Abstract
Temperature is a major driver of biological phenomena, from metabolism to ecological interactions and rates of evolutionary diversification. However, species vary greatly in their thermal tolerance, as well as the temperature under which they perform best. This study aimed to investigate the effect of experimental manipulation of environmental temperatures on the individual mortality and phenotypic composition of colonies of Melipona interrupta. To fulfill these objectives, 30 colonies in equivalent developmental conditions were artificially subjected to different temperatures. Temperatures were monitored by thermo-hygrometers, and immature mortality and sex and caste ratios were observed in brood combs during 14 months. A strong effect of external temperature on immatures was detected on deviations from 28 to 30 °C (the natural average temperature inside the colony), causing an increase in mortality. Likewise, a significant effect of temperature on sex ratio was detected, with male:female ratio decreasing at temperatures below and above 28–30 °C. Lastly, there was no clear evidence for an effect of temperature on caste ratio, although queens appeared to become relatively more frequent at warmer temperatures. The results of this study allow us to conclude that anthropogenic changes, whose effect can be extrapolated to the similar natural changes, that modify the environmental temperatures to which M. interrupta colonies are exposed are likely to compromise their survival, mainly through individual mortality.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Temperature is a major driver of biological phenomena, from metabolism to ecological interactions (Brown et al. 2004) and rates of evolutionary diversification (Allen et al. 2006). However, species vary greatly in their thermal tolerance, as well as the temperature under which they perform best. While species diversity generally increases closer to the tropics (Mittelbach et al. 2007), tropical ectotherms tend to have narrower thermal tolerances and experience environmental temperatures closer to their tolerance limits (Deutsch et al. 2008). Thus, even relatively modest changes in their thermal regime may have important effects on their populations (Palmer et al. 2017). Such changes are likely given the ongoing tropical deforestation (DeFries et al. 2010) and global climate change (Parmesan 2006), but recent investigations suggest complex responses of organisms to shifts in thermal regime, stressing the current uncertainty in predicting such responses (Clusella-Trullas et al. 2011; Bellard et al. 2012) and the need for studies on thermal biology in order to help forecasting the fate of species and ecosystems (Chown et al. 2010).
Stingless bees (Meliponini) comprise a diverse clade of eusocial insects which are important pollinators in the tropics (Heard 1999; Kerr et al. 2001). Stingless bees evolved a cryptic nesting biology, so that colonies typically build nests in well-insulated cavities, such as tree hollows (Roubik 2006). With nesting behavior playing a major role in thermoregulation, selection for active mechanisms of thermoregulation may have been relaxed (Engels et al. 1995; Jones and Oldroyd 2006). Indeed, although nest internal temperatures appear to be higher and more stable than external ones, they fluctuate along with evironmental temperatures (Roubik and Peralta 1983). Usually, this is perhaps of little functional significance, as colonies inhabiting tropical forests are unlikely to experience extreme temperatures consistently. However, stingless bee assemblages are highly affected by land-use change in the tropics, particularly by forest clearing (Brosi et al. 2007), which is likely to subject colonies to wider thermal amplitudes and longer extreme temperatures. This, coupled to climate change, may pose important threats to their persistence.
Deviations of optimal nest temperatures are expected to impact the physiological performance of individuals and, eventually, their survival (Couto and Camillo 2007; Lopes et al. 2011). However, bee colonies are composed of different phenotypes that may respond differently to temperature variation. Typically, a stingless bee colony is composed of a mated queen, a few hundred female workers, and dozens of males. Depending on the time of the year, up to 15% of the individuals in a colony may be males, which are determined by arrhenotokous parthenogenesis (Kerr 1996; Francini et al. 2012). However, female differentiation into queen or worker phenotype varies within eusocial bees (Kerr et al. 1966, 1996; Nogueira-Neto 1997).
In Melipona, one of the most diverse Neotropical genera of stingless bees, queen production, has been proposed by Kerr (1950, 1974) to be under a genetic predisposition mechanism combined with diet. According to this hypothesis, caste would be determined by two genes, each with two allelles, so that double heterozygosis would code for the queen phenotype. Thus, a queen:worker ratio of up 1:4 is predicted if the larvae receive enough food. In fact, such ratio is not commonly observed in nature, suggesting that the expression of the queen phenotype may be influenced by environmental factors such as food availability and temperature (Brito et al. 2013).
Although temperature can affect both survival and phenotpic composition of colonies, no study has directely compared them, and thus, their relative impacts remain unclear. In this study, we investigated the effect of experimental manipulation of environmental temperatures on the individual mortality and phenotypic composition of colonies of Melipona interrupta. Considering the known influence of temperature on other stingless bee species, we expected that immature mortality would increase along with deviations from those temperatures normally experienced by colonies. We further hypothesized that temperature would influence colony composition in terms of both sex and caste, either due to differences in thermal tolerance between phenotypes or by directly affecting caste polyphenism. Thus, we expected queen:worker ratio to decrease with temperature.
Material and methods
Biological material
Thirty colonies of M. interrupta were chosen from the meliponary of the Grupo de Pesquisasem Abelhas (GPA) at the Instituto Nacional de Pesquisas da Amazônia (INPA), Manaus, Brazil. Colonies were in equivalent conditions of development, according to the pattern of grades established by Kerr et al. (1996) (i.e., grades 5 and 6 that are self-sufficient colonies). Bees were maintained in INPA model standardized modular boxes made of wood, measuring internally 15 × 15 × 15 cm (Carvalho-Zilse et al. 2012).
Sampling design and experiment assembly
Colonies were haphazardly assigned to different temperatures, in order to cover a gradient spanning temperatures below and above the average of 30 °C (Table 1) recorded within colonies of Melipona fasciculata by Kerr (1996), as well as the natural range of thermal variation in the region (see below). Six colonies were maintained in the meliponary at ambient temperature to represent natural thermal conditions (from now on referred as environmental temperature), whereas the remaining colonies were maintained in laboratory under experimentally thermal regimes. Under natural, regional conditions, extreme temperatures occur mainly as daily peaks (cool nights and warmer midday), so that colonies may be exposed to extreme temperatures for relatively short periods. However, in the experiment, such temperatures were maintained consistently over time, in order to reveal bee responses to heat and cool stress. This was achieved by placing the wood standardized modular boxes inside polystyrene boxes (44 × 35 × 40 cm) covered with cardboard lids where incandescent lamps (25, 40, 60 W) were coupled (Fig. 1). The microambient created between the polystyrene box and the wood box from now on will be referred as controlled external temperature.
Schematic illustration of the Melipona interrupta colonies treated under laboratory conditions. Air conditioner was used for cooling the laboratory environment at 25 °C; light bulbs inside polystyrene boxes were used for heating the colonies from 31 to 38 °C. In the illustration, the colony kept between 31 and 33 °C was used as an example. The colonies’ external and internal maximum and minimum temperatures were measured with the thermo-hygrometers attached to the outside of the wood box with an internal sensor, represented here by the black square with the strip
Temperatures of those microambients were regulated manually by adjusting the lamp’s power and height using a thermometer for reference. This method allowed the generation of a temperature gradient ranging, on average, from 24.5 to 37.3 °C around colonies (controlled external temperatures) (see “Temperature measurement” section).
Laboratory colonies were coupled to a tube about 40 cm long connecting it to the outside environment through a hole in the wall of the lab, so that the bees had free access to the external natural environment for foraging. To prevent ant attacks to the colonies, a mixture of talc and powdered sulfur was placed at the bottom of the standardized boxes.
The experiment lasted 14 months (from August 2012 to October 2013). However, the time colonies lasted in the experiment depended on their survival under the induced thermal conditions, so that there were some variations in the time of the year each colony began to be monitored. To control for any effect this may have had on the experiment, time of the year was used as a covariate in the analyses (see “Statistical analyses” section).
Temperature measurement
The colonies external and internal maximum and minimum temperatures were monitored daily with calibrated thermo-hygrometers attached to the outside of the wood box with an internal sensor. Temperatures were monitored at 17:00 o’clock, after the maximum and the minimum of the day (daily peaks).
The daily maximum and minimum temperatures were used to compute daily average temperatures, which were then similarly averaged throughout the duration of each colony in the experiment. This average controlled external temperature varied from 24.57 to 37.33 °C among colonies, and was strongly correlated to both the average internal maximum (r = 0.89) and minimum (r = 0.75). Thus, it was used to represent the temperature gradient in all subsequent analyses.
Average daily temperature in the region where the study was conducted ranged from 24 to 31.98 °C during the experiment (INMET 2014). Thus, the artificially created thermal gradient included the natural range of temperature, as well as more extreme temperatures mimicking conditions of heat stress.
Monitoring sex and caste ratios
After the experiment was set up, colonies were monitored daily until they started building uppermost combs and laying eggs on them (Fig. 2a). Then, approximately 30 days were counted in order to remove the uppermost comb, which is the period required for individuals to reach the pupal stage in Melipona (Kerr et al. 1996). At this stage, the identification of sex and caste becomes possible. After removal, the colony was monitored until it produced another uppermost comb. One to three combs were removed sequentially from each colony (Table 2), depending on their availability throughout the experiment.
The brood cells of removed combs were uncapped (Fig. 2b) and inspected for dead individuals, which were identified by their lack of movement and/or dried out aspect; these were all eggs or larvae. The pupae were visually identified to sex and caste (Fig. 2c) under stereomicroscope using morphological traits such as eye size, interorbital distance, presence of corbicula in the tibia of the third pair of legs, and bifurcation of the nails (Carmargo et al. 1967). The numbers of dead immatures, workers, queens, and males were counted. Voucher specimens were deposited at the Entomological Collection of INPA.
Statistical analyses
Analyses were performed using colonies as sampling units. As variation in the experiment occurred at two levels (among combs of the same colony, and among colonies), we assessed the relative magnitude of variation deriving from these sources by computing the coefficient of variation (CV) for all counts per comb, as well as the CV of average counts per colony.
The dependent variables used were the average number of dead immatures, the average sex ratio, and the average caste ratio. Sex ratio was defined as the ratio of males to females, and caste ratio as the ratio of queens to workers. For a few colonies, such ratios could not be computed (e.g., if the computation involved dividing by zero), and those were excluded from the respective analyses.
To assess the effect of average external temperature on individual mortality and composition, we employed generalized additive models (GAM). Such models extended traditional regression models in that they assume no a priori shape for the relations being modeled; rather, they attempt to find the best description for the data (Zuur et al. 2009). As this runs the risk of overfitting the data, a constraint on model complexity is imposed. Here, we used function “gam” from package “mgcv” (Wood 2006) of the program R 3.0.2 (R Development Core Team 2013) to model each dependent variable as a function of temperature. We set basis dimension “k” to 3, which allows the fitting of reasonable curves while guarding against excessive model complexity (Zuur et al. 2009). In all analyses, we included the time at which the colony began to be monitored (coded as a quantitative variable, with an integer attributed to each month), in order to control for any natural changes in colony mortality or caste/sex ratios throughout the year. All remaining computations and plots were also performed in R 3.0.2.
Results
The amplitude of environmental temperatures experienced by colonies in natural ambient (control group) ranged from 6.21 to 9.04 °C, being on average 7.24 °C, whereas the amplitude experienced by laboratory colonies (treatments) ranged from 1.54 to 2.83 °C, with an average of 2.18 °C. Maximum daily external temperature, averaged for each colony throughout its duration in the experiment, ranged from 25.6 to 37.1 °C, while minimum daily external temperature ranged from 21.7 to 27.9 °C (supplementary data). The colonies internal temperatures had a lower amplitude, however under clear influence of the external temperature, therefore was used the external temperatures in the analysis.
Throughout the experiment, between-colony variability was consistently larger than that among combs of the same colony: the coefficient of variation of average number of dead immatures per colony was 3.57, while its average for counts within colonies was 1.48, ranging from 0.59 to 3.03. Thus, between-colony variation was 2.4 times larger than within-colony variation. Likewise, variation in queen and worker numbers among colonies was 2 to 2.5 times higher than that among combs of the same colony: the coefficient of variation of worker numbers between colonies was 2.43, while the average coefficient of variation of worker numbers in combs of the same colony was 1.25 (ranging from 0.31 to 2.82). For queens, the coefficient of variation numbers between colonies was 2.99 and in combs of the same colony was 1.59 (ranging from 0.69 to 3.03). Comparatively, for males, count among colonies was 6.29, while the average coefficient of variation numbers among combs of the same colony was 2.43 (ranging from 0.83 to 3.03).
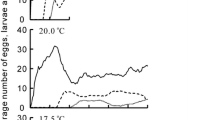
The influence of external temperature on immature mortality was evidenced by the generalized additive model, which detected a marked curvilinear pattern (Fig. 3a and Table 3). In particular, the model explained 87% of the variation in mortality, with deviations from around 28 to 30 °C causing an increase in mortality. Likewise, a significant effect of temperature on sex ratio was detected, while controlling for an effect of period of the year (Fig. 3b and Table 3). Namely, male:female ratio tended to decrease at temperatures below and above the range 28–30 °C. However, this effect was weaker than that detected for mortality, with the model explaining only 38% of the variation in sex ratio. Lastly, there was no evidence for an effect of temperature on caste ratio (Fig. 3c and Table 3), although a single colony appeared to deviate from the majority of the data (bottom right of Fig. 3c). A post hoc analysis using the same model but excluding this colony suggested a marginally significant effect of temperature on caste ratio (F = 3.28, P = 0.06), with queens becoming relatively more frequent at warmer temperatures.
Discussion
To sustain successful reproduction, bees need to maintain a homeostatic nest. This depends heavily on the temperature of the brood area, which is supported in stingless bees by nesting in insulated cavities, building an insulating involucrum within the nest, and by the brood itself, which generates heat that helps maintaining nest temperature (Roubik and Peralta 1983; Engels et al. 1995). Here, we have shown that consistent change in controlled temperatures simulating environmental temperatures caused marked mortality of immatures in colonies of M. interrupta, despite their thermoregulatory capacity. Namely, a high percentage of dead immatures (media over 70%) was observed in colonies exposed to temperatures 4 °C above the average temperature experienced by untreated colonies. The high mortality of individuals weakened especially the colonies exposed to temperatures above 36 °C that eventually died (Fig. 4). In a pilot essay, the colonies were exposed to temperatures above 39 °C, in which, the queen stopped the oviposition drastically, cerumen melted culminating with the colony collapse in only 24 h. This is in line with previous observations that environmental temperature can have important impacts on immature development and survival (Couto and Camillo 2007; Lopes et al. 2011). Interestingly, the fact that maximum average daily temperature in the region where the study was performed was slightly higher (31.98 °C) than the average external temperatures where mortality was smaller (28–30 °C) suggests that M. interrupta is currently experiencing environmental conditions close to its upper optimal thermal limit, as suggested for tropical species relative to those occurring at higher latitudes (Deutsch et al. 2008).
The decrease in male:female ratio beyond the temperature range experienced by untreated colonies suggests that deviations from the thermal optimum either cause a relative decrease in male production or a relative increase in female production. The coefficients of variation endorse that the measured effect was not natural difference between combs but influence of the controlled external temperature on the colonies. However, temperature is not expected to directly affect sex determination in hymenopterans because males are mostly haploid and, thus, genetically determined (Whiting 1943; Kerr 1997). On the other hand, the relative numbers emerging of each sex may differ if they have distinct thermal tolerances. Although thermal properties of stingless bees can be predicted from morphological features such as body size and coloration (Pereboom and Biesmeijer 2003), males and females cannot be readily differentiated during immature stages, just from pupal stage onwards (Kerr 1974; Brito et al. 2015). This suggests that, if the temperature-dependent variation in sex ratio observed in this study is due to difference in thermal tolerance between sexes, such difference has mainly a physiological basis. Notoriously in our study, a larger number of queens was observed in elevated temperatures, which may suggest that they are more resistant to high temperatures compared to the workers. Such possibility should be further investigated.
The influence of temperature on stingless bee caste ratio has been reported for M. quadrifasciata anthidioides (Kerr et al. 1966), in which there was a decrease in the emergence of queens at elevated temperatures (33 and 34 °C). In work with M. scutellaris, Nunes-Silva et al. (2006) subjected individual in cocoon spinning larvae to heat shocks (42 °C for three times of 30 min interspersed with 30 min at 30 °C) and observed a dramatic decrease in the production of queens, indicating that temperature increase inhibits the development of queens. As only the queen produces fertile offspring, any effects of temperature on queen production could have long-term effects on colony reproduction and, thus, population maintenance. However, temperature had no clear effects on caste ratio in our experiment; even after excluding an outlying colony, any effect was only marginally significant, and queen:worker ratio actually appeared to increase at higher temperatures (Fig. 3c). Thus, at the colony level, any effects of temperature on caste ratio seem modest and, similarly to sex ratio, may be related to differential survival of castes rather than direct effects on caste differentiation.
Overall, our results suggest that even mild modification of the current thermal environment of M. interrupta may have strong, deleterious impacts on its populations. Such effects are likely to be driven by physical habitat change, such as deforestation. Indeed, there is evidence for strong effects of deforestation on Melipona diversity and distribution in the tropics, even where signifcant forest patches remain (Brown and Albrecht 2001). Further, stingless bee diversity seems more related to forest cover than to floral resource availability (Brosi 2009), suggesting that the populations may be more affected by physical features of the environment, such as the local thermal regime. These data make predictions that climate change will accelerate the risk of extinctions more alarming (Urban 2015), especially with the loss of habitat and food availability with changes in land use (Settele et al. 2016). In this context, our data prove that M. interrupta will be at great risk.
In our study, some points should be considered. First, part of the observed variation between colonies may come from differences in the age of physogastric queens, which can influences the size and composition of the colony and, thus, its functioning as Barbosa-Costa and Carvalho-Zilse (2013) observed for another stingless bee, Scaptotrigona xanthotricha. Although this could not be completely controlled in the experiment, using colonies with similar grades of development as we did is likely to minimize any such effect. Second, ambient colonies were kept outside of laboratory and, thus, experienced a range of environmental variation that was not experienced by laboratory colonies. While this may cause colony mortality and composition to vary as function of factors other than temperature, it is clear from the results (Table 3) that average external temperature explained most of the variation in immature mortality. This strongly suggests that temperature was the main factor driving variation among colonies in the experiment, at least with respect to mortality.
Our experiment has shown that external temperatures lower than 28 °C and higher than 30 °C increased the mortality of immatures in colonies of M. interrupta, and also provided clear evidence for an effect on sex ratio. While caste composition is expected to have an important role in colony functioning, our results clearly show that temperature effects on mortality are much stronger than those on both sex and caste ratios. Therefore, the expected increase in tropical deforestation (DeFries et al. 2010) and climate change (IPCC 2013) are likely to impose severe threats to M. interrupta populations.
References
Allen AP, Gillooly JF, Savage VM, Brown JH (2006) Kinetic effects of temperature on rates of genetic divergence and speciation. Proc Natl Acad Sci U S A 103(24):9130–9135. https://doi.org/10.1073/pnas.0603587103
Barbosa-Costa K, Carvalho-Zilse GA (2013) Processo de oviposição da abelha da Amazônia ScaptotrigonaxanthotrichaMoure, 1950. In: Bermúdez EGC, Teles BR, Keppler RF (eds) Entomologia na Amazônia brasileira, 1st edn. Editora INPA, Manaus, pp 91–106
Bellard C, Bertelsmeier C, Leadley P, Thuiller W, Courchamp F (2012) Impacts of climate change on the future of biodiversity. Ecol Lett 15:365–377. https://doi.org/10.1111/j.1461-0248.2011.01736.x
Brito DV, Nunes RA, Pequeno PACL, Nunes-Silva CG, Carvalho-Zilse GA (2013) Differential environmental effects on caste allocation in two Amazonian Melipona bees. Apidologie 44(6):666–672. https://doi.org/10.1007/s13592-013-0215-8
Brito DV, Silva CGN, Hasselmann M, Viana LS, Astolfi-Filho S, Carvalho-Zilse GA (2015) Molecular characterization of the gene feminizer in the stingless bee Meliponainterrupta (Hymenoptera: Apidae) reveals association to sex and caste development. Insect Biochem Mol Biol 66:24–30
Brosi BJ, Daily GC, Shih TM, Oviedo F, Durán G (2007) The effects of forest fragmentation on bee communities in tropical countryside. J Appl Ecol 45(3):773–783. https://doi.org/10.1111/j.1365-2664.2007.01412.x
Brosi BJ (2009) The complex responses of social stingless bees (Apidae: Meliponini) to tropical deforestation. For Ecol Manag 258(9):1830–1837. https://doi.org/10.1016/j.foreco.2009.02.025
Brown J, Gillooly J, Allen A, Savage V, West G (2004) Toward a metabolic theory of ecology. Ecology 85(7):1771–1789 Retrieved from:http://www.esajournals.org/doi/abs/10.1890/03-9000
Brown JC, Albrecht C (2001) The effect of tropical deforestation on stingless bees of the genus Melipona (Insecta: Hymenoptera: Apidae: Meliponini) in central Rondonia, Brazil. J Biogeogr 28:623–634. https://doi.org/10.1046/j.1365-2699.2001.00583.x
Carmargo JMF, Kerr WE, Lopes CR (1967) Morfologia externa de Melipona (Melipona) marginata Lepeletier (Hymenoptera, Apidae). Pap Avulsos Zool 20:229–258
Carvalho-Zilse GA, Vilas-Boas HC, Costa KB, Nunes-Silva CG, Souza MT, Fernandes RS (2012) Meliponicultura na Amazônia. INPA, Manaus
Chown S, Hoffmann A, Kristensen T, Angilletta M, Stenseth N, Pertoldi C (2010) Adapting to climate change: a perspective from evolutionary physiology. Clim Res 43(1):3–15. https://doi.org/10.3354/cr00879
Clusella-Trullas S, Blackburn TM, Chown SL (2011) Climatic predictors of temperature performance curve parameters in ectotherms imply complex responses to climate change. Am Nat 177(6):738–751. https://doi.org/10.1086/660021
Couto RM, Camillo E (2007) Influence of temperature on the immatures mortality of Centris (Heterocentris) analis (Hymenoptera, Apidae, Centridini). Iheringia, Sér Zool 97(1):51–55. https://doi.org/10.1590/S0073-47212007000100008
DeFries RS, Rudel T, Uriarte M, Hansen M (2010) Deforestation driven by urban population growth and agricultural trade in the twenty-first century. Nat Geosci 3:178–181. https://doi.org/10.1038/ngeo756
Deutsch C A, Tewksbury J J, Huey R B, Sheldon K S, Ghalambor C K, Haak D C, Martin P R (2008) Impacts of climate warming on terrestrial ectotherms across latitude. Proc Natl Acad Sci U S A. Retrieved from: http://www.pnas.org/content/105/18/6668.short
Engels W, Rosenkranz P, Engels E (1995) Thermoregulation in the nest of the Neotropical stingless bee Scaptotrigonapostiça and a hypothesis on the evolution of temperature homeostasis in highly eusocial bees. Stud Neotrop Fauna E30(4):193–205. https://doi.org/10.1080/01650529509360958
Francini IB, Nunes-Silva CG, Carvalho-ZilseG A (2012) Diploid male production of two Amazonian Melipona bees (Hymenoptera: Apidae). Psyche 1:1–7
Heard TA (1999) The role of stingless bees. Annu Ver Entomol 44:183–206. https://doi.org/10.1146/annurev.ento.44.1.183
INMET (2014) http://www.inmet.gov.br. Accessed 5 Nov 2014
IPCC (2013) Climate change 2013: the physical science basis. Contribution of working group I to the fifth assessment report of the intergovernmental panel on climate change. Stocker T F, Qin D, Plattner G K, Tignor M, Allen S K, Boschung J, Nauels a, Xia Y, Bex V, Midgley P M (ed). Cambridge University press, Cambridge, 1535p
Jones J, Oldroyd B (2006) Nest thermoregulation in social insects. Adv Insect Physiol 33(06):153–191. https://doi.org/10.1016/S0065-2806(06)33003-2
Kerr WE (1950) Genetic determination of castes in the genus Melipona. Genetics 35(2):143–152 Retrieved from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1209477&tool=pmcentrez&rendertype=abstract
Kerr WE (1974) Sex determination in bees. III Caste determination and genetic control in Melipona. Insect Soc 21:357–368
Kerr WE (1996) Biologia e manejo da tiúba: a abelha do Maranhão. EDUFMA, São Luis
Kerr WE (1997) Sex determination in honey bees (Apinae and Meliponinae) and its consequences. Braz J Genet 20:601–611
Kerr WE, Carvalho GA, Nascimento VA (1996) Abelha Uruçu: Biologia, Manejo e Conservação. Fundação Acangaú, Belo Horizonte
Kerr WE, Carvalho GA, Coletto-Silva A, Assis MGP (2001) Aspectos Pouco Mencionados da Biodiversidade Amazônica.InBiodiversidade, Pesquisa e Desenvolvimento na Amazônia. Parcerias Estratégicas, Ministério da Ciência e Tecnologia 12:20–41
Kerr WE, Stort AC, Montenegro MJ (1966) Importância de alguns fatores ambientais na determinação das castas no gênero Melipona. An Acad Bras Ciênc 38(1):151–168
Lopes MTR, Barbosa AL, Neto JMV, Pereira FM, Camargo RCR, Ribeiro VQ, Souza BA (2011) Alternativas de sombreamento para apiários. Pesquisa Agropecuária Tropical 41(3):299–305. https://doi.org/10.5216/pat.v41i3.8919
Mittelbach GG, Schemske DW, Cornell HV, Allen AP, Brown JM, Bush MB, Harrison SP, Hurlbert AH, Knowlton N, Lessios H, McCain CM, McCune AR, McDade L, McPeek M, Near TJ, Price TD, Ricklefs RE, Roy K, Sax DF, Schluter D, Sobel JM, Turelli M (2007) Evolution and the latitudinal diversity gradient: speciation, extinction and biogeography. Ecol Lett 10(4):315–331. https://doi.org/10.1111/j.1461-0248.2007.01020.x
Nogueira-Neto P (1997) Vida e criação de abelhas indígenas sem ferrão. Editora Nogueirapis, São Paulo
Nunes-Silva CG, Kerr WE, Bonetti AM, Carvalho-Zilse GA (2006) Effect of juvenile hormone III and heat shock in caste determinations in Melipona scutellaris Latreille, 1811 (Hymenoptera, Apidae). Magistra 18(4):277–280
Palmer G, Platts PJ, Brereton T, Chapman JW, Dytham C, Fox R, Pearce-Higgins J, Roy DB, Hill JK, Thomas CD (2017) Climate change, climatic variation and extreme biological responses. Phil Trans R Soc B 372:20160144. https://doi.org/10.1098/rstb.2016.0144
Parmesan C (2006) Ecological and evolutionary responses to recent climate change. Annu Rev Ecol Evol Syst 37(2006):637–669. https://doi.org/10.2307/annurev.ecolsys.37.091305.30000024
Pereboom JJM, Biesmeijer JC (2003) Thermal constraints for stingless bee foragers: the importance of body size and coloration. Oecologia 137(1):42–50. https://doi.org/10.1007/s00442-003-1324-2
Development Core Team R (2013) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna ISBN 3-900051-07-0, http://www.R-project.org/
Roubik DW (2006) Stingless bee nesting biology. Apidologie 37:124–143. https://doi.org/10.1051/apido:2006026
Roubik D, Peralta F (1983) Thermodynamics in nests of two Melipona species in Brasil. Acta Amazon 13(2):453–466 Retrieved from: http://agris.fao.org/agris-search/search.do?recordID=US201302048790
Settele J, Bishop J, Potts SG (2016) Climate change impacts on pollination. Nature plants 2(7):16092. https://doi.org/10.1126/science.aaa4984
Urban MC (2015) Accelerating extinction risk from climate change. Science 348(6234):571–573. https://doi.org/10.1038/nplants.2016.9210.1038/nplants.2016.92
Whiting, P W (1943) Multiple Alleles in complementary sex determination of Habrobracon. Genetics 28(5):365–382
Wood SN (2006) Generalized additive models: an introduction with R. Chapman and Hall/CRC
Zuur AF, Ieno EM, Walker NJ, Saveliev AA, Smith GM (2009) Mixed effects models and extensions in ecology with R. Springer, New York
Acknowledgments
We are grateful to the anonymous referees for the constructive suggestions. We acknowledge the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for a MSc scholarship to the first author and the Instituto Nacional de Pesquisas da Amazônia (INPA), Graduate Program in Entomology (PPG-Entomologia) for the logistical support.
Funding
We thank the sponsoring agencies FAPEAM and CNPq and CENBAM (Instituto Nacional de Ciência e Tecnologia-Centro de Estudos Integrados da Biodiversidade Amazônica) for the financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by: Sven Thatje
Electronic supplementary material
ESM 1
(DOCX 43 kb)
Rights and permissions
About this article
Cite this article
Becker, T., Pequeno, P.A.C.L. & Carvalho-Zilse, G.A. Impact of environmental temperatures on mortality, sex and caste ratios in Melipona interrupta Latreille (Hymenoptera, Apidae). Sci Nat 105, 55 (2018). https://doi.org/10.1007/s00114-018-1577-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00114-018-1577-6