Abstract
Herpes simplex virus (HSV) encephalitis can induce an autoimmune encephalitis mediated by autoantibodies against the N-methyl-D-aspartate receptor (NMDAR). Post-HSV NMDAR encephalitis and de novo NMDAR encephalitis have been more commonly described in children and young adults. We describe the case of a 67-year-old woman with post-HSV NMDAR encephalitis and review the relevant literature. Clinical, serological, neurophysiological, and imaging evaluations were undertaken in the evaluation of this patient. A literature review was performed. Nearly 2 months after a typical course of HSV encephalitis confirmed by HSV polymerase chain reaction studies from the spinal fluid and treated with intravenous acyclovir, a 67-year-old woman suffered neurological deterioration. There was no evidence of active HSV infection, but NMDAR antibodies were found in her serum and spinal fluid. The patient improved after initiation of immunosuppressive therapy. All patients who experience new or recurrent neurological symptoms following recovery from HSV encephalitis should be evaluated for post-infectious autoimmune encephalitis, including NMDAR encephalitis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Relapses of herpes simplex virus encephalitis (HSVE) have been reported in up to 12 % of adults and 14–35 % of children with the disease, often despite full-course treatment with acyclovir (Sköldenberg et al. 2006; De Tiége et al. 2003; Schleede et al. 2013; Hacohen et al. 2014). Whereas the recurrent neurologic symptoms have been previously interpreted as relapses of HSVE, in many of these cases, repeat testing for HSV1/2 via polymerase chain reaction (PCR) has been negative. Recently, reports have emerged of some of these patients having autoantibodies against the GluNR1 subunit of the N-methyl-D-aspartate receptor (NMDAR). Thus, rather than representing a true relapse of HSVE, these cases have highlighted the previously unrecognized entity of HSVE-induced autoimmune encephalitis. The pathogenesis of this process remains uncertain (De Tiége et al. 2003). However, recognizing its existence can have important implications for the treatment of recurrent neurologic symptoms after HSVE. Whereas prior cases have been reported primarily in children and young adults, this case report describes NMDAR encephalitis after HSVE in an immunocompetent post-menopausal woman.
Case report
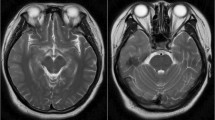
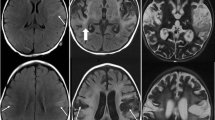
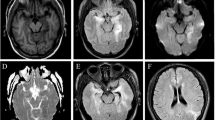
A 67-year-old woman presented to another hospital with 3 days of confusion, personality changes, lethargy, headache, and fever. A lumbar puncture was performed, and cerebrospinal fluid (CSF) analysis revealed a cell count of 730 white blood cells/mm3 with a differential of 95 % lymphocytes, 61 red blood cells/mm3, protein of 164 mg/dL, and glucose of 66 mg/dL. Magnetic resonance imaging (MRI) of the brain showed increased T2/fluid attenuation inversion recovery (FLAIR) intensity in the right temporal lobe, medial/inferior frontal lobe, and insula, with restricted diffusion in the right temporal lobe and insula. Electroencephalography (EEG) was notable for right frontal lateral periodic discharges. Herpes simplex virus type 1 (HSV-1) PCR was positive in the CSF. She was treated with a 21-day course of intravenous acyclovir, 10 mg/kg every 8 h. Following discharge, she had improvement in her mental status, with residual mild personality changes, short-term memory deficits, and fatigue, preventing her return to prior employment. Outpatient follow-up at this hospital 6 weeks later revealed isolated deficits in short-term recall and rare orofacial dyskinesias.
One week later (3 months after initial presentation), the patient presented to this hospital with 3 days of worsening confusion, disorientation, a mild occipital headache, and neck pain. Examination revealed only mild disorientation and impairment of short-term recall. Repeat MRI showed gliosis in the same distribution of the right frontotemporal areas originally affected. Repeat CSF analysis revealed 10 white blood cells/mm3, 228 red blood cells/mm3, protein of 135 mg/dL, glucose of 89 mg/dL, increased intrathecal IgG production, and two oligoclonal bands. CSF HSV-1 PCR was negative, but she was treated empirically with acyclovir due to a concern for recurrent HSV encephalitis. Her mental status deteriorated. She failed to regard or track. Her speech alternated between unintelligible mumbling and marked echolalia. She developed fixed nihilistic delusions as well as visual, auditory, and tactile hallucinations. The orofacial dyskinesias became more prominent. She suffered several generalized tonic-clonic seizures with electrographic correlates and was treated with antiepileptic medications, but her behavior continued to deteriorate despite clinical and electrographic seizure control. Indirect tissue immunofluorescence and cell-based assays were positive for NMDAR antibodies in both serum and CSF. She was treated with a five-day course of intravenous immunoglobulin (IVIG), followed by rituximab (1000 mg IV twice, 2 weeks apart). Due to continued deterioration of the patient’s mental status to near catatonia despite these treatments, CT with contrast and FDG-PET of the chest-abdomen-pelvis were performed; these were negative for occult malignancy. Monthly intravenous cyclophosphamide (750 mg/m2) was added to her regimen for 3 months. Her symptoms improved slowly over several weeks. She was readmitted once for agitation and psychosis, which improved with olanzapine. She had no further seizures. At her last follow-up, 10 months after initial HSV encephalitis onset, her neurologic examination was normal, including formal neuropsychological testing. Her daughter reported only mild persistent personality changes.
Discussion
Debate as to the etiology of presumed HSVE relapses has centered on two main hypotheses, either viral reactivation or a post-infectious autoimmune process. Inadequate doses or duration of acyclovir have been implicated by several studies and point to recurrent infection as the mechanism (Van Landingham et al. 1988; Kimura et al. 1992; Ito et al. 2000). In some cases, a repeated course of acyclovir has proved beneficial, more so in cases where the initial course was short or when corticosteroids were co-administered with the first course (Pike et al. 1991; Knezevic and Carroll 1983). Further proof of viral reactivation is derived from pathologically proven active HSV-1 replication from biopsy or autopsy specimens from patients who relapsed (Davis and McLaren 1983; Yamada et al. 2003).
In an early investigation of HSVE relapse after treatment with vidarabine (Whitley et al. 1986), viral reactivation was considered the most likely explanation, but failure to grow out HSV from biopsy tissue culture, lack of response to subsequent courses of vidarabine, and microscopic findings of perivenous inflammatory demyelination were all suggestive of a post-infectious autoimmune process (Koenig et al. 1979). Indeed, evidence of active HSV infection during relapse is often lacking, whether by imaging, serum or CSF antibody identification, CSF PCR, tissue culture, or pathological examination of biopsy specimens (Sköldenberg et al. 2006; De Tiége et al. 2003; Hacohen et al. 2014; Ito et al. 2000; Barthez-Carpentier et al. 1995; Dennett et al. 1996). Concordantly, many patients do not respond to further antiviral therapy. HSVE induces a robust intrathecal inflammatory response, which is attenuated by acyclovir. A persistent inflammatory response has been demonstrated in animal models and humans with HSVE after recovery and has been linked to insidious long-term deterioration (Aurelius et al. 1993; Sellner et al. 2005). The full profile of inflammatory markers during presumed relapses of HSVE has not been studied.
Post-infectious immune-mediated targeting of the nervous system is well recognized. Many authors have cited a positive response to corticosteroids in presumed HSVE relapses as support for an autoimmune, as opposed to an infectious, pathogenesis for the recurrent neurologic symptoms. (De Tiége et al. 2003; Prüss et al. 2012; Armangue et al. 2014; Leypoldt et al. 2013; De Tiege et al. 2005; Joos et al. 2003) This etiology may account for the better outcomes seen when glucocorticoid treatment is delayed. In one series of HSVE patients, NMDAR antibodies were not present during the acute HSVE but rather appeared after 1–4 weeks (Armangue et al. 2014). Delaying corticosteroid therapy may therefore allow for effective treatment of the acute infection, while still preventing the subsequent development of neural autoantibodies.
Others arguing against recurrent neurologic symptoms representing a true “relapse” of HSVE point out that clinical features in “relapses” may be distinct from the original HSVE presentation, especially in children. Since originally reported in 1961, choreoathetosis has been recognized as a common, late symptom of HSVE relapse (De Tiége et al. 2003; Ross and Stevenson 1961; Abramson et al. 1984; Shanks et al. 1991; Baxter et al. 1994; Wang et al. 1994; Schlesinger et al. 1995; Hargrave and Webb 1998; Valencia et al. 2004; Devrim et al. 2008). Anti-NMDAR encephalitis is known to commonly present with prominent abnormal movements in children, as compared to behavioral and psychiatric changes in adults (Hacohen et al. 2014; Armangue et al. 2014; Dalmau et al. 2008; Armangué et al. 2013). Thus, post-HSVE choreoathetosis may actually be a manifestation of anti-NMDAR encephalitis, and its presence may even predict the presence of neural autoantibodies (Prüss et al. 2012). Several case reports support the notion that at least some HSVE relapses in children and adults may be due to HSVE-induced anti-NMDAR encephalitis (Hacohen et al. 2014; Armangue et al. 2014; Leypoldt et al. 2013; Armangué et al. 2013; Dalmau et al. 2007; Desena et al. 2014).
Since 2007, anti-NMDAR encephalitis has been recognized as a frequent cause of encephalitis, especially in children and young adults (Dalmau et al. 2007). The California Encephalitis Project reported that the frequency of anti-NMDAR encephalitis is greater than that of any viral encephalitis in patients less than 30 years old (Gable et al. 2012). The hallmark symptoms include prominent neuropsychiatric changes, decreased consciousness, seizures, dyskinesias including choreoathetosis, and autonomic instability (Dalmau et al. 2008). Given the clinical overlap, it is not surprising that anti-NMDAR encephalitis and HSVE are commonly considered on the differential diagnosis for a given patient. A patient with HSVE was found to also harbor NMDAR antibodies, prompting a retrospective review of 44 patients with HSVE, in whom NMDAR antibodies (IgA, IgM, IgG) were detected in 13 (30 %) (Prüss et al. 2012). Some of the patients had NMDAR antibodies identified at hospital admission, while others developed antibodies after a week of illness. As only the IgG subtype has been shown to be associated with anti-NMDAR encephalitis, a later retrospective analysis of HSVE samples for IgG NMDAR antibodies identified the antibodies in three out of 34 patients (9 %) (Armangue et al. 2014). The age of the patient reported above, 67, is unusual for anti-NMDAR encephalitis, and it also differs from previously reported cases of HSVE-induced anti-NMDAR encephalitis. This likely reflects a different mechanism of induction from more typical cases of anti-NMDAR encephalitis.
Anti-NMDAR encephalitis is known to occur after nonspecific viral prodromes, as well as serologically proven acute influenza, Epstein-Barr virus, and especially mycoplasma pneumoniae infections (Dalmau et al. 2008; Baltagi et al. 2010; Xu et al. 2011; Gable et al. 2009; Prüss et al. 2010). How a preceding viral infection may induce anti-NMDAR encephalitis remains a mystery. In the setting of HSVE-induced autoimmune encephalitis, the three primary mechanisms proposed are molecular mimicry, nonspecific B cell activation as seen in other neurological diseases such as multiple sclerosis, and the development of synaptic autoimmunity secondary to presentation of previously sheltered neuronal antigens exposed by virally induced neuronal lysis and inflammatory compromise of the blood–brain barrier (Prüss et al. 2012; Armangue et al. 2014). While the latter explanation is favored, questions persist regarding why anti-NMDAR encephalitis is not also seen after other processes that similarly lead to necrosis and breakdown of the blood–brain barrier, such as stroke or bacterial meningitis (Prüss et al. 2012). The recent discovery of autoantibodies against the dopamine-2 receptor in choreoathetotic encephalitic relapse following HSVE, both concomitant with and independent of the presence of anti-NMDAR antibodies, highlights that HSVE seems to induce the production of a range of anti-neural autoantibodies (Mohammad et al. 2014). The full repertoire of HSVE-triggered antibodies remains to be prospectively studied. Moreover, anti-NMDAR encephalitis has now been reported after VZV encephalitis, and thus, this phenomenon does not appear to be restricted to HSV (Schäbitz et al. 2014).
Conclusion
We have described a case of HSVE-induced anti-NMDAR encephalitis in a previously healthy 67-year-old woman. Relapses after HSVE have long been reported and may be due to persistent or reactivated viral infection, especially in the setting of inadequate duration or dosing of antiviral therapy. However, there is a growing body of literature which suggests that what were previously thought to be as relapses of HSVE may actually be HSVE-triggered autoimmune encephalitis. Thus, when any patient with recent HSVE develops new or recurrent neurologic symptoms, there should be a high suspicion for post-infectious autoimmune encephalitis. In addition to testing the CSF for HSV1/2 PCR, one should also test it for neurally directed autoantibodies, including NMDAR antibodies. It is particularly important to test the CSF as well as the serum for these antibodies, as it has been shown that testing CSF is more sensitive and specific than testing serum for NMDAR antibodies (Dalmau et al. 2011; Gresa-Arribas et al. 2014). Treatment with steroids during the initial phase of HSVE in order to prevent autoimmunity is controversial and has yet to be studied in a clinical trial. Treatment of patients with new or recurrent neurologic symptoms after HSVE with immunosuppression should be guided by the clinical picture, the results of CSF HSV1/2 PCR testing, and the presence of intrathecal anti-NMDAR antibodies. Now that this phenomenon has been recognized, it will be important to follow these patients long-term, to clarify whether the double-hit of anti-NMDAR encephalitis following HSVE has important ramifications for overall functional outcomes, and how these cases of autoimmune encephalitis may differ from more traditional cases. In our patient’s case, appropriate diagnosis and aggressive immunotherapy resulted in a near-full functional recovery.
References
Abramson JS, Roach ES, Levy HB (1984) Postinfectious encephalopathy after treatment of herpes simplex encephalitis with acyclovir. Pediatr Infect Dis J 3:146–147
Armangué T, Titulaer MJ, Málaga I et al (2013) Pediatric anti-N-methyl-D-aspartate receptor encephalitis-clinical analysis and novel findings in a series of 20 patients. J Pediatr 162:850–856
Armangue T, Leypoldt F, Malaga I et al (2014) Herpes simplex virus encephalitis is a trigger of brain autoimmunity. Ann Neurol 75:317–323
Aurelius E, Forsgren M, Skoldenberg B et al (1993) Persistent intrathecal immune activation in patients with herpes simplex encephalitis. J Infect Dis 168:1248–1252
Baltagi S, Shoykhet M, Felmet K et al (2010) Neurological sequelae of 2009 influenza A (H1N1) in children: a case series observed during a pandemic. Ped Crit Car Med 11:179–184
Barthez-Carpentier MA, Rozenberg F, Dussaix E et al (1995) Relapse of herpes simplex encephalitis. J Child Neurol 10:363–367
Baxter R, Forsyth RJ, Eyre JA (1994) Movement disorder after herpes simplex virus encephalitis. Dev Med Child Neurol 36:275–276
Dalmau J, Tuzun E, Wu HY et al (2007) Paraneoplastic anti-N-methyl-D aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol 61:25–36
Dalmau J, Gleichman AJ, Hughes EG et al (2008) Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol 7:1091–1098
Dalmau J, Lancaster E, Martinez-Hernandez E et al (2011) Clinical experience and laboratory investigations in patients with anti-NMDAR encephalitis. Lancet Neurol 10:63–74
Davis LE, McLaren LC (1983) Relapsing herpes simplex encephalitis following antiviral therapy. Ann Neurol 13:192–195
De Tiége XD, Rozenberg F, Des Portes V et al (2003) Herpes simplex encephalitis relapses in children: differentiation of two neurologic entities. Neurology 61:241–243
De Tiege X, De Laet C, Mazoin N, et al. (2005) Postinfectious immune-mediated encephalitis after pediatric herpes simplex encephalitis. Brain Dev 27-304-7
Dennett C, Klapper PE, Cleater GM (1996) Polymerase chain reaction in the investigation of ‘relapse’ following herpes simplex encephalitis. J Med Virol 48:129–132
Desena A, Graves D, Warnack W, Greenberg BM (2014) Herpes simplex encephalitis as a potential cause of anti-N-methyl-D-aspartate receptor antibody encephalitis. JAMA Neurol 71:344–346
Devrim I, Tezer H, Haliloglu G et al (2008) Relapsing herpes simplex virus encephalitis despite high-dose acyclovir therapy: a case report. Turk J Pediatr 50:380–382
Gable SM, Gavali S, Radner A et al (2009) Anti-NMDA receptor encephalitis: report of ten cases and comparison with viral encephalitis. Eur J Clin Microbiol Infect Dis 28:1421–1429
Gable MS, Sheriff H, Dalmau J, Tilley DH, Glaser CA (2012) The frequency of autoimmune N-methyl-D-aspartate receptor encephalitis surpasses that of individual viral etiologies in young individuals enrolled in the California Encephalitis Project. Clin Infect Dis 54:899–904
Gresa-Arribas N, Titulaer M, Torrents A et al (2014) Antibody titres at diagnosis and during follow-up of anti-NMDA receptor encephalitis: a retrospective study. Lancet Neurol 13:167–177
Hacohen Y, Deiva K, Pettingill P et al (2014) N-Methyl-D-aspartate receptor antibodies in post-herpes simplex virus encephalitis neurological relapse. Mov Disord 29:90–96
Hargrave DR, Webb DW (1998) Movement disorders in association with herpes simplex virus encephalitis in children: a review. Dev Med Child Neurol 40:640–642
Ito Y, Kimura H, Yabuta Y et al (2000) Exacerbation of herpes simplex encephalitis after successful treatment with acyclovir. Clin Infect Dis 30:185–187
Joos AAB, Ziyeh S, Rauer S et al (2003) Postinfectious autoimmune-mediated encephalitis eight months after herpes simplex encephalitis. Eur Neurol 50:54–56
Kimura H, Aso K, Kuzushima K et al (1992) Relapse of herpes simplex encephalitis in children. Pediatrics 89:891–894
Knezevic W, Carroll WM (1983) Relapse of herpes simplex encephalitis after acyclovir therapy. Aust N Z J Med 13:625–626
Koenig H, Rabinowitz SG, Day E et al (1979) Post-infectious encephalomyelitis after successful treatment. NEJM 300:1089–1093
Leypoldt F, Titulaer MJ, Aguilar E et al (2013) Herpes simplex virus-1 encephalitis can trigger anti-NMDA receptor encephalitis: case report. Neurology 81:1637–1639
Mohammad SS, Sinclair K, Pillai S et al (2014) Herpes simplex encephalitis relapse with chorea is associated with autoantibodies to N-methyl-D-aspartate receptor or dopamine-2-receptor. Mov Disord 29:117–122
Pike MG, Kennedy CR, Neville BGR et al (1991) Herpes simplex encephalitis with relapse. Arch Dis Child 66:1242–1244
Prüss H, Dalmau J, Harms L (2010) Retrospective analysis of NMDA receptor antibodies in encephalitis of unknown origin. Neurology 751735–9
Prüss H, Finke C, Höltje M (2012) N-methyl-D-aspartate receptor antibodies in herpes simplex encephalitis. Ann Neurol 72:902–911
Ross CAC, Stevenson J (1961) Herpes-simplex meningoencephalitis. Lancet 2:682–685
Schäbitz WR, Rogalewski A, Hagemeister C, Bien CG (2014) VZV brainstem encephalitis triggers NMDA receptor immunoreaction. Neurology 83:2309–2311
Schleede L, Bueter W, Baumgartners-Sigl S et al (2013) Pediatric herpes simplex virus encephalitis: a retrospective multicenter experience. J Child Neurol 28:321–331
Schlesinger Y, Buller RS, Brunstrom JE et al (1995) Expanded spectrum of herpes simplex encephalitis in children. J Pediatr 126:234–241
Sellner J, Dvorak F, Zhou Y et al (2005) Acute and long-term alteration of chemokine mRNA expression after anti-viral and anti-inflammatory treatment in herpes simplex virus encephalitis. Neurosci Lett 374:197–202
Shanks DE, Blasco PA, Chason DP (1991) Movement disorder following herpes simplex encephalitis. Dev Med Child Neurol 33:343–355
Sköldenberg B, Aurelius E, Hjalmarsson A et al (2006) Incidence and pathogenesis of clinical relapse after herpes simplex encephalitis in adults. J Neurol 253:163–170
Valencia I, Miles DK, Melvin J et al (2004) Relapse of herpes encephalitis after acyclovir therapy: report of two new cases and review of the literature. Neuropediatrics 35:371–376
Van Landingham KE, Marsteller HB, Ross GW et al (1988) Relapse of herpes simplex encephalitis after conventional acyclovir therapy. JAMA 259:1051–1053
Wang HS, Kuo MF, Huang SC et al (1994) Choreoathetosis as an initial sign of relapsing of herpes simplex encephalitis. Pediatr Neurol 11:341–345
Whitley RJ, Alford CA, Hirsh MS et al (1986) Vidarabine versus acyclovir therapy in herpes simplex encephalitis. NEJM 314:144–149
Xu CL, Liu L, Zhao WQ et al (2011) Anti-N-methyl-D-aspartate receptor encephalitis with serum and anti-thyroid antibodies and IgM antibodies against Epstein-Barr virus viral capsid antigen: a case report and one year follow-up. BMC Neurol 11:149–155
Yamada S, Kameyama T, Nagaya S (2003) Relapsing herpes simplex encephalitis: pathological confirmation of viral reactivation. J Neurol Neurosurg Psychiatry 74:262–264
Conflict of interest
Nicholas A. Morris, MD, Tamara Kaplan, MD, and Jenny Linnoila, MD, PhD declare that they have no competing interests.
Tracey Cho, MD received compensation as a consultant for Optum Insight, guest editor for Continuum: Lifelong Learning in Neurology, and guest editor for Seminars in Neurology.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Morris, N.A., Kaplan, T.B., Linnoila, J. et al. HSV encephalitis-induced anti-NMDAR encephalitis in a 67-year-old woman: report of a case and review of the literature. J. Neurovirol. 22, 33–37 (2016). https://doi.org/10.1007/s13365-015-0364-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13365-015-0364-9