits for cold filter plugging point, but each country can establish limits according to time of year and local climate [48]. Moreover, other properties of SCO, including cloud point, pour point, long-chain saturated factor, higher heating value, and saponification index value, were comparable between transesterified goat tallow and SCO. Thus, it was inferred that the SCO-based biodiesels from oleaginous yeast, Y. lipolytica, could be used as fuel blends [55].
4 Conclusion
In this study, the utilization of slaughterhouse lipid waste, goat tallow, as the substrate for single cell oil (SCO) production from Yarrowia lipolytica MTCC 9520, was explored. The SCO extracted from this oleaginous strain could be considered as a promising feedstock for biodiesel production. The optimum time, pH, substrate concentration, inoculum size, and C/N ratio were observed to be 96 h, pH 7.0, 1.5% (v/v), 5% (v/v), and 100, respectively. These optimized process conditions resulted in the maximum biomass and lipid yield, as 2.6 g/L and 3.8 g/L, respectively, with the lipid content of 69.3% (g/g). Palmitic acid, stearic acid, and oleic acid were found to be the major fatty acids of the trans-esterified SCO, and the predicted properties based on the FAME composition complied with the international standard specifications. The present study paves the way for scale-up studies with improved controlled conditions to establish a sustainable biodiesel production process. Further research in this area can aid to benefit the utilization of microbial-derived SCO as an alternative feedstock for the production of biodiesel and in eliminating the need for petroleum-based fuels to a great extent.
References
Guerfali M, Ayadi I, Sassi HE, Belhassen A, Gargouri A, Belghith H (2020) Biodiesel-derived crude glycerol as alternative feedstock for single cell oil production by the oleaginous yeast Candida viswanathii Y-E4. Ind Crop Prod 145:112103
Nguyen VP, Nguyen HH, Nguyen DT, Nguyen HL, Huynh TM (2018) Optimization of biodiesel production from waste cooking oil using static mixer technology in Vietnam. Biofuels 9(5):567–574
Venu H, Madhavan V (2017) Influence of diethyl ether (DEE) addition in ethanol-biodiesel-diesel (EBD) and methanol-biodiesel-diesel (MBD) blends in a diesel engine. Fuel 189:377–390
Leung DY, Wu X, Leung MK (2010) A review on biodiesel production using catalyzed transesterification. Appl Energy 87(4):1083–1095
Prabhu K, Jayakumar A, Sreelakshmi KP, Raha A, Maitra M, Radha P (2019) Utilization of microbial oil produced from Pichia kudriavzevii NCIM 3653 using paper mill sludge as an alternative substrate for biodiesel synthesis. Biofuels:1–8
Muniraj IK, Uthandi SK, Hu Z, Xiao L, Zhan X (2015) Microbial lipid production from renewable and waste materials for second-generation biodiesel feedstock. Environ Technol 4(1):1–6
Poli JS, da Silva MA, Siqueira EP, Pasa VM, Rosa CA, Valente P (2014) Microbial lipid produced by Yarrowia lipolytica QU21 using industrial waste: a potential feedstock for biodiesel production. Bioresour Technol 161:320–326
Aghbashlo M, Demirbas A (2016) Biodiesel: hopes and dreads. Biofuel Res J 3(2):379
Abomohra AE, Elsayed M, Esakkimuthu S, El-Sheekh M, Hanelt D (2020) Potential of fat, oil and grease (FOG) for biodiesel production: a critical review on the recent progress and future perspectives. Prog Energy Combust Sci 81:100868
Signori L, Ami D, Posteri R, Giuzzi A, Mereghetti P, Porro D, Branduardi P (2016) Assessing an effective feeding strategy to optimize crude glycerol utilization as sustainable carbon source for lipid accumulation in oleaginous yeasts. Microb Cell Factories 15(1):75
Dobrowolski A, Mituła P, Rymowicz W, Mirończuk AM (2016) Efficient conversion of crude glycerol from various industrial wastes into single cell oil by yeast Yarrowia lipolytica. Bioresour Technol 207:237–243
Lopes M, Gomes AS, Silva CM, Belo I (2018) Microbial lipids and added value metabolites production by Yarrowia lipolytica from pork lard. J Biotechnol 265:76–85
Ochsenreither K, Glück C, Stressler T, Fischer L, Syldatk C (2016) Production strategies and applications of microbial single cell oils. Front Microbiol 7:1539
Donot F, Fontana A, Baccou JC, Strub C, Schorr-Galindo S (2014) Single cell oils (SCOs) from oleaginous yeasts and moulds: production and genetics. Biomass Bioenergy 68:135–150
Louhasakul Y, Cheirsilp B, Intasit R, Maneerat S, Saimmai A (2020) Enhanced valorization of industrial wastes for biodiesel feedstocks and biocatalyst by lipolytic oleaginous yeast and biosurfactant-producing bacteria. Int Biodeterior Biodegradation 148:104911
Groenewald M, Boekhout T, Neuvéglise C, Gaillardin C, Van Dijck PW, Wyss M (2014) Yarrowia lipolytica: safety assessment of an oleaginous yeast with a great industrial potential. Crit Rev Microbiol 40(3):187–206
Sauer M, Porro D, Mattanovich D, Branduardi P (2008) Microbial production of organic acids: expanding the markets. Trends Biotechnol 26(2):100–108
André A, Chatzifragkou A, Diamantopoulou P, Sarris D, Philippoussis A, Galiotou-Panayotou M, Komaitis M, Papanikolaou S (2009) Biotechnological conversions of bio-diesel-derived crude glycerol by Yarrowia lipolytica strains. Eng Life Sci 9(6):468–478
Saygün A, Şahin-Yeşilçubuk N, Aran N (2014) Effects of different oil sources and residues on biomass and metabolite production by Yarrowia lipolytica YB 423-12. J Am Oil Chem Soc 91(9):1521–1530
Blazeck J, Hill A, Liu L, Knight R, Miller J, Pan A, Otoupal P, Alper HS (2014) Harnessing Yarrowia lipolytica lipogenesis to create a platform for lipid and biofuel production. Nat Commun 5(1):1–10
Subhash GV, Mohan SV (2015) Sustainable biodiesel production through bioconversion of lignocellulosic wastewater by oleaginous fungi. Biomass Convers Bio 5(2):215–226
Beopoulos A, Nicaud JM (2012) Yeast: a new oil producer? Oléagineux, Corps gras. Lipides. 19(1):22–28
Singh D, Sharma D, Soni SL, Sharma S, Sharma PK, Jhalani A (2019) A review on feedstocks, production processes, and yield for different generations of biodiesel. Fuel 116553
Papanikolaou S, Aggelis G (2011) Lipids of oleaginous yeasts. Part I: biochemistry of single cell oil production. Eur J Lipid Sci Technol 113(8):1031–1051
Beligon V, Christophe G, Fontanille P, Larroche C (2016) Microbial lipids as potential source to food supplements. Curr Opin Food Sci 7:35–42
American Public Health Association (1998) Water Environment Federation, Standard methods for the examination of water and wastewater. Stand Methods 541
Sadasivam S, Manickam A (1992) Biochemical methods for agricultural sciences. Wiley eastern limited
Ren X, Zhao X, Turcotte F, Deschênes JS, Tremblay R, Jolicoeur M (2017) Current lipid extraction methods are significantly enhanced adding a water treatment step in Chlorella protothecoides. Microb Cell Factories 16(1):26
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37(8):911–917
Mlíčková K, Roux E, Athenstaedt K, D’Andrea S, Daum G, Chardot T, Nicaud JM (2004) Lipid accumulation, lipid body formation, and acyl coenzyme A oxidases of the yeast Yarrowia lipolytica. Appl Environ Microbiol 70(7):3918–3924
Ghanavati H, Nahvi I, Roghanian R (2014) Monitoring growth and lipid production of new isolated oleaginous yeast Cryptococcus aerius UIMC65 on glucose and xylose cultures. Biotechnol Bioprocess E 19(3):468–477
Booramurthy VK, Kasimani R, Subramanian D, Pandian S (2020) Production of biodiesel from tannery waste using a stable and recyclable nano-catalyst: an optimization and kinetic study. Fuel 260:116373
da Silva Nonato N, Escobar EL, Kochepka DM, Derner RB, D’Oca MG, Corazza ML, Ramos LP (2020) Extraction of Muriella decolor lipids using conventional and pressurized solvents and characterization of their fatty acid profile for biodiesel applications. J Supercrit Fluids 158:104750
Radha P, Prabhu K, Jayakumar A, Abilash Karthik S, Ramani K (2020) Biochemical and kinetic evaluation of lipase and biosurfactant assisted ex novo synthesis of microbial oil for biodiesel production by Yarrowia lipolytica utilizing chicken tallow. Process Biochem 95:17–29
Wang LB, Yu HY, He XH, Liu RY (2012) Influence of fatty acid composition of woody biodiesel plants on the fuel properties. J Fuel Chem Technol 40(4):397–404
Guldhe A, Singh P, Kumari S, Rawat I, Permaul K, Bux F (2016) Biodiesel synthesis from microalgae using immobilized Aspergillus niger whole cell lipase biocatalyst. Renew Energy 85:1002–1010
Talebi AF, Tabatabaei M, Chisti Y (2014) BiodieselAnalyzer: a user-friendly software for predicting the properties of prospective biodiesel. Biofuel Res J 1(2):55–57
Fickers P, Benetti PH, Waché Y, Marty A, Mauersberger S, Smit MS, Nicaud JM (2005) Hydrophobic substrate utilisation by the yeast Yarrowia lipolytica, and its potential applications. FEMS Yeast Res 5(6-7):527–543
Sestric R, Munch G, Cicek N, Sparling R, Levin DB (2014) Growth and neutral lipid synthesis by Yarrowia lipolytica on various carbon substrates under nutrient-sufficient and nutrient-limited conditions. Bioresour Technol 164:41–46
El Bialy H, Gomaa OM, Azab KS (2011) Conversion of oil waste to valuable fatty acids using oleaginous yeast. World J Microbiol Biotechnol 27(12):2791–2798
Louhasakul Y, Cheirsilp B, Prasertsan P (2016) Valorization of palm oil mill effluent into lipid and cell-bound lipase by marine yeast Yarrowia lipolytica and their application in biodiesel production. Waste Biomass Valori 7(3):417–426
Juanssilfero AB, Kahar P, Amza RL, Miyamoto N, Otsuka H, Matsumoto H, Kihira C, Thontowi A, Ogino C, Prasetya B, Kondo A (2018) Effect of inoculum size on single-cell oil production from glucose and xylose using oleaginous yeast Lipomyces starkeyi. J Biosci Bioeng 125(6):695–702
Jiru TM, Groenewald M, Pohl C, Steyn L, Kiggundu N, Abate D (2017) Optimization of cultivation conditions for biotechnological production of lipid by Rhodotorula kratochvilovae (syn, Rhodosporidium kratochvilovae) SY89 for biodiesel preparation. 3 Biotech 7(2):145
Kuttiraja M, Douha A, Valéro JR, Tyagi RD (2016) Elucidating the effect of glycerol concentration and C/N ratio on lipid production using Yarrowia lipolytica SKY7. Appl Biochem Biotechnol 180(8):1586–1600
Deeba F, Pruthi V, Negi YS (2017) Fostering triacylglycerol accumulation in novel oleaginous yeast Cryptococcus psychrotolerans IITRFD utilizing groundnut shell for improved biodiesel production. Bioresour Technol 242:113–120
Wang J, Hu M, Zhang H, Bao J (2017) Converting chemical oxygen demand (COD) of cellulosic ethanol fermentation wastewater into microbial lipid by oleaginous yeast Trichosporon cutaneum. Appl Biochem Biotechnol 182(3):1121–1130
Chebbi H, Leiva-Candia D, Carmona-Cabello M, Jaouani A, Dorado MP (2019) Biodiesel production from microbial oil provided by oleaginous yeasts from olive oil mill wastewater growing on industrial glycerol. Ind Crop Prod 139:111535
Ramos MJ, Fernández CM, Casas A, Rodríguez L, Pérez Á (2009) Influence of fatty acid composition of raw materials on biodiesel properties. Bioresour Technol 100(1):261–268
Leiva-Candia DE, Ruz-Ruiz MF, Pinzi S, García-Ruiz JR, Dominguez J, García IL, Dorado MP (2013) Influence of nitrogen fertilization on physical and chemical properties of fatty acid methyl esters from Brassica napus oil. Fuel 111:865–871
de Freitas ON, Rial RC, Cavalheiro LF, dos Santos Barbosa JM, Nazário CE, Viana LH (2019) Evaluation of the oxidative stability and cold filter plugging point of soybean methyl biodiesel/bovine tallow methyl biodiesel blends. Ind Crop Prod 140:111667
Bhatti HN, Hanif MA, Qasim M (2008) Biodiesel production from waste tallow. Fuel 87(13-14):2961–2966
Amini Z, Ong HC, Harrison MD, Kusumo F, Mazaheri H, Ilham Z (2017) Biodiesel production by lipase-catalyzed transesterification of Ocimum basilicum L.(sweet basil) seed oil. Energy Convers Manag 132:82–90
Puhan S, Gopinath A, Nagarajan G (2009) Combustion, performance and emission characteristics of a DI CI engine using biodiesel with varied fatty acid composition. IJRET 1:81
Arous F, Jaouani A, Mechichi T (2019) Oleaginous microorganisms for simultaneous biodiesel production and wastewater treatment: a review. In: Microbial Wastewater Treatment. Elsevier, pp 153–174
Rivaldi JD, Carvalho AKF, da Conceicao LRV, de Castro HF (2017) Assessing the potential of fatty acids produced by filamentous fungi as feedstock for biodiesel production. Prep Biochem Biotechnol 47(10):970–976
Acknowledgments
The authors are grateful to the Dept. of Biotechnology, School of Bioengineering, SRM Institute of Science and Technology (SRMIST) for the support provided to carry out this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 610 kb)
Rights and permissions
About this article
Cite this article
Radha, P., Narayanan, S., Chaudhuri, A. et al. Synthesis of single-cell oil by Yarrowia lipolytica MTCC 9520 utilizing slaughterhouse lipid waste for biodiesel production. Biomass Conv. Bioref. 13, 1–12 (2023). https://doi.org/10.1007/s13399-020-01132-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-020-01132-y