Abstract
This paper is a study on effects of separation procedures on yield and characteristics of biocrude derived from hydrothermal liquefaction (HTL) of Tetraselmis sp. microalgae. The algae was grown and cultivated in outdoor open raceway ponds. The HTL experiments were performed using 1 l custom built high pressure–temperature reactor with inbuilt magnetic stirrer. HTL experimental studies were conducted at reaction temperature of 350 °C and 15 min holding time using alga solids loading of 16 w/v%. HTL product mixture diluted with dichloromethane (ratio 1:1) was allowed to stand for 1 h, 3 h, 6 h, 7 h, 8 h, 9 h, 10 h, 11 h, 12 h and 15 h at room temperature. The result showed that varying stand times for product mixture separation influenced yields in biocrude, solid residue and dissolved aqueous solids. Biocrude yields were in the range of 30 wt% to 56 wt% characterised with higher heating value of ~ 35 to 37 MJ/kg and hydrogen to carbon atomic ratios of 1.56 to 1.95. Maximum yield of biocrude was obtained after 9 h stand time for product mixture and dichloromethane (PM–DCM) mixture. Although, varying PM–DCM mixture stand times showed variation in product yields, there was no clear trend in distribution of elemental contents. Majority of alkali metals distributed in aqueous phase and solid residue, which could be used as nutrients, an alternative to conventional fertiliser.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Biomass availability and accessibility across the World is considered as one of the main feedstocks for producing renewable energy and value-added chemicals [1, 2]. Due to microalgae faster growth rate, higher oil content, higher biomass productivity and its renewability, microalgae have been considered as promising feedstocks for biofuel production when compared to terrestrial biomass [3]. Hydrothermal liquefaction (HTL), also referred to as direct conversion, is one of the promising technologies for conversion of biomass to biofuels.
HTL decomposes large organic molecular-weight compounds to small molecules [4]. Specifically, HTL is performed at moderate temperature (200 to 370 °C) and high pressure (2 MPa to 25 MPa) with or without catalyst [5] using 5 to 20 wt% algae biomass solids loading. HTL main product is an organic liquid referred to as biocrude having an energy content in the range of 30 to 40 MJ/kg. Biocrude is produced through different reaction pathways such as depolymerisation, decomposition and reformation [6, 7]. The resultant biocrude is characterised with undesired high heteroatoms such as oxygen and nitrogen; hence, upgrading is necessary in order to meet standard fuel specifications [8]. Solid residue, aqueous and gas phases are other products derived from HTL.
A review of scientific literatures showed that several research studies have been investigated on HTL of algae, ranging from effects of reaction conditions, kinetic modelling, solvent types, algal strains, upgrading and review papers [7, 9,10,11,12,13,14,15,16]. These studies mainly aimed at optimising yield and properties of biocrude. These studies have demonstrated the feasibility of future commercialization of HTL algal biorefinery. Despite these numerous investigations, the separation methods for effective recovery of HTL products are still unclear [17]. Recovering of biocrude from HTL–product mixture (HTL–PM) could be categorised into two separation methods [17, 18]. One method is to allow the PM to separate spontaneously into biocrude, residue and aqueous phases as a result of their immiscibility and differences in density [10, 18, 19]. The second method is the addition of an organic solvent to the cooled PM, which dissolves the biocrude and enhances its separation from solid and aqueous phases [12, 14, 20]. Higher molecular weight organic compounds are extracted in biocrude using latter method [9], leading to an increase in total biocrude yields [10, 16, 19]. Hence, almost all HTL experimental studies in the literature applied this approach to recover biocrude from HTL–PM [2, 11,12,13]. Moreover, dichloromethane (DCM) is the most frequently used organic solvent [4, 14, 17, 18, 21], which could be due to its moderate dielectric constants and capability to dissolve both polar and non-polar chemical compounds [22]. Vlaskin et al. [9] and Yang et al. [23] reported that DCM led to highest yields in biocrude and of better quality compared to other solvents such as acetone, hexane, chloroform and tetrahydrofuran.
Typically, after the HTL reaction step, a product mixture is produced, where an organic solvent (such as dichloromethane (DCM)) is mixed with the product mixture. Thereafter, the mixture of product mixture and DCM (now referred to as PM–DCM) is allowed to stand for phase separation. However, almost all HTL studies have not reported on the allowed stand time for PM–DCM mixture. Although, Bi et al. [5] reported 4 h PM–DCM stand time when investigating catalytic effects on biocrude yields from treatment of partially defatted Cryptococcus curvatus at 300 °C and 350 °C. Eboibi et al. [11] reported 8 h PM–DCM stand time for thermal upgrading of biocrude produced from HTL of Spirulina sp. and Tetraselmis sp. These studies reported influence of catalysts and upgrading on biocrude yields, but no report on effects of PM–DCM stand time on biocrude yield and properties.
The reason for differences in allowed stand time for PM–DCM mixture in previous reports is not clear; hence, further investigations may be required. It is believed there is transfer of molecules between layers towards formation of biocrude phase, residue and aqueous phases. Therefore, the allowed stand time for PM–DCM mixture to equilibrate prior to separation of layers may influence yields and properties of products, particularly biocrude. To the best of my knowledge, there is no published report on this aspect of study. If biocrude yield and properties are affected by product mixture and solvent stand time, then reported data in literature may be a concern. To a very large extent, biocrude yields are important data used to evaluate performance of HTL algal biorefinery [23, 24]. Thus, it is essential to examine impact of stand time of PM–DCM on biocrude recovery from HTL product mixture. This could provide more information and potentially discover hidden effects for more economical and efficient separation methods for HTL algal biorefinery.
Moreover, after liquefaction reaction, Boens et al. [12] added distilled water and DCM into the reactor containing product mixture and then stirred for 1 h 30 min. The stirred solution was transferred to a separating funnel for phase separation. This procedure enhanced biocrude yield; however, allowed stand time was not reported. Biller and Ross [13] added 50 ml each of DCM and water to the product mixture, of which two layers were obtained. Although yields in biocrude, solid residue and aqueous phase were reported, there was no data on stand time. Also, centrifugation technique has been used in previous HTL product and separation procedures to extract biocrude from solid and aqueous phase mixture [4, 14]. However, such practices may not be applicable at large scale. Even after using a centrifuge, time is normally given for contents in a vial to equilibrate before using pipette to withdraw product fractions. Hence, knowing a suitable time may be necessary during separation of products from PM–DCM mixture.
Sheng et al. [14] and Wang et al. [25] after mixing DCM with product mixture immediately filtered (using microporous membranes 0.45 μm) the PM–DCM mixture to remove solids. Then, the filtrate was transferred to a separating funnel where it was allowed to stand for certain period; however, time allowed was not reported. In related studies, Huang et al. [26], Jindal and Jha [27] and Yang et al. [28] initially filtered solid residue from product mixture before mixing DCM to the filtrate (containing water and organic phase). The filtered solid residue has been reported to contain residual organics [16, 29]; hence, such procedure may reduce total amount of biocrude and increase in yield of solid residue.
Based on reviewed scientific literature, it could be agreed that different separation procedures have been used to obtain yields after liquefaction. Also, extraction of biocrude from product mixture has been carried out arbitrary, in terms of product mixture and solvent stand time. Moreover, mixing solvent with product mixture and allowed to stand for certain period enhances dissolution, thereby improving recovery of hydrocarbons and reduction in solid residue and aqueous phase by-products. Hence, knowledge of PM–DCM stand time is necessary as it would help to formulate specific recommends for HTL separation procedure. Also, it would help for recovery of optimum yield in biocrude of better quality during downstream processing of HTL products. Therefore, the main aim of this reported study was to investigate the effects of stand time for the mixture of PM–DCM on yields and properties of biocrude after hydrothermal liquefaction.
2 Materials and methods
2.1 Materials
Tetraselmis sp. microalga biomass was used in the present study. The alga was grown and cultivated in outdoor open raceway ponds at Pilot Plant, Biotechnology Division, Aban Infrastructure Pvt. Limited, Chennai, India. Details of the alga biomass culturing, harvesting and characterisation have been reported elsewhere [30]. Harvested biomass slurry having ~ 16 w/v% alga solids was stored (− 8 °C) in refrigerator prior to liquefaction experiments. Dichloromethane (DCM) of 99% purity was used as the organic solvent.
2.2 Hydrothermal liquefaction: product mixture production
Production of product mixture was achieved by HTL of the harvested alga using a custom built Inconel batch reactor of 1 l capacity. The reactor has a designed capacity of 500 °C and 350 bar as operating temperature and pressure, respectively, with an inbuilt magnetic stirrer. HTL experiment was conducted at reaction temperature of 350 °C at 15 min holding time. Typically, in each run, 500 g alga slurry was loaded in the reactor. Then, the reactor was sealed and heated to 350 °C (± 4 °C) using an inbuilt electrical heating jacket. Holding time started when reaction temperature reached 350 °C and then maintained for 15 min. After complete holding time, the reactor was switched off and cooled to room temperature.
2.3 Product separation and quantification procedure
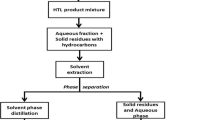
The gas phase was released via the gas valve after cooling to room temperature. Then, the reactor content now referred to as product mixture was transferred to a beaker. About 200 ml (50 ml of water and 150 ml DCM) was used to rinse products stuck to the reactor wall and internal parts (magnetic stirrer) and then transferred to the beaker containing the rest product mixture. The water helps to wash/remove nonoil solution while DCM for oily substance. Thereafter, DCM was mixed with the product mixture in ratio 1:1 and then stirred at 300 rpm for 5 min. The mixed product mixture and DCM is defined as PM–DCM. Then, equal volumes (100 ml) of PM–DCM were transferred to separating funnels SF1, SF3, SF6, SF7, SF8, SF9, SF10, SF11, SF12 and SF15 and sealed with rubber cork. Separating funnel SF1, SF3, SF6, SF7, SF8, SF9, SF10, SF11, SF12 and SF15 were allowed to stand for 1 h, 3 h, 6 h, 7 h, 8 h, 9 h, 10 h, 11 h, 12 h and 15 h, respectively, for phase separation.
After each predefined stand time, the PM–DCM was separated into three layers, a bottom layer (residue), middle layer (biocrude phase) and a top layer (aqueous phase or also known as wastewater). Then, each layer was decanted into separate beaker and labelled accordingly. The residue and aqueous phases were rinsed with DCM three times, in order to enhance extraction of hydrocarbons stuck to pores. The washed fractions were allowed to stand for 3 h after decanting and added to respective beakers containing biocrude, residue and aqueous phases. The biocrude phase was vacuum evaporated at ~ 40 °C in order to remove DCM and residual water, and the remnant was defined as biocrude [2, 17]. The residue was dried at ~ 100 °C, the dried fraction referred to as solid residue [11, 29]. Similarly, the aqueous phase was dried at ~ 100 °C, and dried fraction was defined as dissolved aqueous solids [16, 24]. The experimental runs and separation procedures were conducted in triplicate and average yields reported. A schematic diagram of production and separation procedure is shown in Fig. 1.
HTL production and separation procedures with respect to stand time. SF1 − n: separating funnels at time intervals. DCM: dichloromethane. B1 − n: biocrude obtained from separating funnels with respect to time. SR1 − n: solid residue obtained from separating funnels with respect to time. DAS1 − n: dissolved aqueous solids obtained from separating funnels with respect to time
2.4 Analysis
The gravimetric yields in biocrude (ash free dry weight), solid residue and aqueous phase were determined by relating the mass of product to mass of algal slurry loaded into the reactor, whereas yields in gas phase + loss were determined by difference using the calculated yield of remaining fractions. Samples of biocrude, solid residue and dissolved aqueous solids were analysed with regard to elemental content of carbon (C), hydrogen (H), nitrogen (N) and sulphur (S) using an elemental analyser (VariolEL III elemental analyser system GmbH) in accordance with ASTM D5291. The elemental content of oxygen (O) was determined by difference. Based on CHNSO data, the higher heating value was estimated using a unified correlation (Eq. (1)) proposed by Chinnawala and Parikh [31]. Equation (1) has been used previously to estimate HHV of biocrudes from HTL of algae [2, 11, 16, 24].
where C, H, S, O, N and A represent elemental carbon, hydrogen, sulphur, oxygen, nitrogen and ash. The amount of energy recovered (ER) in biocrudes from SF1 − n was estimated using Eq. (2) [29, 32].
In Eq. (2), 19.2 MJ/kg was used as the HHV of algal slurry [11, 24]. The H/C, N/C and O/C atomic ratios were estimated according to the methods explained in previous report [14].
To know the compositional changes in resultant biocrudes, sample of biocrudes (diluted with acetone to 2.5 v/v%) obtained from SF1 − n were analysed for chemical compounds using Gas Chromatography-Mass Spectroscopy (GC-MS) (Agilent HP-5 column of 50m x 200μm x 0.33μm) according to method reported previously [24]. In addition, the ash fractions of biocrudes derived from SF1 − n were analysed for their metallic composition using Inductively Couple Plasma Mass Spectroscopy (ICP-MS) (Agilent 7500 Series). Ash fractions were determined in accordance with the method explained in previous report [11].
3 Results and discussion
3.1 HTL product yields
The obtained mass yields in biocrude, solid residue, dissolved aqueous solids and gas + loss are presented in Fig. 2. The data obtained showed substantial variation in fractional yields, suggesting stand time could have influenced PM–DCM mixture during phase separation. The yields in biocrude yield increased from 30 to 56 wt% when stand time increased from 1 to 9 h. However, biocrude yields decreased from 56 to 42 wt% with further increased in stand time. Although there could be several reasons behind variation in yields, one of the reasons could be that mixture having different molecular weights and densities needs suitable time to equilibrate. Suitable time is important to allow molecules of similar density in a mixture to equilibrate.
The solid residue decreased from 20 to 14 wt% when stand time increased from 1 to 9 h. Although there were no substantial differences in solid residue with further increase in time, the numerical differences suggest side reactions could have occurred. Such side reactions seem to have favoured formation of solid residue. Decomposition of cyclic oxygenates leads to formation of residue. In addition, Mailallard and dehydration reactions favour intermolecular restructuring [33]. Also, it leads to an increase residue formation and reduction in biocrude yield. This finding suggests decomposition and repolymerizations occur if PM–DCM mixture is maintained for a certain period. Moreover, the dissolved aqueous solids decreased from 16 to 12 wt%, whereas the gas + loss was between 18 and 36 wt%.
3.2 Elemental composition
The elemental composition (CHNSO) of biocrudes obtained from SF1 − n is illustrated in Fig. 3a–e. As shown in Fig. 3, there were little variations in the elemental contents. The carbon contents were in the range of 72 to 75 w/w% (shown in Fig. 3a). As illustrated in Fig. 3b, hydrogen content varies between 9.80 and 10.2 w/w%, whereas nitrogen was in range of 2 to 2.4 w/w% (Fig. 3c). Elemental sulphur distribution is presented in Fig. 3c. As shown in Fig. 3d, the sulphur content was between 0.6 and 0.7 w/w%, and oxygen was 12 to 15.6 w/w% (Fig. 3d). The data presented in Fig. 3 showed no clear trend in variation of elemental distributions, though there were some changes in compositions. In addition, impact of stand time on PM–DCM mixture for phase separation could not produce biocrude of better quality for direct use. Thus, hydrotreatment is necessary, in order to improve biocrude fuel properties. Moreover, despite variation in stand time, similar trends in elemental carbon and distribution in biocrudes were found when compared with previous reports [16, 20, 24, 25, 29]. Generally, carbon content has been reported to be in the range of 67 to 79 w/w%, 6.5 to 10.5 w/w% for hydrogen, 3.5 to 6.5 w/w% for nitrogen, < 0.9 w/w% for sulphur and 10 to 16 w/w% for oxygen. Although the nitrogen content in present study is lower than 3.5 to 6 w/w%, it is still relatively high due to its environmental implications and potentially poisonous to catalyst used in refining process [13, 34].
a Carbon content of biocrude with respect to PM–DCM stand time. b Hydrogen content of biocrude with respect to PM–DCM stand time. c Nitrogen content of biocrude with respect to PM–DCM stand time. d Sulphur content of biocrude with respect to PM–DCM stand time. e Oxygen content of biocrude with respect to PM–DCM stand time
3.3 Energy analysis
The higher heating value and energy recovered in biocrude are presented in Fig. 4. Due to little variations in CHNSO contents, there were some changes in HHVs and ER in biocrudes. Biocrude HHVs were between ~ 35 and ~ 37 MJ/kg, whereas the energy recovered was in the range of 54.9% and 88%. In this study, the ER was found to be within range of previous reports investigating hydrothermal liquefaction of microalgae. For example, Shakya et al. [20] reported an ER of 83% from HTL of Nannochloropsis sp., 78% from Scenedesmus sp. and 47% from Chlorella sp. at reaction temperature of 220 to 320 °C at 30 min reaction time. An ER of 43 to 61% from liquefaction of Nannochloropsis sp. at 300 °C for 30 min was reported by Wang et al. [35], whereas Eboibi et al. [11] reported up to 87% ER. This suggests that similar amount of ER could still be recovered from PM–DCM considering stand time.
This study has shown that PM–DCM stand time may influence product yield and properties. Based on the data presented in Fig. 4, in addition to that in Fig. 2, the optimum PM–DCM stand time was found to be after 9 h. The hydrogen-to-carbon ratio of biocrudes was between 1.63 and 1.65, which was found to be similar to 1.56 to 1.95 from HTL of Galdieria sulphuraria and Nannochloropsis salina [34] and 1.4 to 2.0 for petroleum [36]. Similarly, the nitrogen-to-carbon ratio of 0.2 to 0.5 was found to be within the range of 0.05 of petroleum. The oxygen-to-carbon ratio of 0.13 to 0.16 was substantially lower than 0.67 of algae, however higher than 0.05 of conventional petroleum. Therefore, upgrading biocrude is necessary, in order to improve its fuel properties, as mentioned previously.
3.4 Biocrude chemical composition: GC-MS analysis
GC-MS analysis shows that biocrude produced from HTL of algae is a complicated mixture consisting numerous compounds. The chemical compounds identified with GC-MS having relative abundance area greater that 1% are presented in Table 1. It should be noted that only light compounds unlike high boiling point compounds such as asphaltene that can vaporise through the GC column could only be identified with the GC-MS [17].
Based on the data presented in Table 1, there were variations in composition relative to time, suggesting possibility of reaction during extraction of biocrude from the product mixture. For example, heptadecene, 17-chloro, 9-hexadecenoic acid, methyl ester, oxiraneoctanoic acid and B8-octadecenal were not detected at 1 h stand time but were detected with incraese in stand time. This finding suggests that PM–DCM mixture continuously undergoes side reactions during recovery of biocrude from solid residue and aqueous phases.
3.5 Metal composition in biocrude
One of the important factors in HTL algal biorefinery is the composition of metals in biocrude produced. Low metallic composition in biocrude is desirable in order to improve combustion and avoiding refining issues [29, 37]. Therefore, the fate of metals during liquefaction of alga, particularly marine algae with high alkali metals, is a concern. The metals calcium (Ca), potassium (K), magnessium (Mg), sodium (Na), nickel (Ni) and zinc (Zn) were assessed, and the metal balance is presented in Fig. 5.
Most of the metals were distributed to either the solid residue and dissolved aquoeus solids. Approximately 50 wt% Ca, 65 wt% Mg and 55 wt% Ni were recovered in the solid residue. The aqueous fraction was high in Na (72 wt%), K (75 wt%) and Zn (60 wt%), which are essential nutrients for algae cultivation that can be recycled to algae pond to reduce cost of cultivation and energy production [38]. The resultant biocrude was found to contain 7 wt% Ca, 5 wt% K, 12 wt% Mg, 5 wt% Na, 8 wt% Ni and 4 wt% Zn. About 11 to 18 wt% of these metals fractionated in the gas phase. The low composition of these metals in biocrude suggests that biocrude could be refined avoiding issues associated with fouling and slagging [34, 37].
4 Conclusion
In this reported study, the effects of stand time for product mixture and solvent on yields and properties from hydrothermal liquefaction of Tetraselmis sp. algal were investigated. The first demonstration of stand time effects on PM–DCM mixture to form layers has been shown to have effects on yields of biocrude, solid residue and aqueous phase. PM–DCM stand time seems to have no substantial effects on quality of biocrude. This finding suggests HTL product mixture is unstable; hence, more attention is needful to develop effective downstream separation methods for recovery of HTL product fractions.
References
Zhang Y, Xiong Q, Chen Y, Pei ML, Jin P, Yan Y, Pan J (2018) Synthesis of ceria and sulfated zirconia catalysts supported on mesoporous SBA-15 toward glucose conversion to HMF in a green isopropanol-medicated system. Ind Eng Chem Res 57:1968–1979. https://doi.org/10.1021/acs.iecr.7b04671
Eboibi BE, Lewis DM, Ashman PJ, Chinnasamy S (2015) Integrating anaerobic digestion and hydrothermal liquefaction for renewable energy production: an experimental investigation. Environ Prog Sustain Energy 34:1662–1673
Hu H-S, Wu Y-L, Yang M-D (2018) Fractionation of bio-oil produced from hydrothermal liquefaction of microalgae by liquid-liquid extraction. Biomass Bioenergy 108:487–500
Jiang J, Savage PE (2017) Metals and other elements in biocrude from fast and isothermal hydrothermal liquefaction of microalgae. Energy Fuel 32:4118–4126
Bi Z, Zhang J, Zhu Z, Liang Y, Wiltowski T (2018) Generating biocrude from partially defatted Cryptococcus curvatus yeast residues through catalytic hydrothermal. Appl Energy 209:435–444
Tian C, Liu Z, Zhang Y (2017) Hydrothermal liquefaction (HTL): a promising pathway for biorefinery of algae in Gupta et al., “Algal biofuels”. Springer, Switzerland, pp 361–391
Toor SS, Rosendahl L, Rudolf A (2011) Hydrothermal liquefaction of biomass: a review of subcritical water technologies. Energy 36:2318–2342
Giaconia A, Caputo G, Ienna A, Mazzei D, Schiavo B, Scialdone O, Galia A (2017) Biorefinery process for hydrothermal liquefaction of microalgae powered by a concentrating solar plant: a conceptual study. Appl Energy 208:1139–1149
Vlaskin MS, Grigorenko AV, Kostyukevich YI, Nikolaev EN, Vladimirov GN, Chernova NI, Kiseleva SV, Popel OS, Zhuk AZ (2018) Influence of solvent on the yield and chemical composition of liquid products of hydrothermal liquefaction of Arthrospira platensis as revealed by Fourier transform ion cyclotron resonance mass spectrometry. Eur J Mass Spectrom 24:363–374. https://doi.org/10.1177/1469066718771209
Barreiro DL, Prins W, Ronsse F, Brilman W (2013) Hydrothermal liquefaction (HTL) of microalgae for biofuel production: state of the art review and future prospects. Biomass Bioenergy 53:113–127
Eboibi BE, Lewis DM, Ashman PJ, Chinnasamy S (2014) Hydrothermal liquefaction of microalgae for biocrude production: improving the biocrude properties with vacuum distillation. Bioresour Technol 174:212–221
Boëns B, Pilon G, Bourdeau N, Adjallé K, Barnabé S (2016) Hydrothermal liquefaction of a wastewater native Chlorella sp. bacteria consortium: biocrude production and characterization. Biofuels 7:611–619. https://doi.org/10.1080/17597269.2016.1168027
Biller P, Ross AB (2011) Potential yields and properties of oil from hydrothermal liquefaction of microalgae with different biochemical content. Bioresour Technol 102:215–225
Alba GL, Torri C, Samorì C, van der Spek J, Fabbri D, Kersten SRA, Brilman DWF (2011) Hydrothermal treatment (HTT) of microalgae: evaluation of the process as conversion method in an algae biorefinery concept. Energy Fuel 26:642–657
Sheng L, Wang X, Yang X (2018) Prediction model of biocrude yield and nitrogen heterocyclic compounds analysis by hydrothermal liquefaction of microalgae with model compounds. Bioresour Technol 247:14–20
Eboibi BE (2018) Effects of separation methods on yield and quality of biocrude after thermochemical liquefaction of a marine microalgae. NIJOTECH 37(3):679–691
Barreiro DL, Riede S, Hornung U, Kruse A, Prins W (2015) Hydrothermal liquefaction of microalgae: effect of the product yields of the addition of an organic solvent to separate the aqueous phase and the biocrude. Algal Res 12:206–212
Xu D, Savage PE (2014) Characterization of biocrudes recovered with and without solvent after hydrothermal liquefaction of algae. Algal Res 6:1–7
Elliott DC, Hart TR, Schmidt AJ, Neuenschwander GG, Rotness LJ, Olarte MV, Zacher AH, Albrecht KO, Hallen RT, Hollada JE (2013) Process development for hydrothermal liquefaction of algae feedstocks in a continuous-flow reactor. Algal Res 2:445–454
Shakya R, Adhikaria S, Mahadevan R, Shanmugam SR, Nam H, Hassan EB, Dempster TA (2017) Influence of biochemical composition during hydrothermal liquefaction of algae on product yields and fuel properties. Bioresour Technol 243:1112–1120
Xu D, Savage PE (2018) Supercritical water upgrading of water-insoluble and water-soluble biocrudes from hydrothermal liquefaction of Nannochloropsis microalga. J Supercrit Fluids 133:683–689
Smallwood I, (2012) Handbook of organic solvent properties. Arnold, London, NW1 3BH
Yang J, He Q, Corscadden K, Niu H (2018) The impact of downstream processing methods on the yield and physiochemical properties of hydrothermal liquefaction bio-oil. Fuel Process Technol 178:353–361
Eboibi BE, Lewis DM, Ashman PJ, Chinnasamy S (2015) Influence of process conditions on pretreatment of microalgae for protein extraction and production of biocrude during hydrothermal liquefaction of pretreated Tetraselmis sp. RSC Adv 5:20193–20207
Wang W, Zhang S, Yu Q, Lin Y, Yang N, Han W, Zhang J (2017) Hydrothermal liquefaction of high protein microalgae via clay material catalysts. RSC Adv 7:50794–50801
Huang Y, Chena Y, Xie J, Liu H, Yin X, Wu C (2016) Bio-oil production from hydrothermal liquefaction of high-protein high-ash microalgae including wild Cyanobacteria sp. and cultivated Bacillariophyta sp. Fuel 183:9–19
Jindal MK, Jha MK (2016) Effect of process parameters on hydrothermal liquefaction of waste furniture sawdust for bio-oil production. RSC Adv 6:41772–41780
Yang W, Li X, Zhang D, Feng L (2017) Catalytic upgrading of bio-oil in hydrothermal liquefaction of algae major model components over liquid acids. Energy Convers Manage 154:336–343
Eboibi BE, Lewis DM, Ashman PJ, Chinnasamy S (2014) Effect of operating conditions on yield and quality of biocrude during hydrothermal liquefaction of halophytic Tetraselmis sp. alga. Bioresour Technol 174:20–29
Fon Sing S, Isdepsky M, Borowitzka A, Lewis DM (2014) Pilot scale continuous recycling of growth medium for the mass culture of a halotolerant Tetraselmis sp. in raceway ponds under increasing salinity: a novel protocol for commercial microalgal biomass production. Bioresour Technol 161:47–54
Channiwala SA, Parikh PP (2012) A unified correlation for estimating HHV of solid, liquid, and gaseous fuels. Fuel 81:1051–1063
Posmanik R, Labatut RA, Kim AH, Usack JG, Tester JW, Angenent LT (2017) Coupling hydrothermal liquefaction and anaerobic digestion for energy valorization from model biomass feedstocks. Bioresour Technol 233:134–143
Hu Y, Wang S, Li J, Wang Q, He Z, Feng Y, Abomohra AF, Afonaa-Mensah S, Hui C (2018) Co-pyrolysis and co-hydrothermal liquefaction of seaweeds and rice husk: comparative study towards enhanced biofuel production. J Anal Appl Pyrolysis 129:162–170
Speight G (1999) The chemistry and technology of petroleum, third edn. Marcel Dekker Inc, New York
Wang W, Xua Y, Wanga X, Zhang B, Tiana W, Zhang J (2018) Hydrothermal liquefaction of microalgae over transition metal supported TiO2 catalyst. Bioresour Technol 250:474–480
Cheng F, Cui Z, Mallick K, Nirmalakhandan N, Brewera CE (2018) Hydrothermal liquefaction of high- and low-lipid algae: mass and energy balances. Bioresour Technol 258:158–167
Anastasakis K, Ross AB (2011) Hydrothermal liquefaction of the brown macro-alga Laminaria saccharina: effect of reaction conditions on product distribution and composition. Bioresour Technol 102:4876–4883
Alba GL, Torri C, Fabbri D, Kerstena SRA, Brilman DWF (2013) Microalga growth on the aqueous phase hydrothermal liquefaction of the same microalgae. Chem Eng J 228:214–223
Acknowledgements
The technical assistance of the Biotechnology Division of Aban Infrastructure Pvt. Limited, Chennai, India is acknowledged. In addition, the author is grateful for the guidance and support of Prof. David Lewis and Prof. Peter Ashman, both of School of Chemical Engineering, the University of Adelaide, Australia, and Dr. Senthil Chinnasamy of Aban Infrastructure Pvt. Ltd., Chennai, India.
Funding
This work received support from the Australian Research Council’s Linkage Projects Funding Scheme (Project LP100200616), industry partner SQC Pty Ltd. and the Australian Biofuels Investment Readiness program funding agreement no. Q00150, as well as financial support in the form of the Postgraduate Research Award provided by Education Trust Fund of the Federal Republic of Nigeria via Delta State University, Nigeria.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Eboibi, B.E. Impact of time on yield and properties of biocrude during downstream processing of product mixture derived from hydrothermal liquefaction of microalga. Biomass Conv. Bioref. 9, 379–387 (2019). https://doi.org/10.1007/s13399-019-00371-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-019-00371-y