Abstract
For Lasius japonicus Santschi (Hymenoptera: Formicidae), a common ant species in Japan, diapause induction is temperature dependent. To elucidate the geographic variation of its temperature dependence, new L. japonicus queens were collected immediately after nuptial flight from four sites at different latitudes: Kitami (44.1°N), Hakodate (41.8°N), Shizuku-ishi (39.7°N), and Okayama (34.7°N). The collected queens were reared for 100 days at four constant-temperature conditions (25 °C, 20 °C, 17.5 °C, and 15 °C) under a 12L:12D photoperiod. The queens began founding colonies immediately in all conditions. For all temperature conditions, daily change patterns of the average numbers of eggs, larvae, and pupae were similar among the Hakodate, Shizuku-ishi, and Okayama populations, but the development patterns of the Kitami colonies differed from those of the other three sites. Low temperatures strongly suppressed colony development of the Kitami population; development was weak even at moderate temperatures. For new queens of L. japonicus, solitary overwintering without rearing larvae is regarded as serving an important role in successful colony foundation under cool climate conditions. Results reported herein suggest that local temperatures exert important selective pressure on the timing of diapause induction and on colony development in northern populations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Under unfavorable environmental conditions, many insects cease their development and enter diapause. Their development does not resume thereafter unless proper conditions prevail. Insects adjust their developmental stage and regulate their annual life cycle within a certain range that is suitable for survival (Bradshaw and Holzapfel 2007; Danilevskii 1961; Danks 1987; Denlinger 2009; Tauber et al. 1986). Diapause is an adaptation to annual environmental change in a given climate.
In temperate regions, insects anticipate the coming of unfavorable seasons even when current environmental conditions are suitable for growth and reproduction. Therefore, they enter diapause in response to specific environmental stimuli such as short day length or low temperature (Danilevskii 1961; Danks 1987; Lees 1955; Tauber et al. 1986). Day length is a reliable environmental cue indicating seasonal change. For that reason, many insects sense day length as a signal to enter diapause. This diapause-inducing response exhibits geographical variations along the habitat latitude. For example, noctuid moths Acronicta rumicis (Linnaeus) from habitats at higher latitudes have longer critical day length for induction of pupal diapause (Danilevskii 1961). Latitudinal variations in photoperiodic responses have also been reported for several Japanese insects (Masaki 1961). In general, the critical day length increases by 1–2 h for every 5° of increased habitat latitude (Danilevskii 1961). In the predatory bug Orius sauteri (Poppius), the critical photoperiod for the induction of diapause increases by approximately 1 h for each 5° of increased latitude (Ito and Nakata 2000). In each habitat of O. sauteri, air temperature falling to below 20 °C corresponds with the timing of diapause induction.
Temperature is a less reliable cue than day length, but some insects use temperature as a primary determinant signal for diapause induction. In the parasitoid Leptopilina boulardi Barbotin, Carton and Kelner-Pillault, prepupal diapause is induced by low temperature (17.5 °C) but it is absent at 25 °C; moreover, it is independent of photoperiod. Four populations from different latitudes (4°S to 44°N) exhibit identical responses to temperature (Carton and Claret 1982). The temperature response for diapause induction in Trichogramma dendrolimi Matsumura shows geographical variation, but it does not simply correspond with latitude (Zhang et al. 2017). For the spider mite Eotetranychus smithi Pritchard & Baker, although not insect, diapause is induced by low temperature alone (≤ 17.5 °C). At high temperatures (≥ 20 °C), E. smithi lay non-diapause eggs. Photoperiod has little effect on diapause induction (Gotoh and Kameyama 2014). Furthermore, temperature can modify the diapause-inducing response to day length (Danks 1987). Relations between temperature and photoperiodic response for diapause induction differ among A. rumicis of different latitudinal habitats. Northern populations enter diapause at higher temperatures than southern populations (Danilevskii 1961). Similar modifications to photoperiodic responses according to temperature are observed for other insects as well (Beck 1980).
The seasonal regulation of the ant life cycle is known to be affected more strongly by temperature than by day length (Kipyatkov 1993, 2001). Ants in warm temperate regions enter adult and larval diapause in winter (Kipyatkov 1993, 2001). In warm climates, low temperature is the primary environmental stimulus for the induction of diapause (Kipyatkov 1993, 2001). Oviposition by queens and pupation of larvae in colonies cease when temperatures decline, thereby indicating that adults and larvae enter diapause. When the temperature rises in spring, the ants resume colony development (e.g., Lasius niger (Linnaeus), Aphaenogaster sinensis Wheeler (= A. japonica Forel), and Myrmica rubra (Linnaeus); Kipyatkov 1993, 2006). The queen restarts oviposition; the larvae enter pupation. In some Pogonomyrmex lineages, only queens that have experienced cold can produce new queens (Schwander et al. 2008).
In several ant species, geographical variation along the latitude of habitat is found in the queen’s oviposition activity. In two Myrmica species, the oviposition period of the queen is shorter and the eggs are fewer in populations at high latitudes than in populations at low latitudes (Kipyatkov and Lopatina 2002). In Myrmica ruginodis Nylander, shorter oviposition periods of queens and longer larval diapause are found in populations living at high latitudes. These changes are likely to be adaptations to short summers in the high-latitude habitats.
Kamitani et al. (2015) examined the effects of photoperiod and temperature on the colony development of Lasius japonicus Santschi (Hymenoptera: Formicidae) collected in Okayama. For the number of pupae produced by the colony, they found no significant difference between long-day and short-day conditions. For queens reared at 25 °C shortly after nuptial flight, rapid colony development with cyclic emergence of pupae was observed. By contrast, diapause was induced in larvae and queens at lower temperatures (Kamitani et al. 2015). These observations demonstrate that temperature is the primary factor affecting L. japonicus colony development. Populations of other areas have not been examined to date, although L. japonicus is distributed widely in eastern Asia including the islands of Japan, the Korean Peninsula, and Taiwan (JADG 2003). It remains unclear how the habitat latitude affects the temperature response leading to diapause induction in this species.
Diapause is usually induced at species-specific developmental stages (Danks 1987). Insects cannot survive through adverse seasons if they fail to enter diapause stages with appropriate timing. Consequently, diapause is induced before adverse environmental conditions occur (Danilevskii 1961; Danks 1987; Tauber et al. 1986). Winter diapause is often induced several months before the temperature falls below the developmental threshold of an insect. In ants, queens invariably experience adverse seasons because they can survive for more than several years. Before the arrival of winter, queens switch their physiological state from reproductive to diapause. Nakamura et al. (2017) report that L. japonicus queens in the Okayama population lay eggs continuously at 17.5 °C, but enter reproductive diapause at 15 °C under a short day length. The diapause-inducing temperature is equivalent to the average air temperature in early November in Okayama. This fact suggests that queens enter diapause directly in response to low temperature, instead of anticipating the arrival of winter a few months before the cold season. We infer, for the temperature response related to the induction of L. japonicus diapause, that the reproductive diapause of queens shows no geographical variation along with latitude. Diapause is induced when the temperature decreases below a certain temperature, irrespective of latitudinal differences (a first hypothesis).
At high latitudes, ant queens often enter diapause after a certain period of reproduction, irrespective of environmental stimuli. They react similarly even when subjected to warm, stable conditions in the laboratory (Kipyatkov 1993, 2001). For developing ant colonies during 1–5 years, diapause occurs at various temperatures under several constant photoperiodic conditions. In several Formica species such as Formica cinerea Mayr, F. clara Forel, F. fusca Linnaeus, F. japonica Motschoulsky, and F. lemani Bondroit, periods of one oviposition cycle are roughly 10–20 weeks at constant temperatures of 17–28 °C, or under thermoperiods of 15/25 °C, 16/30 °C, and 20/30 °C (Kipyatkov and Lopatina 1993). Temperature affects the start of the oviposition cycle, although the cycle duration is unaffected by external factors such as temperature (Kipyatkov and Lopatina 1993). The queens of Formica aquilonia Yarrow and F. polyctena Foerster show long oviposition cycles from a few months to more than a year. Both the start and end of the oviposition cycle are unaffected by any external factor (Kipyatkov 2006). These observations suggest that some endogenous mechanisms maintain oviposition cycles and regulate the seasonal development of the entire colony (Kipyatkov 2006). Kuroki et al. (2018) reported that L. japonicus queens show endogenous periodicity in their oviposition activity under high light intensity, suggesting a probable relation to a biological clock. Endogenous periodicity of queens’ reproduction is commonly observed in ants at high latitudes (Kipyatkov 1993, 2001). Cyclic oviposition in L. japonicus enables queens to terminate oviposition activity before the arrival of winter in a stable underground environment. Therefore, we formulated a second hypothesis that L. japonicus queens of high-latitude populations stop oviposition after a certain active period even when subjected to warm, stable conditions in the laboratory.
We tested the hypotheses presented above by rearing ants of different latitudinal populations under different temperature conditions. Based on findings obtained from our experiments, we can discuss the seasonal and geographical adaptations of the life cycle in L. japonicus.
Materials and methods
During late June–late August in 2007–2011, we collected new queens of L. japonicus that were attracted to night lights after nuptial flight, at sites of four latitudes: Kitami, Hakodate, Shizuku-ishi, and Okayama, Japan (Table 1). The collected queens were brought back to our laboratory within 24 h after shedding wings (day 0). For ease of observation, each queen was put in a shallow transparent plastic container (l × w × d = 7.2 × 4.5 × 2.3 cm), the bottom of which was covered with approximately 1-cm plaster containing activated charcoal powder. The containers were then placed under four constant-temperature conditions of 25 °C, 20 °C, 17.5 °C, and 15 °C under a 12L:12D photoperiod (n = 17–56 for each group). Ohta et al. (2017) reported that the oviposition activity of L. japonicus queens was affected by temperature but not by photoperiod. Therefore, the photoperiodic condition was set as that of early October, when L. japonicus enter diapause in nature. Then the effects of various temperatures on colony development were investigated.
Each colony was supplied with a few milliliters of water twice a week to maintain moisture. For colonies that produced workers, each container was annexed with the same-sized second-story transparent plastic container. In each, dried bloodworms (Propsilocerus akamusi (Tokunaga)) and insect jelly (High-protein lactic acid jelly WS; Fujikon Co., Ltd., Japan) were placed so that the workers were able to feed the queen.
During the first 10 days after the onset of breeding, the queen’s condition in each container was checked every day. The number of eggs in the container was recorded. Thereafter, each container was observed twice a week: the queen’s condition was checked; the numbers of eggs, larvae, and pupae were recorded. Observations were continued for 100 days or until the queen’s death. Colonies in which the queen died before day 60 were excluded from data analyses. When the experiment was completed, the ratio of colonies that produced pupae was calculated for each conditioned population. Ants have no pupal diapause (Kipyatkov and Lopatina 1993). Therefore, the proportion of such colonies is an important determinant of overwintering.
Data of local monthly average and maximum temperatures at the meteorological stations nearest the collection sites were obtained from a webpage of the Japan Meteorological Agency (http://www.data.jma.go.jp/obd/stats/etrn/index.php?prec_no=&block_no=&year=&month=&day=&view=, accessed on 29 March, 2017).
Average numbers of eggs, larvae, and pupae were compared among the populations at each temperature condition using one-way ANOVA. Multiple comparisons were made using Tukey tests (Zar 2010). Percentages of colonies that produced pupae were analyzed using chi-square tests. Then multiple comparisons were made using Tukey tests after arcsine transformation of data (Zar 2010). Populations that produced no larvae/pupae were excluded from analyses.
Results
Geographical variation in colony development
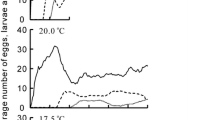
Colony development patterns of four populations at 25 °C are presented in Fig. 1. Average numbers of eggs in the Kitami population increased soon after the start of rearing, and peaked at day 13. Subsequently, the egg number decreased; larvae emerged. A second peak of egg number was not observed clearly. The average number of larvae increased gradually between days 10 and 20. Thereafter, the larval number was stable until the end of the experiment, at day 100. Most larvae did not pupate during the experimental period. In the Hakodate, Shizuku-ishi, and Okayama populations, the average numbers of eggs increased similarly and peaked around day 15. Subsequently, the egg number decreased, but it increased again around day 40. In the Oka-yama population, the second and later egg peaks, which were not clear, were higher than the first peak, unlike those in the other populations. Larvae began to appear at around day 12. The average number of larvae peaked around day 20 in each population. In accordance with the decrease of larvae, pupae began to increase. The numbers of larvae and pupae repeatedly increased and decreased until the end of the experiment.
At 20 °C, differences among the four populations in colony development patterns were smaller than the differences found at 25 °C (Fig. 2). In all populations, eggs began to increase immediately after the onset of breeding. They decreased around day 30 in accordance with the hatching of larvae. The second peak of the number of eggs was not clear in every population, although the numbers of eggs after day 50 in the southern two populations were larger than those in the northern ones. The number of larvae was stable after approximately day 40. The pupae were much fewer than at 25 °C in every population.
At 17.5 °C, in the southern three populations, changing patterns of the numbers of eggs and larvae were similar to those at 20 °C (Fig. 3). Specifically, no second peak in oviposition activity was apparent. The number of larvae was constant in most colonies. Pupation rarely occurred during the experimental period. In the Kitami population, the average number of larvae was much smaller than those of the southern populations, suggesting that hatching was prevented.
At 15 °C, the Hakodate population could not be investigated because of the small number of collected queens. Among the remaining three populations, no clear difference was detected in the pattern of colony development (Fig. 4). Larval hatching was seldom observed despite the fact that a decrease of the egg number was apparent after approximately day 30.
Average numbers of eggs, larvae, and pupae
Table 2 presents the numbers of eggs, larvae, and pupae around the respective first peak days in the four L. japonicus populations reared under different temperature conditions. At 25 °C, marked differences in the egg number were apparent among the four populations. The eggs in the Kitami population were significantly fewer than those in the southern populations (Tukey test, p < 0.05). At 20 °C and 17.5 °C also, the average number of eggs in the Kitami population was less than those in the southern populations. At 15 °C, the egg number in the Shizuku-ishi population was greater than in either of the other two populations.
Regarding the number of emerging larvae, significant differences were found among the populations at 25 °C, 20 °C, and 17.5 °C. The larvae in the Kitami population were significantly fewer than those in the other three populations (Tukey test, p < 0.05). At 15 °C, very few larvae emerged only in the Shizuku-ishi population. For pupal appearance, significant differences were found between the Kitami and other populations at 25 °C (Tukey test, p < 0.05). At 20 °C, no pupa appeared in the Kitami population at day 60; the Hakodate population produced only a few pupae. The numbers of pupae in the other two populations were small and did not differ significantly (Tukey test, p < 0.05). At 17.5 °C and 15 °C, no pupa was produced in any population at day 60.
Differences in pupa production among populations
Some colonies of L. japonicus produced pupae during the 100-day breeding period. Others did not. The percentages of colonies that produced pupae differed among the populations (Table 3). At 25 °C, the percentage in the Kitami population was 40%. This value was significantly lower than those in the other southern populations (Tukey test, p < 0.05). The proportions in the Hakodate and Okayama populations were regarded as equal.
At 20 °C, no colony in the Kitami population produced a pupa. Only 10% of the colonies in the Hakodate population produced pupae. However, in the two southern populations, more than 65% colonies produced pupae. At 17.5 °C, the colonies that produced pupae were few in these two southern populations. At 15 °C, no pupa was observed in any population during the 100-day experimental period.
Discussion
Geographic variation of temperature response for diapause induction
In ectothermic organisms such as insects, the suitable period for development and reproduction varies according to the latitude of their habitat. Winter arrives early at high latitudes. The warm period is short. Consequently, in the northern hemisphere, insects of the northern populations enter diapause earlier than those of southern populations. The photoperiodic responses for diapause induction display clear geographical variations (Danilevskii 1961; Danks 1987; Tauber et al. 1986). For example, the noctuid moth A. rumicis shows a linear positive correlation between the habitat latitude and critical day length for the induction of pupal diapause (Danilevskii 1961). However, temperature responses leading to diapause induction in some parasitoid wasps show different features according to geographical variation (Carton and Claret 1982; Zhang et al. 2017).
The present study did not clarify any geographical variation in the colony development of L. japonicus related to latitude. Daily changing patterns in the average numbers of eggs, larvae, and pupae were similar among the three southern populations, supporting our first hypothesis. In these populations, oviposition, hatching, and pupation occurred repeatedly throughout the experimental period at 25 °C (Fig. 1). At 20 °C and 17.5 °C, the numbers of larvae were stable (Figs. 2 and 3). Pupae at 20 °C were not numerous even in the two southern populations. Only a few colonies of these two southern populations produced a few pupae at 17.5 °C (Table 3). Therefore, larval diapause was likely to have been induced in these populations. At 15 °C, hatching rarely occurred (Fig. 4). Moreover, the number of eggs decreased gradually to zero in most colonies, suggesting that reproductive diapause is induced in queens.
It is particularly interesting that the responses to temperature in the Shizuku-ishi and Hakodate populations were quite similar to that found in the Okayama population, even though Shizuku-ishi and Hakodate are, respectively, 550 km and 780 km north of Okayama. These northern collection sites have day lengths that are much longer than those in Okayama. In many Japanese insects, geographical variations in diapause characteristics are apparent. The higher the habitat latitude, the longer the critical day length for diapause induction (Masaki 1961). The critical photoperiod for diapause induction in individuals of the flower bug O. sauteri collected at 42.0°N is longer by approximately 2 h than that of individuals collected at 33.3°N (Ito and Nakata 2000; Kohno 1997). The latitudinal difference in that case is similar to that between Hakodate (41.8°N) and Okayama (34.7°N) found in the present study. The present results suggest strongly that L. japonicus shows only slight geographical variation in temperature response leading to the induction of diapause. This temperature response in L. japonicus differs greatly from those in many other insects, as we expected. The temperature-dependent response leading to diapause induction in the parasitoid L. boulardi also shows no clear geographical variation (Carton and Claret 1982). Moreover, in the egg parasitoid T. dendrolimi, the geographical variation of the diapause rate, which is affected by temperature, cannot be interpreted simply as a latitude-related phenomenon (Zhang et al. 2017). The mechanism of temperature response apparently differs from that of photoperiodic response.
For both reproductive diapause of queens and larval diapause, the response to temperature was similar among the three southern populations (Figs. 1, 2, 3, 4). However, the percentages of the colonies that produced pupae differed among these three populations. At 20 °C, the percentage in the Hakodate population was much lower than those in the Shizuku-ishi and Okayama populations (Table 3). Such a difference between the Hakodate and the other two populations was also observed at 17.5 °C. These results suggest that L. japonicus larvae of the northern populations are sensitive to low temperatures.
Ecological significance of geographical variation in temperature response
Figure 5 presents 30-year averages (1981–2010) of monthly changes of both maximum and average monthly air temperatures at the four collection sites. In Okayama, average temperatures from late June through September are higher than 25 °C. Warm climate enables L. japonicus to continue colony development for a long period of months, as evidenced by the oviposition activity of queens and larval development at 25 °C (Fig. 1).
In Shizuku-ishi, average monthly temperatures are lower than 25 °C, even in summer. However, the maximum monthly temperature rises to approximately 30 °C in August. Possibly, new queens produce workers before winter of the first year. In the Shizuku-ishi population, continuous oviposition and larval development at 25 °C must be adaptive for successful colony foundation.
In Hakodate also, the maximum temperature in August is higher than 25 °C, although the average temperature is lower than 25 °C. Warm temperatures in summer are likely to enable workers to emerge before winter. Active colony development at 25 °C (Fig. 1) agrees with this inference, although natural colony development has not been examined outdoors.
The colony development pattern of the Kitami population differed from those of the Hakodate, Shizuku-ishi, and Okayama populations. The eggs, larvae, and pupae were fewer than those of the other populations, irrespective of temperature (Table 2). At 25 °C, the daily change in the number of larvae was slight. In addition, pupae were few (Fig. 1), indicating that larval diapause was induced even at warm temperatures. At other lower temperatures, even hatching was inhibited strongly (Figs. 2, 34; Table 2). These results indicate that colony development in the Kitami population is suppressed strongly in the first year of the queen’s life.
Average monthly temperatures in Kitami are lower than those of the other three localities (Fig. 5). The average temperature and maximum temperature in August are, respectively, 19.7 °C and 24.3 °C. Under such cool climate conditions, adult workers are unlikely to emerge before the arrival of winter, even if queens started to found colonies soon after nuptial flight. Queens of L. japonicus eat no food until the emergence of the first workers. They use reserved nutrients to rear the larvae of the first brood (Kamitani et al. 2015; Nakamura et al. 2017). It must be important for colony foundation whether adult workers emerge before winter or not. Larva-rearing queen mortality would soar if they were to pass the winter with no workers. Therefore, even at high temperatures, the induction of diapause in both queens and larvae is likely to be an adaptive strategy in the absence of workers, in addition to reduced oviposition. To pass winter as solitary queens without larvae might play an important role in the successful foundation of colonies under cool climate conditions.
These results indicate that, for temperature response related to colony development of L. japonicus, an apparent geographical difference exists between the two northern localities: Kitami and Hakodate. Variations among southern populations are not large, although some difference is observed between the Hakodate and Shizuku-ishi populations. In a closely related species, L. niger, colonies of two Russian populations develop differently (Kipyatkov et al. 2004). The proportion of the colonies that produced workers in the Saint Petersburg (59.6°N) population is much lower than that of the colonies in the Belgorod (50.4°N) population. In ant populations of high latitudes, the suitable period for larval development is short. Therefore, local temperatures are likely to exert important selective pressure on the timing of diapause induction and on colony development.
Endogenous induction of diapause
Lasius japonicus queens show endogenous periodicity in oviposition activity at moderate or low temperatures (Nakamura et al. 2017), or under high light intensity (Ohta et al. 2017). Such propensities are the same as those observed for other ant species inhabiting high-latitude regions (Kipyatkov 1993, 2001). Kuroki et al. (2018) reported that this endogenous periodicity is probably related to a biological clock. Oviposition periodicity regulated by the innate clock would enable queens to terminate oviposition before winter in a stable underground environment. In the present study, L. japonicus queens of the Kitami population ceased oviposition even at warm temperatures such as 25 °C and 20 °C (Figs. 1 and 2), supporting our second hypothesis. The endogenous oviposition cycle is important for the termination of colony development in autumn (Kuroki et al. 2018).
In conclusion, for new queens of L. japonicus, solitary overwintering without rearing larvae, not consuming nutrients, likely plays an important role in the foundation of colonies in high-latitude regions. For populations of L. japonicus in high-latitude regions, where successful termination of oviposition is crucially important, endogenous induction of queens’ reproductive diapause is extremely important, even at high temperatures.
References
Beck SD (1980) Insect photoperiodism, 2nd edn. Academic Press, New York, p 387
Bradshaw WE, Holzapfel CM (2007) Evolution of animal photoperiodism. Annu Rev Ecol Evol Syst 38:1–25
Carton Y, Claret J (1982) Adaptative significance of a temperature induced diapause in a cosmopolitan parasitoid of Drosophila. Ecol Entomol 7:239–247
Danilevskii AS (1961) Photoperiodism and seasonal development of insects. (Translated by Hidaka T, Masaki S in 1966 from the Russian edition, Leningrad University Press, Leningrad.) University of Tokyo Press, Tokyo (in Japanese)
Danks HV (1987) Insect dormancy: an ecological perspective. Biological survey of Canada monograph series no. 1
Denlinger DL (2009) Diapause. In: Resh VH, Cardé RT (eds) Encyclopedia of insects, 2nd edn. Academic Press, San Diego, pp 267–271
Gotoh T, Kameyama Y (2014) Low temperature induces embryonic diapause in the spider mite, Eotetranychus smithi. J Insect Sci 14:1–8
Ito K, Nakata T (2000) Geographical variation of photoperiodic response in the females of a predatory bug, Orius sauteri (Poppius) (Heteroptera: Anthocoridae) from northern Japan. Appl Entomol Zool 35:101–105
JADG (Japanese Ant Database Group) (2003) Ants of Japan. Gakken, Tokyo, 196 pp. (in Japanese)
Kamitani S, Asakura K, Nakamura K (2015) Effects of environmental factors on life cycle regulation in Lasius japonicus Santschi (Formicidae). Sociobiology 62:467–473
Kipyatkov VE (1993) Annual cycles of development in ants: diversity, evolution, regulation. Proc Colloq Soc Insects 2:25–48
Kipyatkov VE (2001) Seasonal life cycles and the forms of dormancy in ants (Hymenoptera: Formicoidea). Acta Soc Zool Bohem 65:211–238
Kipyatkov VE (2006) The evolution of seasonal cycles in cold-temperate and boreal ants: patterns and constraints. In: Kipyatkov VE (ed) Life cycles in social insects: behaviour, ecology and evolution. St. Petersburg University Press, St. Petersburg, pp 63–84
Kipyatkov VE, Lopatina EB (1993) The regulation of annual cycle of development in the ants of the subgenus Serviformica (Hymenoptera, Formicidae). Proc Colloq Soc Insects 2:49–60
Kipyatkov VE, Lopatina EB (2002) Reaction norm in response to temperature may change to adapt rapid brood development to boreal and subarctic climates in Myrmica ants (Hymenoptera: Formicidae). Eur J Entomol 99:197–208
Kipyatkov VE, Lopatina EB, Imamgaliev AA, Shirokova LA (2004) Effect of temperature on rearing of the first brood by the founder females of the ant Lasius niger (Hymenoptera, Formicidae): latitude-dependent variability of the response norm. J Evol Biochem Physiol 40:165–175
Kohno K (1997) Photoperiodic effect on incidence of reproductive diapause in Orius sauteri and O. minutus (Heteroptera: Anthocoridae). Appl Entomol Zool 32:644–648
Kuroki I, Tagawa J, Nakamura K (2018) Endogenous periodicity in egg-number fluctuation in Lasius japonicus (Formicidae). Biol Rhythm Res https://doi.org/10.1080/09291016.2018.1427600
Lees AD (1955) The physiology of diapause in arthropods. Cambridge University Press, London, p 162
Masaki S (1961) Geographic variation of diapause in insects. Bull Fac Agric, Hirosaki Univ 7:66–98
Nakamura K, Fujiyama M, Ohta K (2017) Effect of temperature on queen oviposition and seasonal colony development in Lasius japonicus (Hymenoptera: Formicidae). Appl Entomol Zool 52:107–112
Ohta K, Yuge C, Nakamura K (2017) Effects of photoperiod and light intensity on queen oviposition and colony development in Lasius japonicus (Hymenoptera: Formicidae). Jpn J Appl Entomol Zool 61:17–23 (in Japanese with English abstract)
Schwander T, Humbert JY, Brent CS, Cahan SH, Chapuis L, Renai E, Keller L (2008) Maternal effect on female caste determination in a social insect. Curr Biol 18:265–269
Tauber MJ, Tauber CA, Masaki S (1986) Seasonal adaptations of insects. Oxford University Press, New York, p 426
Zar JH (2010) Biostatistical analysis, 5th edn. Prentice Hall, Upper Saddle River, p 960
Zhang JJ, Desneux N, Benelli G, Zang LS, Du WM, Ruan CC (2017) Geographic variation of diapause induction rates in Trichogramma dendrolimi (Hymenoptera: Trichogrammatidae) in China. J Econ Entomol 110:386–391
Acknowledgements
We thank K. Ohta, M. Fujiyama, T. Ohtsuki, and C. Yuge for helping us in this study. Thanks are also extended to H. Takasaki for manuscript preparation and to M. Natori for statistical advice.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kuroki, I., Tagawa, J. & Nakamura, K. Geographic variation of temperature effects on initial colony development of Lasius japonicus (Hymenoptera: Formicidae). Appl Entomol Zool 54, 175–183 (2019). https://doi.org/10.1007/s13355-019-00610-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13355-019-00610-8