Abstract
Oil palm industry generates different types of waste biomass in the form of oil palm empty fruit bunch (OPEFB), oil palm mesocarp fibers (OPMF), oil palm fronds (OPF), oil palm trunks (OPT), oil palm bark (OPB), oil palm leaves (OPL), and oil palm shell (OPS). These biomass wastes possess a great energy potential to be converted into biofuels, particularly bio-oil. Among all, the OPS have favorable physicochemical characteristics to be converted into bio-oil. Therefore, this paper mainly focuses to review the suitability of OPS as feedstock for bio-oil production compared to other oil palm biomasses. The physicochemical characteristics of the OPS, in terms of heating value, ultimate analysis, proximate analysis, and lignocellulosic composition, are presented and compared to those of the OPEFB, OPMF, OPF, OPT, OPB, and OPL. To illustrate further and signify the stability, the abovementioned properties of OPS bio-oil are also reviewed and compared to those of bio-oils produced from OPEFB, OPF, OPB, OPL, and petroleum fuels. The challenges and future prospects of OPS as a source of bio-oil are addressed and compared with other wastes of oil palm industry. Additionally, methods used for bio-oil production from oil palm industry biomass are discussed and illustrated in detail.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Nowadays, the world is focusing on sustainable biofuel production in order to overcome the environmental issues and to meet the energy demands [1]. Biofuels extracted from palm oil are known as environmentally friendly fuels and these fuels can be supportive in the implementation of cleaner production (Reijnders & Huijbregts, 2008). The two key stakeholders of palm oil in the world’s market are Malaysia and Indonesia, which collectively produce 86% of the world’s palm oil, in which 47% comes from Malaysia [2,3,4,5]. In 2010, 4.6 million hectares of palm was planted by only Malaysia, and these plants generate 17.6 million tons of palm oil and about 0.7 tons of palm kernel oil [1]. The oil palm biomass may be defined as organic materials—edible or non-edible—and in a solid or liquid form which is produced by oil palm industry during harvesting of palm oil, crushing of palm oil, and refining and milling of palm kernel oil [1]. The huge amount of lignocellulosic agricultural waste in the form of oil palm mesocarp fibers (OPMF), palm kernel cake, decanter cake, oil palm fronds (OPF), oil palm trunks (OPT), oil palm empty fruit bunches (OPEFB), and oil palm shell (OPS) is generated from oil palm industry (Abdullah et al., 2011). The palm oil mills collectively generate 55.7 million tons/year of waste. At present, these wastes are incinerated, dumped, or burnt in mills for heat generation and these operations are considered hazardous for the environment. Therefore, it is important to utilize these wastes properly. Together with a solution to environmental problems, a large amount of revenue also might be generated by their proper use. Different processing steps in typical biorefinery for converting oil palm biomass to the biofuels are shown in Fig. 1.
Various process steps involved in biorefinery for palm oil and palm oil base biomass wastes [6]. Copyright permission from Elsevier
A possible way of utilization of these wastes is to convert them into biofuels such as methanol, biodiesel, biogas, liquid bio-oil, and solid biochar. There are two main technologies, thermochemical and biochemical technologies, which are widely used for converting biomass into biofuels. Thermochemical technologies include pyrolysis, gasification, combustion, and hydrothermal processes. Hydrothermal methods are further divided into hydrothermal liquefaction, hydrothermal gasification, and hydrothermal carbonization. On the other hand, the widely practiced biochemical processes are anaerobic digestion, fermentation, composting, and transesterification [1]. In the biochemical technologies, the microorganisms and enzymes are used as a catalyst to transform biomass into biofuels.
Among wastes of oil palm industry, OPS contains lower sulfur and ash contents, and higher energy content showing its potential for biofuel production. In Malaysia, around 2.4 million tons of OPS are generated annually, which is equivalent to 45.85 PJ of energy unit [7, 8]. OPS is an industrial waste of oil palm industry produced from milling of the oil palm fruits. Around 0.3 tons of OPS waste is generated from the extraction of 1 ton of oil [9]. Higher calorific value and lower ash and oxygen values of OPS make its applications wider: as a fuel to burn in oil palm mills for power generation and it may be converted into biofuels. To date, different efforts have been done to convert this waste into bio-oil, biogas, biochar, hydrochar, activated carbon, and dielectric material and to recover energy [7, 8, 10,11,12,13,14,15]. These efforts show the suitability of OPS from oil palm industry to utilize it as a green energy biomass.
Bio-oil is a fluid dark in color, is polar and viscous, and has a higher density. It contains a complex mixture of deoxygenated compounds including alcohols, ketones, carboxylic acids, esters, benzoids, and aldehydes [16]. Bio-oil has different applications such as it can be used as a liquid fuel due to its extraordinary combustion characteristics in gas turbines and boilers and it might be used as a transportation fuel after proper upgradation; further, bio-oil may be converted into valuable hydrocarbon chemicals for industrial purposes [17,18,19,20,21]. Bio-oil can be extracted from a variety of biomaterials including agro-waste, and industrial and municipal solid waste. In fact, OPS, a waste of oil palm industry, has got much attention from the researchers to get it converted into bio-oil due to its lignocellulosic composition. Around 50% of the OPS comprises of cellulose and hemicellulose composition indicating the suitability of OPS as a feed material for bio-oil production. The hemicellulose and cellulose are easy to degrade hence easily get converted into bio-oil, whereas lignin remains as a residue. Therefore, the main objective of this study is to overview OPS as feed material for bio-oil production. The physicochemical characterization of OPS is studied and compared to those of different sources of oil palm biomass. Furthermore, the recent advancements in thermochemical methods, including liquefaction and pyrolysis of bio-oil production from OPS, are highlighted and the yield percentages of bio-oil from OPS using liquefaction and pyrolysis are compared. Additionally, the physicochemical properties of OPS bio-oil are compared to bio-oil from OPEFB, OPT, OPF, OPB, and liquid petroleum fuels to find the suitability of OPS bio-oil as a substitute for liquid petroleum fuels. Further, the future prospects and major challenges in the utilization of OPS as a feed material for bio-oil extraction are discussed and some suggestions are provided to tackle these challenges upfront.
2 OPS and its physicochemical characteristics compared to OPEFB, OPF, OPT, OPMF, OPB, and OPL
OPS in an endocarp layer surrounding the kernels, seeds, and palm kernels. It is produced by milling of the palm seeds during the mechanical crushing of residue nuts for kernel extraction from screw press [9]. The demand and production of palm oil are increasing annually due to its higher yield of oil, in the result; a huge amount of biomass waste is produced. The production of biomass waste increased from 4 million tons to 18.8 million tons from 1980 to 2012 [22]. At the same time, the OPS generation also increased from 2.5 and 4.3 million tons in 2004 and 2006, respectively, to 11.1 million tons in 2014 [1]. It is believed that only 30% of the OPS biomass has been utilized currently for the purpose of power production from oil mills [9].
OPS is a lignocellulosic material comprising of cellulose, hemicellulose, lignin, and some extractive materials. The extractive materials include fatty acids, protein, wax, and minerals [9]. The lignocellulosic material is considered a potential biomass feedstock to produce biofuels or to generate heat and power. The lignocellulosic composition of a different waste of oil palm industry including OPS, OPEFB, OPF, OPT, OPMF, oil palm bark (OPB), and oil palm leaves (OPL) is listed and compared in Table 1. The lignocellulosic composition of OPS is 20.8–39.7% cellulose, 16.9–22.9% hemicellulose, 32.5–53.4% lignin, and 4.8–19.1% extractive material as shown in Table 1. The cellulose and hemicellulose contents of OPS make it a potential feed material to get converted into biofuels, especially into bio-oil. The cellulose and hemicellulose are easy to decompose than lignin, thus gets converted into bio-oil at lower temperatures whereas lignin remains as residue [54,55,56]. It is reported that the cellulose starts to decompose at 240 °C, and the hemicellulose degrades at approximately 200–260 °C, whereas lignin degrades at a higher temperature in the range of 280–500 °C due to its complex chemical composition [57]. In comparison to lignocellulosic composition of OPS, the cellulose, hemicellulose, lignin, and extractive contents of OPEFB are 32.1–59.7%, 22.1–35.3%, 17.8–25.9%, and 2.7–27.9%; of OPF are 30.4–50.3%, 17.1–40.4%, 20.2–22.9%, and 1.7–3.5%; of OPT are 31.7–45.8%, 17.7–34.4%, 22.6–35.9%, and 3.1–12.0%; of OPMF are 23.1%, 17–34.0%, 13–30.6%, and 24.1%; of OPB are 18.9%, 49.28%, 21.8%, and 10.0%; and of OPL are 32.5–44.5%, 7.5–23.0%, 26.0–27.4%, and 20.6%, respectively.
Proximate analysis quantifies the determination of fixed carbon, volatile matter, moisture content, and ash contents in any fuel or biomass [58]. The proximate analysis of any biomass varies depending on the growing environment, age, and its time [59,60,61]. Proximate analysis is an important characterization which determines the energy content of any fuel or biomaterials by finding the ratio of combustibles (explosive matter and fixed carbon) to non-combustibles (ash and moisture content) [9]. As shown in Table 1, the volatile contents of different wastes of oil palm industry are 36.8–77.5%, 55.9–83.9%, 82.5–82.7%, 82.6–83.9%, 66.8–78.0%, and 66.8% for OPS, OPEFB, OPF, OPT, OPMF, and OPL, respectively. The ignition of fuels/biomass containing higher volatile matter is easier [62]; therefore, the ignition of oil palm waste will be easier. Further, the presence of volatile matters in large quantity supports the maximum production of bio-oil [23]. It is reported that higher volatile contents offer the benefits of high reactivity and volatility which support the liquid fuel production [62]. The volatile matter yield is significantly affected by heating rate and temperature [63]. The fixed carbon contents for OPS, OPEFB, OPF, OPT, OPMF, and OPL are 13.2–54.7%, 8.65–19.9%, 1.5–3.2%, 5.0–16.1%, 7.6–24.4%, and 11.9%, respectively. The OPS contains higher fixed carbon values than other studied wastes of oil palm industry suggesting that the OPS will generate a significant amount of heat by its burning [9]. A higher percentage of fixed carbon in OPS than other materials is also of significance, because a material containing higher fixed carbon content will yield a product of higher carbon content [23]. The higher amount of moisture contents is undesirable because it reduces the calorific value of the material. Moreover, its presence in higher quantity is a crucial issue in converting biomass into bio-oil because the total water content in biomass goes to bio-oil during bio-oil production, thus causing an increase of water in bio-oil [24]. It is reported that the moisture content can be easily removed from the end product by drying it in an oven at 105 °C for 24 h [46]. Although moisture is undesirable, it has some advantages also such as the temperature profile will be maintained in the presence of higher moisture content; together with that, it also reduces the emissions of NOx [64]. The ash is an important property of biomass helping to predict/determine the distribution of yield percentage, into solid, liquid, and gaseous products [46]. It is proved that an increase in ash content of biomass will increase the yield percentage of solid and gaseous products, whereas the liquid yield percentage will be decreased [65]. Abnisa et al. [46] witnessed that the OPL having higher content of ash produced lower bio-oil yield percentage and higher char and gaseous products. It is evident from Table 1 that the OPS contained less amount of ash than OPEFB, OPMF, and OPL, while OPT and OPF contained less ash content than OPS. The ultimate (elemental) analysis measures the quantifiable determination of carbon, hydrogen, sulfur, nitrogen, and oxygen contents present in any biomass or fuel [58]. The ultimate analysis determines the quantity of gas needed for burning of the fuel or biomass together with analyzing the quantity and composition of gas emitting during combustion of the fuel [22]. The comparative carbon compositions of different biomass of oil palm industry including OPS, OPEFB, OPF, OPT, OPMF, and OPL are 26.9–56.1%, 40.9–49.1%, 42.8–47.2%, 42.7–47.5%, 43.2–50.3%, and 40.4%, respectively. In addition to that, the hydrogen content is also higher in OPS than other oil palm biomass studied. The higher carbon and hydrogen contents of OPS than all other studied oil palm biomass advocate that the OPS is a good fuel than others. The higher carbon content and lower oxygen content improve the combustion properties of fuel [58, 66]. It is reported in the literature that the calorific value is directly proportional to the carbon content of fuel or biomass [67]. The oxygen is undesirable in any fuel because it reduces the calorific value. Though its presence in higher quantity is not good, its presence has some advantages also such as higher oxygen contents reduce the emission of carbon dioxide (CO2) because it tends to reduce the combustion properties [68]. The heating values of OPS, OPEFB, OPT, OPF, and OPMF are listed and compared in Table 1. The lower HHV values of oil palm wastes are linked to higher oxygen and moisture contents of biomass. These results can be attributed to the higher ratio of combustible to non-combustible substances [9]. It can be noted from Table 1 that the OPS contained greater HHV than all other studied wastes of oil palm industry suggesting that the OPS is a better fuel than the rest. The HHVs of oil palm biomass are comparable to those of softwood and hardwood proposing that the oil palm wastes will provide the same benefits as wood in terms of HHV [69, 70]. Further, the XRF analysis for different oil palm biomass waste is shown in Table 2.
3 Recent advancement in technologies for conversion of OPS into bio-oil
Thermochemical technology is a widely accepted technology for the conversion of biomass into solid, gaseous, and liquid biofuels as well as for the generation of heat. The thermochemical methods generally can be divided into four types, namely combustion, pyrolysis, gasification, and hydrothermal processes (hydrothermal gasification, hydrothermal carbonization, and hydrothermal liquefaction) depending upon the quantity of heat and oxygen used [72]. Among thermochemical processes, the pyrolysis and hydrothermal liquefaction are the most commonly used methods for conversion of any source of biomass into liquid fuels. Pyrolysis deploys dry biomass; therefore, the water present in biomass has a negative effect on the pyrolysis process. Ultimately, it needs the higher heat of vaporization [73]. Generally, pyrolysis is preferred for the biomass having a moisture content of less than 40%. In the case of higher moisture content biomass, the pretreatment is needed to cater the moisture content issue and make it suitable for pyrolysis process. Pyrolysis process also releases volatile matters [9]. These issues limit the options of biomass feedstocks and the overall economy of the pyrolysis process. Therefore, the pyrolysis process is not considered an environmentally friendly and economical process.
On the other hand, hydrothermal liquefaction (also known as solvolysis [22]) can handle any type of biomass with any level of moisture content [73]. Hydrothermal liquefaction can suitably liquefy the biomass with greater moisture content such as tropical biomass (80–85% moisture content) and aquatic species (moisture content around 90%) due to higher salvation power of fluids at hydrothermal conditions [73]. Fluids under hydrothermal conditions attain properties which support liquefaction of biomass like high heat and density, fast decomposition, mass transfer capabilities, and extraction [74, 75].
3.1 Pyrolysis
Pyrolysis is an endothermic reaction carried out at elevated temperature to convert biomass into solid, gaseous, and liquid products through rapid heating in the absence of oxygen or in the presence of limited oxygen [22, 76,77,78,79]. In pyrolysis, the biomass experiences a number of primary and secondary reactions involving the mechanism of mass and heat transfer. The primary reactions taking place during pyrolysis are formation of the primary and intermediate products by decomposition of hemicellulose, cellulose, and lignin content of biomass feedstock. Then, the formed intermediates undergo secondary cracking through charring and dehydration reactions of primary products and volatilization and decomposition of intermediate products [73].
Pyrolysis can be divided into six main types depending on process parameters: slow pyrolysis, flash pyrolysis, fast pyrolysis, intermediate pyrolysis, vacuum pyrolysis, and hydropyrolysis. Slow pyrolysis is one of the oldest processes used for getting higher biochar yield from pyrolysis at relatively low temperature and low heating rate. The formation of biochar in greater amounts during slow pyrolysis takes place due to higher vapor residence time in slow pyrolysis. These vapors react with each other continuously in vapor phase, which results in higher biochar production [80]. The flash pyrolysis is carried out with the help of a canister of packed bed of feedstock which is kept in pressure vessel. Both the biochar and bio-oil can be produced from flash pyrolysis but the bio-oil is considered a main product as 75% of biomass gets converted into bio-oil during flash pyrolysis process [81]. Fast pyrolysis produces all three types of the product namely solid, liquid, and gaseous products. During fast pyrolysis, 10–20% of dry biomass is converted into gaseous products, 15–25% into bio-oil, and 75% into bio-oil [82, 83] suggesting that the bio-oil is the main product of fast pyrolysis process. Fast pyrolysis reaction is carried out at high transfer rate and temperature, fast cooling of vapors of pyrolysis, lower residence time of vapors, and fine feed materials [84, 85]. Intermediate pyrolysis is conducted between operating conditions of fast pyrolysis and slow pyrolysis, i.e., moderate temperature (500 °C), moderate residence time of vapors (2–4 s), and 0.5–25-min residence time of feedstock. The product distribution of intermediate pyrolysis is 40–60% bio-oil, 20–30% noncondensable vapors, and 15–20% biochar [86]. A main feature of intermediate pyrolysis is that its bio-oil is separated readily into an aqueous and organic phase [87]. Vacuum pyrolysis of industrial residues is considered a clean, attractive recycling method which has been subject to various patents also. It generally produces pyrolytic carbon black and liquid hydrocarbons. The vacuum pyrolysis produces more liquid product because it minimizes secondary reactions such as catalytic cracking, gas phase collision, oxidation and reduction reactions, thermal cracking, recondensation, and repolymerization reactions. Hence, when vapor products are quenched, the pyrolysis oil yield increases at the expense of gaseous and solid residue products [88]. It does not emit any hazardous emissions due to mild pyrolysis conditions, i.e., lower temperature and absence of carrier gas [89]. Hydropyrolysis is considered a hybrid of pyrolysis and hydrolysis, which comprises all the terms including thermolysis, liquefaction, gasification, decomposition in hot compressed water and thermal decomposition, etc., for pyrolysis in water [90]. The temperature, heating rate, and residence time of hydropyrolysis are nearly the same to those of fast pyrolysis. Hence, it can be considered fast pyrolysis at higher pressure and in presence of hydrogen/hydrogen-based materials [91]. The typical operating parameters for each type of pyrolysis are shown in Table 3.
The amount of end products and their respective composition obtained from pyrolysis process depend on several factors such as reaction temperature, residence time, particle size, biomass composition, pressure, catalyst, and heating rate [25, 92, 93]. The type and composition (cellulose, hemicellulose, and lignin contents) of the biomass affect the nature and the composition of pyrolysis products. In biomass, lignin supports higher char production because it is more stable and hard to degrade, therefore produces a larger char product. Char production from lignin is because of the fracturing of relatively weak bonds and the formation of a more condensed solid structure [94]. Demirbas [95] studied the pyrolysis of different biomass with different lignin contents and resulted that the biomass with more lignin content produced higher amount of char, whereas the biomass with less lignin content gave the higher yields of bio-oil and biogas.
The biomass is always associated with some amount of moisture content. Higher moisture content is not favorable for the pyrolysis process. Biomass having greater than 30% of moisture content is not suitable for pyrolysis process [95]. Biomass with greater moisture content needs higher energy for pyrolysis to dry biomass [73]. Higher moisture content also reduces the heating rate and, ultimately, requires more time to attain pyrolysis temperature [96]. Pyrolysis is a favorable process for low moisture content biomaterials [91]. The biomaterials having higher moisture content are preferable for bio-oil fuel production and they produce lower amount of biochar. A study [97] researched the various agro-materials having identified moisture content. It was explored from the results that higher amount of bio-fuel and low biochar can be obtained at only high moisture content. Benesoro et al. [98] also identified the similar phenomenon in between the extraction of biochar and moisture content. He examined that increase in water content in sewage sludge results in decreased biochar yield. The moisture affects the energy need of the conversion process but also has an influence on the product quality. The amount of biomass sample also has an influence on biochar yield percentage during the pyrolysis process.
The vapor residence time heating rate and particle size are also considered one of the important parameters to affect yield percentage distribution during the pyrolysis process. The favorable condition for a charcoal production is long residence time at low temperature [99]. A high amount of biochar can be obtained at extensive residence time because a suitable reaction time is desired for the repolymerization of biomaterials. Therefore, repolymerization is directly proportional to residence time. Short residence time will result in lower biochar yield [100]. Particle size controls the rate at which heat is transported into the input biomass. The maximum biochar production can be achieved at an increased particle size because the vapor rise through combustion of biomaterials covers more space over the layers of biochar and provides extra time for the secondary reaction to take place, and results in an increased char yield [91]. Mani et al. [101] investigated the influence of particle size on pyrolysis of wheat straw for biochar yield. It was observed that by increasing particle size, from 0.25 to 0.47 mm, improved biochar yield from 11.85 to 23.28%. Similarly, another study [95] also reported an improvement of 28.3% in charcoal production at the temperature of 900 °C, by varying the particle size of hazelnut from 0.15 to 1.4 mm. Di Blasi et al. [102] observed that increasing particle size of biomass results in high rate of char production. During pyrolysis of beech wood in a fluidized bed reactor at the temperature of 807 K, an increase of 5 wt% was noticed while increasing particle size from 2 to 10 mm. Gronli et al. [103] noticed a decline of 2.8% from 3.3% in char production at the constant heating rate of 400 °C, when the mass was reduced from 0.94 to 0.11 mg. Even though most researches examining the influence of particle size on biochar production report that biochar production can be increased by increasing particle size, there are also conflicting studies reporting that the percentage of biochar production is reduced by increasing particle size [101, 104]. Under these situations, comparatively large particle sizes (i.e., ≥ 14-mm cubes) have no substantial effect on charcoal or fixed carbon yield.
The particle size also affects the properties of the pyrolysis products. It has been observed in previous studies that dow corning simple material holds more ash than fine wood chips, while high-quality denim wood chips contain more ash than dow corning simple material. Increasing the particle size can significantly increase the fixed carbon content. The heating rate plays an influential role in the pyrolysis because the composition and the nature of the final product of pyrolysis are affected by the rate of change of heat. The amount of biochar reduces at higher heating rates. At higher heating rates, the secondary pyrolysis reaction dominates, and it supports gaseous products. In addition, the depolymerization of biomass components in volatile substituents is enhanced at higher heating rates, ultimately resulting in delay in the percentage of coke yield [91]. The reduction in biochar yield was reported by increasing the heating rate from 30 to 50 °C/min. At low heating rates, the natural porosity of the material allows for volatile to release without major morphological changes. However, at high heating rates, there is virtually no cell structure after devolatilization. The slow heating rate of charcoal showed a clear similarity to the original eucalyptus, and the high heating rate of char began to show signs of melting similar to radiata pine.
The reaction temperature has a negative influence on the yield percentage of biochar, i.e., the biochar yield percentage decreases with increasing the reaction temperature because an increase in temperature helps in the thermal cracking of heavy hydrocarbon materials, leading towards the enhanced formation of liquid and gaseous products, and decreased biochar product. The biochar formed during the pyrolysis process passes through secondary reactions and gets converted into liquid and gaseous products leading to the decrease in biochar yield [91]. A 10% decrease in biochar yield percentage of hazelnut shell was observed during pyrolysis of hazelnut shell by increasing pyrolysis temperature from 400 to 700 °C [105].
It is reported in literature that the pressure inside reactor is an important factor which affects the yield distribution percentage during pyrolysis process. A high rate of pressure as compared to ambient pressure also raises the production rate of biochar [106]. It is because the high rate of pressure extends residence period during preparation of secondary char and vapor breakdown on surface of char which finally increases the char yield [107]. High pressure raises the capacity of pressure and temperature of bio-oil, thus postponing its conversion to vapor phase and preferring liquid-phase coking reactions that develop the charcoal. One more research also identified an increase in char production by raising pressure of reactor. An increment of pressure up to 1 MPa from 0.1 MPa can rise the biochar production up 35% from soft and hardwood [108]. The pressure also has effects on the physicochemical characterization of products. The results obtained for fine cowboy and sawdust showed an increase of 22% in the value of fixed carbon from 16% and 27.6 wt% respectively at high pressure. The scanning electron microscopy (SEM) images of red oak biochar material obtained at 0.1 MPa pressure showed the fibrous arrangement of its biomass. Another study of corncob showed that the high pressure can cause molten cell wall, although the pore shape was conserved [109]. So it is confirmed that high pyrolysis pressure will decline the surface area of charcoal. Hence, charcoal produced at low pressure shows microspores while charcoal prepared at high pressure shows macropore structures. Similarly, the study also identified that pressure affects the amount of carbon with an increase of 93.2 wt% at 16 bar which was 86.4 wt% at 1 bar.
The flow rate of carrier gas is also considered one of the important parameters to affect the yield percentage distribution of pyrolysis. It shows the effect on biochar in a negative way. Argon, nitrogen, and water vapors have been reported in the literature as a carrier gas for pyrolysis process but nitrogen is most commonly used for the purging of vapors in pyrolysis process because it is cheap, inert, and readily available [91]. Normally, higher stream rate of carrier gas is not appropriate for charcoal yield in pyrolysis process as it decreases the char production. Higher flow rate throws the vapors out of the reaction zone, which decreases the vapors’ residence time; consequently, the volatiles are driven out rapidly, which causes a decrease in biochar yield. A number of studies have been carried out to study the effect of flow rate of carrier gas on biochar yield percentage, which supports the statement that biochar yield is decreased with increasing flow rate of carrier gas. Zhang et al. [110] noted a slight decrease in biochar yield with an increase in flow rate of nitrogen from 1.2 lit/min to 4.5 lit/min. Heideri et al. [111] also observed a decrease in the yield percentage of biochar with a rise in the flow rate of carrier gas.
3.2 Hydrothermal liquefaction
Hydrothermal liquefaction is a pressurized thermal conversion process in the water/saturated steam media conducted at relatively low temperature of 180–400 °C, and catalysts to enhance the reaction rate and/or to improve the selectivity of the process by using citric acid and metal ions catalyst [3, 110, 112,113,114,115]. Liquefaction of biomass in organic solvents is a unique thermochemical conversion. It yields substances that contain a combination of useful functional groups both coming from the organic solvents and the biomass feedstock, thus obtaining a large variety of polymers [116,117,118]. Liquefaction can also convert biomass with high moisture content, hence eliminating the need of drying of biomass feedstock and often resulting in higher bio-oil yield compared to pyrolysis process [26] about 250–400 °C and higher pressure (5–20 MPa) [119]. Besides bio-oil and biochar, liquefaction processes also produce carbon-containing aqueous by-products (which contained dissolved organics, sugars, and acids) and gaseous by-products (CO2, CO, CH4, H2, and other light hydrocarbons). During liquefaction, lignocellulosic components containing lignin, cellulose, and hemicellulose are broken down into low-molecular-weight compounds. These compounds are highly reactive and are useful in various applications like resin precursor and bio-oil. The advantage of liquefaction is that the bio-oil produced by liquefaction is not miscible with water and has a lower oxygen content, and therefore a higher energy content than pyrolysis-derived oils (Zhang et al., 2009). In liquefaction, the heated and pressurized solvent will break down the complex matrix structure of biomass, producing liquid products that are extracted using organic solvents as bio-oil (Chan et al., 2015). The overall objective of biomass liquefaction is to control the reaction rate and reaction mechanisms, using pressure, and/or catalysts, to produce the finest liquid oil (Zhang et al., 2009). The oil produced by hydrothermal liquefaction is similar to its counterpart in dry thermochemical conversion “flash pyrolysis.” The use of solvent in liquefaction processes enables lower process temperatures. Apart from that, the presence of solvent dilutes the concentration of products and prevents the formation of tar compounds due to cross-linking and recombination reactions. The reaction pathways for hydrothermal liquefaction are shown in Fig. 2. However, oil from hydrothermal liquefaction is dominated by phenols and exhibits a lower content of polar compounds like acids and sugars [115].
Major reaction pathways of hydrothermal liquefaction [120]. Copyright permission from Elsevier
4 Comparison of the properties of OPS bio-oil to the bio-oil from OPEFB, OPF, OPT, OPMF, OPB, and OPL and petroleum fuel oils
Utilization of OPS for bio-oil production serves as double purpose, i.e., to get energy and utilize the industrial waste of oil palm industry mitigating the environmental problems [121]. Table 4 compares the properties of the bio-oil obtained from OPS to those of the bio-oil obtained from OPEFB, OPF, OPT, OPMF, OPB, and OPL as well as to those of petroleum fuel oils. This will help to determine the quality of the OPS bio-oil and its potential applications in various industries. Bio-oils possess a great potential to be utilized as a substitute of fuel oil, diesel, and chemicals. It is reported that the bio-oil has been used as an alternative of fuel oil in power generation with gas turbines, diesel engines and steam power and natural gas plants [64, 134]. It is described that the bio-oils may burn effectively in the standard boilers or slightly modified boilers and engines at the similar rate as commercial fuels [136]. Kim et al. [122] suggests that the pyrolytic bio-oil may be potentially used as a feedstock for biodiesel production through hydrotreating. The hydrotreating is more advantageous than transesterification due to higher compatibility of hydrotreating with present infrastructure, feed flexibility, and engine compatibility [122]. Bio-oil obtained by catalytic pyrolysis may be a suitable candidate for biodiesel feedstock application due to higher amounts of fatty oxygenates and fatty nitriles [137]. The presence of fatty nitriles in bio-oil supports its application for fuel production because carbon numbers of major nitriles (C11–C16) are within the range of carbon numbers of diesel. Further, the nitrile group can easily be removed from nitrogenous compound during hydrotreating at refinery [138]. The nitriles can be utilized for chemical products such as surfactant and polymers [139, 140].
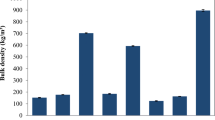
Bio-oil is a dark brown liquid with a smoke-like odor and its composition is very different from petroleum derivatives [7]. The bio-oil mainly consists of two phases, i.e., aqueous and non-aqueous phases [6]. The aqueous phase contains various oxygenated organic compounds of lower molecular weight such as acetone, acetic acid, and methanol. On the other hand, the non-aqueous phase contains oxygenated compounds, single-ring aromatic hydrocarbons, and polycyclic aromatic hydrocarbons [141, 142]. It is evident from the Table 4 that the density of OPS bio-oil is in the range of the bio-oils from other palm tree biomass whereas it is bit higher than that of the petroleum fuels. Kinematic viscosity of OPS bio-oil is 3.2 mm2/s, which is near to that of OPEFB bio-oil (2.3–8.4 mm2/s), OPF bio-oil (1.88 mm2/s), OPT bio-oil (1.99 mm2/s), OPL bio-oil (1.75 mm2/s), diesel/light fuel oil (1.2–7.5 mm2/s), and high sulfur heavy fuel oil (3.51 mm2/s). The flash point of the OPS bio-oil (65 °C) is nearly in the range for that of diesel/light fuel oil (60–98 °C) and is much lower than that of high sulfur heavy fuel oil (100 °C).
It can be noted that the OPS bio-oil has lower moisture, solid content, oxygen content, and ash than the bio-oils produced from other biomass wastes of oil palm industry. On the other hand, the bio-oils from either of the oil palm biomass wastes contain higher moisture, solid, ash, and oxygen contents than those of petroleum fuel oils. These are considered undesired characteristics of the bio-oil as an engine fuel [22]. The higher amounts of moisture content present in bio-oil are a major drawback of the bio-oil for its application as fuel [143]. The higher moisture content present in bio-oil reduces the heating value; high amount of ash declines the combustion rate and enhances the ignition delay. Higher solid in bio-oil challenges in injection systems and pipes, whereas higher oxygen content causes thermal instability, thus impeding the storage stability of bio-oil [144]. The bio-oils generally contain small amount of organic acids and solids (char) which may cause problems. For instance, the organic acids are highly corrosive to common construction materials whereas the solids in bio-oil can cause the blockage of injector and can erode the blades of turbines. Also, reactivity of various components of bio-oil may form large molecules which results in slow combustions and high viscosity [64]. Thus, to overcome these limitations, the bio-oils must be upgraded through different methods. The moisture content and the ash content in bio-oil can be reduced to lower than those in petroleum fuels by fractional distillation and by washing the feedstock prior to using it for bio-oil production, respectively [145]. Although the higher oxygen and higher moisture are unwanted in fuels, they possess some positive effects also. Higher amount of oxygen will support in reducing the carbon dioxide emissions because it enhances combustion characteristics [68]. On the other side, higher moisture content helps in maintaining uniform profile of temperature in cylinders, improves flow characteristics of bio-oil, and decreases NOx emissions [64]. The carbon content in OPS bio-oil is almost similar to OPEFB bio-oil whereas much higher than OPF, OPB, and OPL bio-oils. On the other hand, the carbon content of all the bio-oils is much lower than that of petroleum fuel oils. Further, lower amount of sulfur in OPS bio-oil and OPEFB bio-oil than that of the petroleum fuels makes the bio-oils more health and environment friendly than petroleum fuels. The chemical functional groups present in OPS bio-oil are –OH groups (3200–3400 cm−1) representing alcohols and phenols, C–H functional group (2800–3000 cm−1 and 1350–1450 cm−1) showing alkanes, O–C groups (1680–1750 cm−1) demonstrating ketones, quinones, aldehydes, C–C bond (1500–1645 cm−1) indicating alkenes, and aromatic groups as shown in Table 5.
5 Major challenges and future prospects
Bio-oil is a renewable liquid fuel obtained by conversion of different types of biomass through different processes such as pyrolysis, hydrothermal liquefaction, hydrolysis, and solvolysis. It is drawing substantial attention as potential alternate energy source due to its reproducibility and availability of biomass resources in large amounts worldwide [144]. The research work on bio-oil production is still in its early stages and is limited to laboratory scale and its pilot-scale production is still very limited [143]. Although some studies reported successful utilization of bio-oil as an alternative to fuel oil for power generation, its application as transportation fuel is at its early stages. The bio-oil can be produced from different types of biomass sources such as palm industry wastes.
Over the last two decades, much research and developments have been proposed for the utilizing of different types of biomass wastes coming from oil palm industry for synthesis of bio-oil. These biomass wastes include OPS, OPEFB, OPF, OPB, and OPL. Among others, more work has been reported for OPS and OPEFB. An industrial scale plant for bio-oil production from EFB has been successfully installed and established in Malaysia [22]. The bio-oil production from oil palm biomass is still facing different problems such as low quality of bio-oil including higher moisture, higher ash, higher oxygen, high viscosity, high corrosivity, thermal instability, and non-homogeneity. Other drawbacks are lack of appropriate and economically viable technologies including energy-intensive feedstock preparation techniques, lacking of domestic expertise for process and equipment handling, non-competitive market strategies, and financial barriers which need to be addressed [126, 143]. Other challenges of oil palm industry associated with bio-oil production are biodiversity and conversation, life-cycle assessment, land use, replantation, and health concerns [146, 147]. Despite these challenges and issues, it is necessary to upgrade the quality of bio-oil before its utilization as a substitute for petroleum fuels in order to provide the same benefits as petroleum fuels. The upgradation techniques including hydrocracking, hydrotreating, catalytic upgrading, and steam reforming have been successfully used for upgrading of bio-oil to compete with engine fuel [22, 148, 149]. In addition to that, the public and market acceptance are also considered a critical parameter to support and sustain the oil palm biomass waste utilization for biofuel production. It is a responsibility of the government to provide tax incentives or offer an appropriate financial support [150] for synthesis of bio-oil for setting up a capital intensive, design and fabricate large-scale pyrolysis plants. Additionally, there is much need of increasing the acceptance and demand of biofuels in local markets by developing standards and promoting sustainable agriculture. Also, it is important to offer an appropriate infrastructure for educating people and the farmers [151, 152] about the benefits of cultivation of oil palm and proper utilization of palm oil wastes into biofuels as well as about the advantages of these biofuels. It is also important to gear up innovative technologies which provide a solution or utilize by-products formed during pyrolysis of oil palm biomass [153].
Despite the focus on bio-oil synthesis from oil palm biomass resources, it is also suggested that the biochar production through slow pyrolysis of oil palm biomass also should be given an equal importance for improving soil fertility and taking care of climate change. In this perspective, the Universiti Putra Malaysia has taken an initiative and collaborated with NASMECH technology Sdn. Bhd. in a project of EFB carbonator which can process up to 20 tonnes of EFB per day for production of biochar [154].
In order to properly utilize these wastes of oil palm industry, the Malaysian government has introduced national biofuel program. Currently, a number of projects from small renewable energy program (SREP) are operational in Malaysia which provide electricity to national grid [155]. Recently, the companies are showing an interest to produce biofuels from oil palm biomass resources. For instance, various lab-scale pyrolysis reactors have been developed such as transport board, bubble fluidized bed, circulating fluidized bed, vacuum pyrolysis reactor, rotating cone reactor, screw reactor, and ablative reactor [156, 157]. Large-scale pyrolysis plants are also under progress in Malaysia since 1990s, focusing on energy and bio-oil production. First commercial bio-oil plant was started by collaboration between Biomass Technology Group and Genting Sanyen Bhd. Malaysia in 2005, producing about 2 tonnes of bio-oil per hour from EFB (a palm oil waste) [158]. The Palm Oligo Sdn Bhd. also fabricated a fast pyrolysis plant in 2012 with capacity of 5 tonnes per day bio-oil production from palm kernel cake [154]. Another company, the national key Economic Areas, aims to establish 29 new bio-oil pilot plants for pyrolysis of oil palm biomass by 2020 throughout the country. The tire recycling plant has also been established in Malaysia with the handling capacity of 120 tonnes per day. The end product is distributed into 50% bio-oil, 30% biochar, 10% gas, and 10% steel wires [154].
6 Conclusion
The policymakers and researchers are much interested in oil palm biomass, especially OPS, for bio-oil production due to its abundant availability and favorable physicochemical characteristics. The pyrolysis and hydrothermal liquefaction are two key processes used for conversion of OPS into bio-oil, with the former being a more widely used process whereas the latter is considered the most emerging method. Regardless of the process used for production of bio-oil, the OPS bio-oil has more carbon and hydrogen heating value, whereas lower oxygen, moisture, and ash contents than those of the sibling biomass produced bio-oil comparable to the petroleum fuels. To date, there is limited success for production of bio-oil from OPS confirming that still there are challenges which need to be addressed for anticipating any progress in the field in the near future.
References
Lee KT, Ofori-Boateng C (2013) Sustainability of biofuel production from oil palm biomass. Springer
Mohammed M et al (2012) Gasification of oil palm empty fruit bunches: a characterization and kinetic study. Bioresour Technol 110:628–636
Novianti S et al (2014) Upgrading of palm oil empty fruit bunch employing hydrothermal treatment in lab-scale and pilot scale. Procedia Environ Sci 20:46–54
Mazaheri H et al (2010) Sub/supercritical liquefaction of oil palm fruit press fiber for the production of bio-oil: effect of solvents. Bioresour Technol 101(19):7641–7647
Mazaheri H et al (2010) Subcritical water liquefaction of oil palm fruit press fiber for the production of bio-oil: effect of catalysts. Bioresour Technol 101(2):745–751
Chew TL, Bhatia S (2008) Catalytic processes towards the production of biofuels in a palm oil and oil palm biomass-based biorefinery. Bioresour Technol 99(17):7911–7922
Abnisa F et al (2011) Utilization possibilities of palm shell as a source of biomass energy in Malaysia by producing bio-oil in pyrolysis process. Biomass Bioenergy 35(5):1863–1872
Arami-Niya A et al (2011) Optimization of synthesis and characterization of palm shell-based bio-char as a by-product of bio-oil production process. BioResources 7(1):0246–0264
Nizamuddin S et al (2016) A critical analysis on palm kernel shell from oil palm industry as a feedstock for solid char production. Rev Chem Eng
Nizamuddin S et al (2015) Synthesis and characterization of hydrochars produced by hydrothermal carbonization of oil palm shell. Can J Chem Eng 93(11):1916–1921
Nizamuddin S et al Chemical, dielectric and structural characterization of optimized hydrochar produced from hydrothermal carbonization of palm shell. Fuel
Kean CW, Sahu JN, Daud WW (2013) Hydrothermal gasification of palm shell biomass for synthesis of hydrogen fuel. BioResources 8(2):1831–1840
Abnisa F, Daud WW, Sahu J (2011) Optimization and characterization studies on bio-oil production from palm shell by pyrolysis using response surface methodology. Biomass Bioenergy 35(8):3604–3616
Tripathi M et al (2015) Effect of temperature on dielectric properties and penetration depth of oil palm shell (OPS) and OPS char synthesized by microwave pyrolysis of OPS. Fuel 153:257–266
Sabzoi N, Yong EK, Jayakumar NS, Sahu JN, Ganesan P, Mubarak NM, Mazari S (2015) An optimisation study for catalytic hyfrolysis of oil palm shell using response surface methodology. J Oil Palm Res 47(4):339–351
Bridgwater AV (2003) Renewable fuels and chemicals by thermal processing of biomass. Chem Eng J 91(2–3):87–102
Amen-Chen C, Pakdel H, Roy C (2001) Production of monomeric phenols by thermochemical conversion of biomass: a review. Bioresour Technol 79(3):277–299
McKendry P (2002) Energy production from biomass (part 2): conversion technologies. Bioresour Technol 83(1):47–54
Savage PE et al (1995) Reactions at supercritical conditions: applications and fundamentals. AICHE J 41(7):1723–1778
Naik S et al (2010) Production of first and second generation biofuels: a comprehensive review. Renew Sust Energ Rev 14(2):578–597
Effendi A, Gerhauser H, Bridgwater AV (2008) Production of renewable phenolic resins by thermochemical conversion of biomass: a review. Renew Sust Energ Rev 12(8):2092–2116
Chang SH (2014) An overview of empty fruit bunch from oil palm as feedstock for bio-oil production. Biomass Bioenergy 62:174–181
Abnisa F et al (2013) Characterization of bio-oil and bio-char from pyrolysis of palm oil wastes. BioEnergy Res 6(2):830–840
Asadullah M et al (2013) Production and detailed characterization of bio-oil from fast pyrolysis of palm kernel shell. Biomass Bioenergy 59:316–324
Yang H et al (2006) Pyrolysis of palm oil wastes for enhanced production of hydrogen rich gases. Fuel Process Technol 87(10):935–942
Chan YH et al (2014) Bio-oil production from oil palm biomass via subcritical and supercritical hydrothermal liquefaction. J Supercrit Fluids 95:407–412
Idris SS et al (2010) Investigation on thermochemical behaviour of low rank Malaysian coal, oil palm biomass and their blends during pyrolysis via thermogravimetric analysis (TGA). Bioresour Technol 101(12):4584–4592
Abubakar Z, Ani FN (2013) Microwave-assisted pyrolysis of oil palm shell biomass. Jurnal Mekanikal 36:19–30
Jamaluddin MA et al (2013) Microwave-assisted pyrolysis of palm kernel shell: optimization using response surface methodology (RSM). Renew Energy 55:357–365
Hashim R et al (2011) Characterization of raw materials and manufactured binderless particleboard from oil palm biomass. Mater Des 32(1):246–254
Hesas RH et al (2013) Preparation of granular activated carbon from oil palm shell by microwave-induced chemical activation: optimisation using surface response methodology. Chem Eng Res Des 91(12):2447–2456
Mae K et al (2000) A new conversion method for recovering valuable chemicals from oil palm shell wastes utilizing liquid-phase oxidation with H2O2 under mild conditions. Energy Fuel 14(6):1212–1218
Kelly-Yong TL et al (2007) Potential of hydrogen from oil palm biomass as a source of renewable energy worldwide. Energy Policy 35(11):5692–5701
Mazaheri H, Lee KT, Mohamed AR (2013) Influence of temperature on liquid products yield of oil palm shell via subcritical water liquefaction in the presence of alkali catalyst. Fuel Process Technol 110:197–205
Aziz SMA et al (2013) Bio-oils from microwave pyrolysis of agricultural wastes. Fuel Process Technol 106:744–750
Mushtaq F et al (2015) Optimization and characterization of bio-oil produced by microwave assisted pyrolysis of oil palm shell waste biomass with microwave absorber. Bioresour Technol 190:442–450
Abubakar Z, Salema AA, Ani FN (2013) A new technique to pyrolyse biomass in a microwave system: effect of stirrer speed. Bioresour Technol 128:578–585
Claoston N et al (2014) Effects of pyrolysis temperature on the physicochemical properties of empty fruit bunch and rice husk biochars. Waste Manag Res 32(4):331–339
Sidik DAB, Ngadi N, Amin NAS (2013) Optimization of lignin production from empty fruit bunch via liquefaction with ionic liquid. Bioresour Technol 135:690–696
Parshetti GK, Hoekman SK, Balasubramanian R (2013) Chemical, structural and combustion characteristics of carbonaceous products obtained by hydrothermal carbonization of palm empty fruit bunches. Bioresour Technol 135:683–689
Abdullah N, Gerhauser H (2008) Bio-oil derived from empty fruit bunches. Fuel 87(12):2606–2613
Kongpanya J, Hussaro K, Teekasap S (2014) Influence of reaction temperature and reaction time on product from hydrothermal treatment of biomass residue. Am J Environ Sci 10(4):324
Pua FL et al (2013) Solvolytic liquefaction of oil palm empty fruit bunch (EFB) fibres: analysis of product fractions using FTIR and pyrolysis-GCMS. Sains Malays 42(6):79
Chin K et al (2013) Optimization of torrefaction conditions for high energy density solid biofuel from oil palm biomass and fast growing species available in Malaysia. Ind Crop Prod 49:768–774
Sreekala M, Kumaran M, Thomas S (1997) Oil palm fibers: morphology, chemical composition, surface modification, and mechanical properties. J Appl Polym Sci 66(5):821–835
Abnisa F et al (2013) Utilization of oil palm tree residues to produce bio-oil and bio-char via pyrolysis. Energy Convers Manag 76:1073–1082
Yuliansyah AT et al (2010) Production of solid biofuel from agricultural wastes of the palm oil industry by hydrothermal treatment. Waste Biomass Valorization 1(4):395–405
Geng A (2014) Upgrading of oil palm biomass to value-added products, in Biomass and Bioenergy. Springer, pp 187–209
Varman M, Saka S (2011) A comparative study of oil palm and Japanese beech on their fractionation and characterization as treated by supercritical water. Waste Biomass Valorization 2(3):309–315
Ang S et al (2013) Production of cellulases and xylanase by Aspergillus fumigatus SK1 using untreated oil palm trunk through solid state fermentation. Process Biochem 48(9):1293–1302
Fan S-P et al (2011) Comparative studies of products obtained from solvolysis liquefaction of oil palm empty fruit bunch fibres using different solvents. Bioresour Technol 102(3):3521–3526
Hossain MA et al (2016) Microwave pyrolysis of oil palm fiber (OPF) for hydrogen production: parametric investigation. Energy Convers Manag 115:232–243
Ngo T-A, Kim J, Kim S-S (2014) Characteristics of palm bark pyrolysis experiment oriented by central composite rotatable design. Energy 66:7–12
Lathouwers D, Bellan J (2001) Yield optimization and scaling of fluidized beds for tar production from biomass. Energy Fuel 15(5):1247–1262
Cho J, Davis JM, Huber GW (2010) The intrinsic kinetics and heats of reactions for cellulose pyrolysis and char formation. ChemSusChem 3(10):1162–1165
Lin Y-C et al (2009) Kinetics and mechanism of cellulose pyrolysis. J Phys Chem C 113(46):20097–20107
Mohan D, Pittman CU, Steele PH (2006) Pyrolysis of wood/biomass for bio-oil: a critical review. Energy Fuel 20(3):848–889
Khan A et al (2009) Biomass combustion in fluidized bed boilers: potential problems and remedies. Fuel Process Technol 90(1):21–50
Lewandowski I, Heinz A (2003) Delayed harvest of miscanthus—influences on biomass quantity and quality and environmental impacts of energy production. Eur J Agron 19(1):45–63
Pordesimo L et al (2005) Variation in corn Stover composition and energy content with crop maturity. Biomass Bioenergy 28(4):366–374
Christian DG, Yates NE, Riche AB (2006) The effect of harvest date on the yield and mineral content of Phalaris arundinacea L.(reed canary grass) genotypes screened for their potential as energy crops in southern England. J Sci Food Agric 86(8):1181–1188
Omar R et al (2011) Characterization of empty fruit bunch for microwave-assisted pyrolysis. Fuel 90(4):1536–1544
Guldogan Y, Bozdemir TO, Durusoy T (2000) Effect of heating rate on pyrolysis kinetics of Tuncbilek lignite. Energy Sources 22(4):305–312
Oasmaa A, Czernik S (1999) Fuel oil quality of biomass pyrolysis oils state of the art for the end users. Energy Fuel 13(4):914–921
Venderbosch R, Prins W (2010) Fast pyrolysis technology development. Biofuels Bioprod Biorefin 4(2):178–208
Sevilla M, Maciá-Agulló JA, Fuertes AB (2011) Hydrothermal carbonization of biomass as a route for the sequestration of CO 2: chemical and structural properties of the carbonized products. Biomass Bioenergy 35(7):3152–3159
Nizamuddin S et al (2015) Hydrothermal carbonization of oil palm shell. Korean J Chem Eng:1–9
Jacobson K, Maheria KC, Kumar Dalai A (2013) Bio-oil valorization: a review. Renew Sust Energ Rev 23:91–106
Telmo C, Lousada J (2011) Heating values of wood pellets from different species. Biomass Bioenergy 35(7):2634–2639
Garcia-Perez M et al (2007) Vacuum pyrolysis of softwood and hardwood biomass: comparison between product yields and bio-oil properties. J Anal Appl Pyrolysis 78(1):104–116
Loh SK (2017) The potential of the Malaysian oil palm biomass as a renewable energy source. Energy Convers Manag 141:285–298
Pramanik K (2003) Properties and use of Jatropha curcas oil and diesel fuel blends in compression ignition engine. Renew Energy 28(2):239–248
Akhtar J, Saidina Amin N (2012) A review on operating parameters for optimum liquid oil yield in biomass pyrolysis. Renew Sust Energ Rev 16(7):5101–5109
Basagiannis A, Verykios X (2006) Reforming reactions of acetic acid on nickel catalysts over a wide temperature range. Appl Catal A Gen 308:182–193
Horne PA, Williams PT (1996) Influence of temperature on the products from the flash pyrolysis of biomass. Fuel 75(9):1051–1059
Stehlik P (2012) Up-to-date technologies in waste to energy field. Rev Chem Eng 28(4–6):223–242
Frassoldati A et al (2006) Detailed kinetic modeling of thermal degradation of biomasses. In: Proceeding of the 29th meeting on combustion
Goyal H, Seal D, Saxena R (2008) Bio-fuels from thermochemical conversion of renewable resources: a review. Renew Sust Energ Rev 12(2):504–517
Harris K et al (2013) Characterization and mineralization rates of low temperature peanut hull and pine chip biochars. Agronomy 3(2):294–312
Bridgwater A, Czernik S, Piskorz J (2001) An overview of fast pyrolysis. In: Progress in thermochemical biomass conversion, pp 977–997
Demirbas A (2000) Recent advances in biomass conversion technologies. Energy Educ Sci Technol 6:19–41
Guillain M et al (2009) Attrition-free pyrolysis to produce bio-oil and char. Bioresour Technol 100(23):6069–6075
Jahirul MI et al (2012) Biofuels production through biomass pyrolysis—a technological review. Energies 5(12):4952–5001
Demirbas A, Arin G (2002) An overview of biomass pyrolysis. Energy Sources 24(5):471–482
Tsai W, Lee M, Chang Y (2006) Fast pyrolysis of rice straw, sugarcane bagasse and coconut shell in an induction-heating reactor. J Anal Appl Pyrolysis 76(1–2):230–237
Kebelmann K et al (2013) Intermediate pyrolysis and product identification by TGA and Py-GC/MS of green microalgae and their extracted protein and lipid components. Biomass Bioenergy 49:38–48
Yang Y et al (2014) Intermediate pyrolysis of biomass energy pellets for producing sustainable liquid, gaseous and solid fuels. Bioresour Technol 169:794–799
Roy C, Labrecque B, de Caumia B (1990) Recycling of scrap tires to oil and carbon black by vacuum pyrolysis. Resour Conserv Recycl 4(3):203–213
Pakdel H, Pantea DM, Roy C (2001) Production of dl-limonene by vacuum pyrolysis of used tires. J Anal Appl Pyrolysis 57(1):91–107
Sınaǧ A, Kruse A, Schwarzkopf V (2003) Key compounds of the hydropyrolysis of glucose in supercritical water in the presence of K2CO3. Ind Eng Chem Res 42(15):3516–3521
Tripathi M, Sahu JN, Ganesan P (2016) Effect of process parameters on production of biochar from biomass waste through pyrolysis: a review. Renew Sust Energ Rev 55:467–481
Silitonga A et al (2011) A review on prospect of Jatropha curcas for biodiesel in Indonesia. Renew Sust Energ Rev 15(8):3733–3756
Enweremadu C, Mbarawa M (2009) Technical aspects of production and analysis of biodiesel from used cooking oil—a review. Renew Sust Energ Rev 13(9):2205–2224
Santos RB et al (2013) Wood based lignin reactions important to the biorefinery and pulp and paper industries. BioResources 8(1):1456–1477
Demirbas A (2004) Effects of temperature and particle size on bio-char yield from pyrolysis of agricultural residues. J Anal Appl Pyrolysis 72(2):243–248
Janse A, Westerhout R, Prins W (2000) Modelling of flash pyrolysis of a single wood particle. Chem Eng Process Process Intensif 39(3):239–252
Demirbas A (2004) Effect of initial moisture content on the yields of oily products from pyrolysis of biomass. J Anal Appl Pyrolysis 71(2):803–815
Beneroso D et al (2016) Dielectric characterization of biodegradable wastes during pyrolysis. Fuel 172:146–152
Encinar J et al (1996) Pyrolysis of two agricultural residues: olive and grape bagasse. Influence of particle size and temperature. Biomass Bioenergy 11(5):397–409
Park HJ, Park Y-K, Kim JS (2008) Influence of reaction conditions and the char separation system on the production of bio-oil from radiata pine sawdust by fast pyrolysis. Fuel Process Technol 89(8):797–802
Mani T et al (2010) Pyrolysis of wheat straw in a thermogravimetric analyzer: effect of particle size and heating rate on devolatilization and estimation of global kinetics. Chem Eng Res Des 88(8):952–958
Di Blasi C, Branca C (2003) Temperatures of wood particles in a hot sand bed fluidized by nitrogen. Energy Fuel 17(1):247–254
Grønli M, Antal MJ, Várhegyi G (1999) A round-robin study of cellulose pyrolysis kinetics by thermogravimetry. Ind Eng Chem Res 38(6):2238–2244
Şensöz S, Kaynar İ (2006) Bio-oil production from soybean (Glycine max L.); fuel properties of bio-oil. Ind Crop Prod 23(1):99–105
Pütün A, Özcan A, Pütün E (1999) Pyrolysis of hazelnut shells in a fixed-bed tubular reactor: yields and structural analysis of bio-oil. J Anal Appl Pyrolysis 52(1):33–49
Antal MJ Jr et al (1990) Review of methods for improving the yield of charcoal from biomass. Energy Fuel 4(3):221–225
Antal MJ et al (2000) Attainment of the theoretical yield of carbon from biomass. Ind Eng Chem Res 39(11):4024–4031
Richard J-R, Antal MJ Jr (1993) Thermogravimetric studies of charcoal formation from cellulose at elevated pressures. In: Advances in thermochemical biomass conversion. Springer, pp 784–792
Wang L et al (2011) Is elevated pressure required to achieve a high fixed-carbon yield of charcoal from biomass? Part 1: round-robin results for three different corncob materials. Energy Fuel 25(7):3251–3265
Zhang H et al (2009) Comparison of non-catalytic and catalytic fast pyrolysis of corncob in a fluidized bed reactor. Bioresour Technol 100(3):1428–1434
Heidari A et al (2014) Effect of process conditions on product yield and composition of fast pyrolysis of Eucalyptus grandis in fluidized bed reactor. J Ind Eng Chem 20(4):2594–2602
Anca-Couce A (2016) Reaction mechanisms and multi-scale modelling of lignocellulosic biomass pyrolysis. Prog Energy Combust Sci 53:41–79
Behrendt F et al (2008) Direct liquefaction of biomass. Chem Eng Technol 31(5):667–677
Mäkelä M, Benavente V, Fullana A (2015) Hydrothermal carbonization of lignocellulosic biomass: effect of process conditions on hydrochar properties. Appl Energy 155:576–584
Kruse A, Funke A, Titirici M-M (2013) Hydrothermal conversion of biomass to fuels and energetic materials. Curr Opin Chem Biol 17(3):515–521
Tekin K, Karagöz S, Bektaş S (2014) A review of hydrothermal biomass processing. Renew Sust Energ Rev 40:673–687
Bardhan SK et al (2015) Biorenewable chemicals: feedstocks, technologies and the conflict with food production. Renew Sust Energ Rev 51:506–520
Awalludin MF et al (2015) An overview of the oil palm industry in Malaysia and its waste utilization through thermochemical conversion, specifically via liquefaction. Renew Sust Energ Rev 50:1469–1484
Jena U, Das K (2011) Comparative evaluation of thermochemical liquefaction and pyrolysis for bio-oil production from microalgae. Energy Fuel 25(11):5472–5482
Gollakota A, Kishore N, Gu S (2017) A review on hydrothermal liquefaction of biomass. Renew Sust Energ Rev
Nizamuddin S et al (2016) A critical analysis on palm kernel shell from oil palm industry as a feedstock for solid char production. Rev Chem Eng 32(5):489–505
Kim SW et al (2013) Bio-oil from the pyrolysis of palm and Jatropha wastes in a fluidized bed. Fuel Process Technol 108:118–124
Kim S-J, Jung S-H, Kim J-S (2010) Fast pyrolysis of palm kernel shells: influence of operation parameters on the bio-oil yield and the yield of phenol and phenolic compounds. Bioresour Technol 101(23):9294–9300
Islam MN, Zailani R, Ani FN (1999) Pyrolytic oil from fluidised bed pyrolysis of oil palm shell and itscharacterisation. Renew Energy 17(1):73–84
Omoriyekomwan JE, Tahmasebi A, Yu J (2016) Production of phenol-rich bio-oil during catalytic fixed-bed and microwave pyrolysis of palm kernel shell. Bioresour Technol 207:188–196
Abdullah N, Sulaiman F, Gerhauser H (2011) Characterisation of oil palm empty fruit bunches for fuel application. J Phys Sci 22(1):1–24
Abdullah N, Gerhauser H, Sulaiman F (2010) Fast pyrolysis of empty fruit bunches. Fuel 89(8):2166–2169
Sukiran MA, Chin CM, Bakar NK (2009) Bio-oils from pyrolysis of oil palm empty fruit bunches. Am J Appl Sci 6(5):869–875
Pimenidou P, Dupont V (2012) Characterisation of palm empty fruit bunch (PEFB) and pinewood bio-oils and kinetics of their thermal degradation. Bioresour Technol 109:198–205
Khor K, Lim K, Zainal Z (2009) Characterization of bio-oil: a by-product from slow pyrolysis of oil palm empty fruit bunches. Am J Appl Sci 6(9):1647–1652
Misson M et al (2009) Pretreatment of empty palm fruit bunch for production of chemicals via catalytic pyrolysis. Bioresour Technol 100(11):2867–2873
Fukuda S (2015) Pyrolysis investigation for bio-oil production from various biomass feedstocks in Thailand. Int J Green Energy 12(3):215–224
Lua AC, Guo J (1998) Preparation and characterization of chars from oil palm waste. Carbon 36(11):1663–1670
Chiaramonti D, Oasmaa A, Solantausta Y (2007) Power generation using fast pyrolysis liquids from biomass. Renew Sust Energ Rev 11(6):1056–1086
Mahfud F et al (2007) Biomass to fuels: upgrading of flash pyrolysis oil by reactive distillation using a high boiling alcohol and acid catalysts. Process Saf Environ Prot 85(5):466–472
Yaman S (2004) Pyrolysis of biomass to produce fuels and chemical feedstocks. Energy Convers Manag 45(5):651–671
Kim SW, Koo BS, Lee DH (2014) Catalytic pyrolysis of palm kernel shell waste in a fluidized bed. Bioresour Technol 167:425–432
Ahmad MI, Zhang N, Jobson M (2011) Integrated design of diesel hydrotreating processes. Chem Eng Res Des 89(7):1025–1036
Ostgard D et al (2007) The chemoselective hydrogenation of tallow nitriles to unsaturated 1° fatty amines with carbon modified Ni catalysts. Catal Today 121(1–2):106–114
Mekki-Berrada A et al (2013) Fatty acid methyl esters into nitriles: acid–base properties for enhanced catalysts. J Catal 306:30–37
Bridgwater A (1994) Catalysis in thermal biomass conversion. Appl Catal A Gen 116(1–2):5–47
Williams PT, Nugranad N (2000) Comparison of products from the pyrolysis and catalytic pyrolysis of rice husks. Energy 25(6):493–513
Isahak WNRW et al (2012) A review on bio-oil production from biomass by using pyrolysis method. Renew Sust Energ Rev 16(8):5910–5923
Zhang L et al (2013) Upgrading of bio-oil from biomass fast pyrolysis in China: a review. Renew Sust Energ Rev 24:66–72
Capunitan JA, Capareda SC (2013) Characterization and separation of corn stover bio-oil by fractional distillation. Fuel 112:60–73
Chiew YL, Shimada S (2013) Current state and environmental impact assessment for utilizing oil palm empty fruit bunches for fuel, fiber and fertilizer–a case study of Malaysia. Biomass Bioenergy 51:109–124
Ng WPQ et al (2012) Waste-to-wealth: green potential from palm biomass in Malaysia. J Clean Prod 34:57–65
Xiu S, Shahbazi A (2012) Bio-oil production and upgrading research: a review. Renew Sust Energ Rev 16(7):4406–4414
Mortensen PM et al (2011) A review of catalytic upgrading of bio-oil to engine fuels. Appl Catal A Gen 407(1–2):1–19
Feili H et al (2016) Prioritization of renewable energy systems using AHP method with economic analysis perspective in Iran. In: 2nd international conference on modern Researchs in management, economics and accounting, Kualalampur
Lim M (2010) A case study on palm empty fruit bunch as energy feedstock. SEGi Rev 3(2):3–15
Yuosoff S, Kardooni R (2012) Barriers and challenges for developing RE policy in Malaysia. In: International conference on future environment and energy IPCBEE
Loh SK, Choo YM (2013) Prospect, challenges and opportunities on biofuels in Malaysia. In: Advances in biofuels. Springer, pp 3–14
Kong S-H et al (2014) Biochar from oil palm biomass: a review of its potential and challenges. Renew Sust Energ Rev 39:729–739
Mohammed M et al (2011) Hydrogen rich gas from oil palm biomass as a potential source of renewable energy in Malaysia. Renew Sust Energ Rev 15(2):1258–1270
Lu Q, Li W-Z, Zhu X-F (2009) Overview of fuel properties of biomass fast pyrolysis oils. Energy Convers Manag 50(5):1376–1383
Thomsen T et al (2011) The potential of pyrolysis technology in climate change mitigation–influence of process design and–parameters, simulated in SuperPro designer software
Sulaiman F et al (2011) An outlook of Malaysian energy, oil palm industry and its utilization of wastes as useful resources. Biomass Bioenergy 35(9):3775–3786
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Qureshi, S.S., Nizamuddin, S., Baloch, H.A. et al. An overview of OPS from oil palm industry as feedstock for bio-oil production. Biomass Conv. Bioref. 9, 827–841 (2019). https://doi.org/10.1007/s13399-019-00381-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-019-00381-w