Abstract
A 2,175-bp modified gene (cry11Ba-S1) encoding Cry11Ba from Bacillus thuringiensis subsp. jegathesan was designed and the recombinant protein was expressed as a fusion protein with glutathione S-transferase in Escherichia coli. The recombinant Cry11Ba was highly toxic against Culex pipiens mosquito larvae, being nine and 17 times more toxic than mosquitocidal Cry4Aa and Cry11Aa from Bacillus thuringiensis subsp. israelensis, respectively. Interestingly, a further increase in the toxicity of the recombinant Cry11Ba was achieved by mixing with Cry4Aa, but not with Cry11Aa. These findings suggested that Cry11Ba worked synergistically with Cry4Aa, but not with Cry11Aa, in exhibiting toxicity against C. pipiens larvae. On the other hand, the amount of Cry toxin bound to brush border membrane vesicles (BBMVs) did not significantly change between individual toxins and the toxin mixtures, suggesting that the increase in toxins binding to BBMVs was not a reason for the observed synergistic effect. It is generally accepted that synergism of toxins is a potentially powerful tool for enhancing insecticidal activity and managing Cry toxin resistance in mosquitoes. The mixture of Cry4Aa and Cry11Ba in order to increase toxicity would be very valuable in terms of mosquito control.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mosquito control is a central means by which mosquito-borne diseases such as malaria, viral hemorrhagic fever and lymphatic filariasis can be prevented. Chemical pesticides have traditionally been used for mosquito control, but these agents can have negative impacts on other organisms. Consequently, biological control methods have been promoted as an alternative approach to chemical control. Since the entomopathogenic bacterium, Bacillus thuringiensis subsp. israelensis (Bti) is highly specific for mosquito larvae, and safe for mammals, fish, birds and nondipteran insects, it has been used to control vector mosquitos for many years (Ben-Dov 2014). The mosquitocidal activity of Bti resides in three major Cry toxins (Cry4Aa, Cry4Ba, and Cry11Aa) and a Cyt toxin (Cyt1Aa). These Cry toxins show strong toxicity against Anopheles, Aedes, and Culex mosquito larvae (Otieno-Ayayo et al. 2008). On the other hand, Cyt1Aa has a low mosquitocidal activity and it synergizes with other Cry toxins (Crickmore et al. 1995; Pérez et al. 2005; Wirth et al. 1997, 2007; Wu et al. 1994). The application of Cry toxins is always accompanied by the risk of selecting for insecticide resistance in larval mosquito populations. Although resistance to Bti-based bioinsecticides has not yet been reported in mosquito populations in the field, it has been shown in the laboratory that heavy and continuous application of Bti-derived Cry toxin can induce resistance (Georghiou and Wirth 1997; Wirth et al. 2012). It is widely accepted that co-administration of multiple toxins that differ from each other in their mode of action can prevent or at least delay the onset of resistance. It is therefore desirable to find toxins with modes of action that differ from that of Bti in order to prolong the useful life of Bti-based bioinsecticides.
Bacillus thuringiensis subsp. jegathesan (Btj) has high potency as a bioinsecticide and contains Cry proteins that are distinct from Bti (Delécluse et al. 1995). Four major Cry toxins, Cry11Ba (Delécluse et al. 1995), Cry19Aa (Rosso and Delécluse 1997), Cry24Aa (Kawalek 1998), Cry25Aa (Kawalek 1998), and a Cyt toxin, Cyt2Bb (Cheong and Gill 1997), have been identified and characterized from Btj. In addition, three Cry toxins, Cry30Ca, Cry60Aa, and Cry60Ba, have recently been identified using liquid chromatography-tandem mass spectrometry (Sun et al. 2013). Among these, Cry11Ba is the most toxic against mosquito larvae (Breman et al. 2004), being ten times more toxic than the most active toxin of Bti (Delécluse et al. 1995).
In this study, the potency of Cry11Ba in combination with mosquitocidal Cry toxins from Bti was assessed for its application to mosquito control. A synthetic cry11Ba gene, cry11Ba-S1, was therefore designed and expressed in Escherichia coli cells. The mosquitocidal activity of the recombinant Cry11Ba was then analyzed by bioassay using Culex pipiens pallens Coquillet (Diptera: Culicidae) larvae. In addition, the recombinant Cry11Ba was mixed with the recombinant Cry4Aa or Cry11Aa in various ratios, and the potency of the toxin mixtures was compared against those of individual Cry toxins in order to assess the effect of synergistic toxicity. Our findings will contribute towards improving the insecticidal activity of Bti-based bioinsecticides and the management of insecticide resistance in mosquitoes.
Materials and methods
Construction of the synthetic gene
The 2,175-bp synthetic gene (cry11Ba-S1) encoding the Cry11Ba toxin was designed in accordance with the codon preference of E. coli genes (supplemental Fig. 1). The entire nucleotide sequence of cry11Ba-S1 was deposited in the DNA Data Bank of Japan, GenBank and European Molecular Biology Laboratory databases under accession no. LC153032. The cry11Ba-S1 gene, which was tailed with BamHI and XhoI cleavage sequences at the upstream and downstream termini, respectively, was synthesized using a recursive polymerase chain reaction procedure described previously (Hayakawa et al. 2008). The cry11Ba-S1 gene was inserted between the BamHI and XhoI sites of the expression vector pGEX 4T-2 (Healthcare Bio-Sciences, Uppsala) to generate pGST-Cry11Ba-S1.
Preparation of recombinant Cry toxins
The recombinant Cry11Ba was expressed in E. coli BL21 as a fusion protein with glutathione-S-transferase (GST-Cry11Ba) (supplemental Fig. 2). E. coli cells harboring pGST-Cry11Ba-S1 were cultured at 37°C in Terrific Broth medium containing ampicillin (100 μg/ml) until the optical density at 600 nm reached 0.4-0.6. The expression of GST-Cry11Ba was induced with 0.1 mM isopropyl β-D-1-thiogalactopyranoside at 30°C for 4 h. E. coli cells were harvested by centrifugation and cell pellets were washed with phosphate buffered saline (PBS). Upon disruption of the E. coli cells by sonication, the GST-Cry11Ba was purified using glutathione-Sepharose 4B (Healthcare Bio-Sciences) according to the manufacturer’s instructions.
In this study, we used Cry4Aa and Cry11Aa as the Bti Cry toxin. Although the mosquitocidal activity of Bti resides in at least three major Cry toxins (Cry4Aa, Cry4Ba, and Cry11Aa), the toxicity of Cry4Ba against Culex pipiens larvae is considerably weaker than that of Cry4Aa and Cry11Aa (Abdullah et al. 2003; Delécluse et al. 1993; Poncet et al. 1995). The expression vectors pGST-Cry4Aa-S1 (Hayakawa et al. 2008) and pGST11A (Yamagiwa et al. 2004) were used to prepare GST-Cry4Aa and GST-Cry11Aa, respectively (supplemental Fig. 2). Purified proteins were analyzed by sodium dodecyl sulfate (SDS)–polyacrylamide gel electrophoresis (PAGE). The protein concentration was estimated using a protein assay kit (Bio-Rad, Hercules, CA) with bovine serum albumin (BSA) as a standard, or by densitometric scanning with a cooled charge-coupled-device (CCD) camera system (Ez-Capture; ATTO, Tokyo) and analysis using image analysis software (CS analyzer version 3.0; ATTO, Tokyo).
Determination of mosquito-larvicidal activity
The mosquito-larvicidal activity of GST-Cry4Aa, -Cry11Aa and -Cry11Ba was determined by bioassays using C. pipiens larvae (3rd instar). Forty micrograms of the purified toxin was adsorbed onto 2 mg of latex beads (0.8-μm diameter; Sigma-Aldrich, MO) for 1 h at room temperature and then administered to the mosquito larvae as a diet. Mosquito larvae were reared from eggs supplied by the Research and Development Laboratory, Dainihon Jochugiku (Osaka). Bioassays were carried out in a 96-well microtiter plate with one larva per well and 48 larvae per assay. Mortality was recorded 48 h after the wells were inoculated with serially diluted latex beads to which Cry toxin had been bound. The 50 % lethal dose (LC50) was determined using probit analysis (Finney 1971). The experiments were repeated more than four times, and the average LC50 and SD were calculated.
In addition, GST-Cry11Ba was mixed with Bti Cry toxin (GST-Cry4Aa or -Cry11Aa) in various ratios [GST-Cry11Ba:Bti Cry toxin = 1:9, 3:7, 5:5, 7:3, 9:1 (w/w)], and the resulting toxin mixtures were used in a bioassay structured as described above. To assess the extent of any synergy between Cry11Ba and Bti Cry toxin, the synergistic effect values (LC50 expected/LC50 observed) obtained for each toxin mixture were calculated as described by Tabashnik (1992). In the formula, LC50 expected is the LC50 value of the model without interactive effects between Cry11Ba and Bti Cry toxin, and is calculated based on the LC50 value of individual toxins and the relative proportions in the mixture. For example, the expected LC50 value of the toxin mixture (m) containing toxin a (a) and b (b) at a ratio of x:y (x + y = 1) can be calculated as follows: LC50(m) = [x/LC50(a) + y/LC50(b)]−1. Basically, synergism for the toxicity can be expected in the toxin mixture showing synergistic effect value >1.
Binding of Cry toxins to the brush border membrane vesicle
Brush border membrane vesicles (BBMVs) were prepared from 7- to 10-day-old C. pipiens larvae (4th instar) as described previously (Howlader et al. 2010). Briefly, frozen larvae were resuspended and homogenized in ice-cold buffer (300 mM mannitol, 5 mM ethylene glycol tetraacetic acid, 50 mM TRIS–HCl, pH 8.0; MET) containing 24 mM MgCl2. The MET buffer was supplemented with a protease-inhibitor cocktail (Nacalai Tesque, Kyoto) at the concentration recommended by the manufacturer. The homogenate was centrifuged at 2,500 g for 15 min at 4 °C, and the supernatant was then centrifuged at 30,000 g for 30 min at 4 °C. The pellet was resuspended in MET buffer with 12 mM MgCl2. The suspension was centrifuged at 2,500 g for 15 min at 4 °C, and the supernatant was centrifuged again at 30,000 g for 30 min at 4 °C. The resulting pellet containing BBMVs was resuspended in PBS and the protein concentration was quantified using a protein assay kit (Bio-Rad) with BSA as a standard.
The BBMVs containing 20 µg proteins were mixed with 10 µg of Cry toxins in PBS containing the protease-inhibitor cocktail (Nacalai Tesque) at the concentration suggested by the manufacturer. After incubation for 1 h at room temperature, the Cry toxins bound to the BBMVs were separated from the unbound toxin by centrifugation (30,000 g, 30 min, 4°C). Cry toxins bound to the BBMV were separated using SDS-10 % PAGE. The proteins were transferred onto a nitrocellulose membrane using an iBlot 2 gel transfer device (Life Technologies, Carlsbad, CA), and the membrane was blocked overnight with 4 % (W/V) Block Ace (DS Pharma Biomedical, Osaka) in PBS containing 0.05 % (V/V) Tween 20. Cry toxins on the membrane were detected with goat anti-GST antibody (Amersham Biosciences, Piscataway, NJ) followed by rabbit anti-goat immunoglobulin G antibody conjugated with peroxidase (SIGMA Saint Louis, MO). Cry toxins were then visualized using Immun-Star HRP Chemiluminescent kit (Bio-Rad Laboratories) and recorded by densitometric scanning with a cooled CCD camera system (Ez-Capture; ATTO).
Results
Preparation of the recombinant Cry11Ba
Codon usage of the cry11Ba gene from Btj differed from that in E. coli genes, which meant that the guanine plus cytosine (G + C) content of cry11Ba (33 %) was significantly less than that of E. coli genes (51 % on average). Since these differences could have an adverse effect on the production of the recombinant Cry11Ba in E. coli, the 2175-bp synthetic gene (cry11Ba-S1) encoding Cry11Ba was designed and synthesized in accordance with the codon preference of E. coli genes. The cry11Ba-S1 was successfully constructed and its G + C content increased to 48 %, which was similar to that of E. coli genes (supplemental Fig. 1).
The recombinant mosquitocidal Cry toxins. Proteins were analyzed by sodium dodecyl sulfate (SDS)-10 % polyacrylamide gel electrophoresis (PAGE). a Expression of the glutathione-S-transferase (GST)-Cry11Ba. Escherichia coli cells expressing GST-Cry11Ba were disrupted by sonication and 10 µg of protein lysate was applied (Lysate). The GST-Cry11Ba was purified using glutathione beads and 1 µg of purified protein was applied (Purified). b Preparation of the recombinant Cry4Aa and Cry11Aa. The recombinant Cry toxins were expressed and purified as above and 1 µg of purified protein was applied in each lane
The GST-Cry11Ba was successfully expressed and purified. The molecular mass of the purified protein was estimated to be 109 kDa, which was very similar to the expected mass of GST-Cry11Ba (Fig. 1a). Preparation of GST-Cry4Aa and GST-Cry11Aa was also successful (Fig. 1b). In the case of GST-Cry11Aa, low molecular mass polypeptides (ca. 30 kDa) that appeared to be degraded fragments of GST-Cry11Aa were copurified with the GST-Cry11Aa. The toxicities of Cry toxins were analyzed by the bioassay using C. pipiens larvae (3rd instar). The LC50 values of Cry toxins were calculated based on the larval mortality at 48 h after administration. Among the Cry toxins tested, GST-Cry11Ba showed the highest toxicity against C. pipiens larvae, with an LC50 value of 0.11 ± 0.04 µg/ml (Fig. 2). GST-Cry4Aa also showed relatively high toxicity, with an LC50 value of 0.97 ± 0.34 µg/ml (Fig. 2), which was similar to that for Cry4Aa reported previously (Howlader et al. 2009, 2010). The toxicity of GST-Cry11Aa, which had an LC50 value of 1.91 ± 0.37 µg/ml, was slightly lower than that for GST-Cry4Aa (Fig. 2). The high toxicity of Cry11Ba reported in this study is corroborated by other studies in which Cry11Ba was found to be 37 times more toxic than Cry11Aa against C. pipiens larvae (Delécluse et al. 1995).
Synergy between Cry4Aa and Cry11Ba for toxicity against mosquito larvae
To analyze the effect of synergy among Cry toxins, the GST-Cry4Aa and -Cry11Ba were mixed at various ratios and the resulting toxin mixtures (Cry4Aa:Cry11Ba = 1:9, 3:7, 5:5, 7:3 and 9:1 (w:w)) were assessed using a bioassay. For comparison, the individual toxins were also assessed using the same bioassay. Except for the 9:1 mixture, the toxicities of the toxin mixtures were higher than those of individual toxins (LC50 = 0.97 ± 0.34 µg/ml for Cry4Aa, 0.11 ± 0.04 µg/ml for Cry11Ba). Specifically, the LC50 values for the 1:9, 3:7, 5:5, 7:3 and 9:1 mixtures [Cry4Aa:Cry11Ba (w/w)] were 0.04 ± 0.01, 0.04 ± 0.01, 0.05 ± 0.01, 0.06 ± 0.02 and 0.10 ± 0.03 µg/ml, respectively (Fig. 3a–e). These findings suggested that Cry4Aa and Cry11Ba worked synergistically with respect to their combined toxicity against C. pipiens larvae. Interestingly, most of the toxin mixtures showed higher toxicities with similar LC50 values (Fig. 3a–e), suggesting that the proportion of Cry4Aa and Cry11Ba in the toxin mixture did not strictly affect the overall toxicity of the toxin mixture. In this study, to understand the level of synergy, the synergistic effect value (LC50 expected/LC50 observed) for each toxin mixture was calculated. The synergistic effect values of the 1:9, 3:7, 5:5, 7:3 and 9:1 mixtures were determined to be 3.4 ± 0.4, 5.2 ± 2.0, 4.7 ± 1.2, 4.1 ± 0.5 and 6.8 ± 3.3, respectively (Fig. 3F).
Mosquitocidal activities of the recombinant Cry4Aa, Cry11Ba and different mixtures of the two. The recombinant Cry4Aa and Cry11Ba were mixed in the proportions (w/w) indicated, and administered to Culex pipiens larvae. The experiments were repeated independently more than three times, and the average and the SD of the mortalities observed at 48 h after administration are shown. a Cry4Aa:11Ba = 9:1, b Cry4Aa:11Ba = 7:3, c Cry4Aa:11Ba = 5:5, d Cry4Aa:11Ba = 3:7, e Cry4Aa:11Ba = 1:9, f the synergistic effect values for the different Cry4Aa-Cry11Ba mixtures were calculated individually based on the results of independent experiments, and the average synergistic effect values and the SD are shown
Synergy between Cry11Aa and Cry11Ba and their effect on mosquito larvae
The GST-Cry11Aa and -Cry11Ba were also mixed at different ratios, and the resulting toxin mixtures [Cry11Aa:Cry11Ba = 1:9, 3:7, 5:5, 7:3 and 9:1 (w/w)] were assessed using a bioassay. The toxicities of the Cry11Aa-Cry11Ba mixtures were in between those of Cry11Aa (LC50 = 1.91 ± 0.37 µg/ml) and Cry11Ba (LC50 = 0.11 ± 0.04 µg/ml), and the LC50 values for the 1:9, 3:7, 5:5, 7:3 and 9:1 mixtures (Cry11Aa: Cry11Ba (w/w)) were 0.18 ± 0.04, 0.22 ± 0.04, 0.26 ± 0.05, 0.42 ± 0.14 and 0.94 ± 0.05 µg/ml, respectively (Fig. 4a–e). Unlike the results obtained for the Cry4Aa-Cry11Ba mixtures, the combination of Cry11Aa and Cry11Ba did not show any synergistic toxic effect against C. pipiens larvae (Fig. 4a). There was thus no correlation between synergistic effect values and the 1:9 (synergistic effect = 0.9 ± 0.1), 3:7 (1.0 ± 0.2), 5:5 (1.0 ± 0.3), 7:3 (0.9 ± 0.1) and 9:1 (0.9 ± 0.1) mixtures (Fig. 4f). Our observations were partially supported by previous results in which it was shown that a recombinant strain of Lysinibacillus sphaericus expressing Cry11Aa and Cry11Ba was less toxic than a recombinant strain expressing Cry11Ba alone against Aedes aegypti and C. pipiens larvae (Servant et al. 1999).
Mosquitocidal activities of the recombinant Cry11Aa, Cry11Ba, and different mixtures of the two. The recombinant Cry11Aa and Cry11Ba were mixed in the proportions (w/w) indicated, and administered to C. pipiens larvae. The experiments were repeated independently more than three times, and the average and the SD of the mortalities observed at 48 h after administration are shown. a Cry11Aa:11Ba = 9:1, b Cry11Aa:11Ba = 7:3, c Cry11Aa:11Ba = 5:5, d Cry11Aa:11Ba = 3:7, e Cry11Aa:11Ba = 1:9, f the synergistic effect values of the Cry11Aa-Cry11Ba mixtures were calculated individually based on the results of independent experiments, and the average synergistic effect values and the SD are shown
Binding of the recombinant Cry toxins to C. pipiens BBMVs
Binding of Cry4Aa, Cry11Aa and Cry11Ba to BBMVs prepared from C. pipiens larvae was performed. The amount of Cry toxins that bound to BBMVs varied according to the type of Cry toxin, and appeared not to be correlated with their lethal activity against C. pipiens larvae. For example, among the Cry toxins tested, the highest binding was observed in Cry11Aa, whereas that observed with Cry4Aa was low (Fig. 5). The amount of Cry toxins that bound to BBMVs was similar between assays using individual toxins and the toxin mixtures [Cry4Aa or Cry11Aa:Cry11Ba = 1:1 (w/v)] (Fig. 5).
Binding characteristics of the recombinant Cry4Aa, Cry11Aa and Cry11Ba. a Binding of Cry4Aa, Cry11Ba and their mixture [1:1 (w/w)] to brush border membrane vesicle (BBMV) prepared from C. pipiens larvae. b Binding of Cry11Aa, Cry11Ba and their mixture [1:1 (w/w)] to BBMV prepared from C. pipiens larvae. Cry toxins bound to BBMV were separated by SDS-10 % PAGE. After blotting onto a nitrocellulose membrane, toxins were detected by western blotting using anti-GST antibodies. The experiment was repeated three times and the representative result is shown
Discussion
The synthetic Cry11Ba gene, cry11Ba-S1, was newly designed, and the recombinant Cry11Ba was produced in E. coli. The recombinant Cry11Ba was highly toxic against C. pipiens larvae, being nine and 17 times more toxic than the recombinant Cry4Aa and Cry11Aa, respectively (Fig. 2). The finding of high toxicity in Cry11Ba has been reported previously (Delécluse et al. 1995). Interestingly, the potencies of mixtures containing Cry4Aa and Cry11Ba were considerably higher than when Cry11Ba was assayed alone (Fig. 3a–e). These findings suggest that Cry4Aa and Cry11Ba worked synergistically with respect to toxicity against C. pipiens larvae. In addition, high synergistic effect values ranging from 3.4 to 6.8 were obtained for all toxin mixtures containing Cry4Aa and Cry11Ba (Fig. 3b). Conversely, unlike the combinations involving Cry4Aa and Cry11Ba, the Cry11Aa-Cry11Ba mixtures did not show any synergistic toxicity (Fig. 4), and their action could be explained by a simple additive model as proposed previously (Tabashnik 1992). Similarly, although evidence of synergistic toxicity between Cry4Aa and both Cry4Ba and Cry11Aa has been reported previously, the toxicity of mixtures containing Cry4Ba and Cry11Aa can also be explained by a simple additive model (Poncet et al. 1995). Thus, in both this study and previous observations, synergistic toxicity was observed in toxin mixtures containing Cry4Aa. It therefore seems reasonable to speculate that Cry4Aa may be central to the development of Cry toxin mixtures having higher mosquitocidal activity.
Synergistic toxicity similar to that reported here has been observed in other mosquitocidal toxins. For example, Cyt1Aa from Bti shows low toxicity against mosquito larvae in isolation, but it works synergistically to enhance the toxicity of other Cry toxins (Crickmore et al. 1995; Pérez et al. 2005; Wirth et al. 1997, 2007; Wu et al. 1994). It has been shown that Cyt1Aa synergizes Cry11Aa by acting as a surrogate receptor in the midgut of the larval mosquito (Pérez et al. 2005). In addition, it has shown that the binding of Cry11Aa to Cyt1Aa facilitates the formation of a Cry11Aa pre-pore oligomeric structure that is capable of forming pores in membrane vesicles (Pérez et al. 2007). In this study, we measured the extent of binding of Cry4Aa, Cry11Aa and Cry11Ba to BBMVs and consider that either Cry4Aa or Cry11Ba also acts as a surrogate receptor to enhance the toxicity of the other toxin. However, in reactions involving individual toxins or the toxin mixtures [Cry4Aa or Cry11Aa:Cry11Ba = 1:1 (w/w)], the amount of Cry toxin that bound to BBMVs did not differ significantly (Fig. 5).
In addition to Cyt toxins, Mtx toxins from Lysinibacillus sphaericus can also increase the toxicity of other mosquitocidal toxins and reduce resistance levels (Wirth et al. 2007). Since Cyt, Mtx and Cry toxins differ from each other in both structure and mode of action, it is possible that mixing toxins with different modes of action may be responsible for enhancing the observed insecticidal activity and reduce resistance levels. Among the three Cry toxins examined in this study, Cry11Aa and Cry11Ba are considerably more closely related in terms of structure, than either is to Cry4Aa. In fact, Cry11Aa and Cry11Ba have 58 % identity in terms of their amino acid sequences (Delécluse et al. 1995), while Cry4Aa does not show any marked homology to either Cry11Aa or Cry11Ba. In addition, similar receptor types have been identified for both Cry11Aa and Cry11Ba. Aminopeptidase N (APN) from Aedes aegypti binds with Cry11Aa (Chen et al. 2009), and APNs from Anopheles quadrimaculatus and Anopheles gambiae bind with Cry11Ba (Abdullah et al. 2006; Zhang et al. 2008). Similarly, α-amylase from Anopheles albimanus has been identified to bind to Cry11Aa (Fernandez-Luna et al. 2010), and amylase from A. gambiae binds to Cry11Ba with high affinity (Zhang et al. 2013). Cry11Aa binds to A. aegypti alkaline phosphatase (ALP) with high affinity (Fernandez et al. 2006), and an ALP has been reported to regulate Cry11Ba toxicity in A. gambiae larvae (Zhang et al. 2013). Unlike Cry11Aa and Cry11Ba, the receptors for Cry4Aa have not yet been characterized. On the other hand, despite having very low levels of amino acid sequence similarity, Cry4Aa, Cry11Aa and Cry11Ba all possess a three-dimensional structure composed of three domains (I, II and III) (Boonserm et al. 2006). Therefore, these three Cry toxins may share a similar mechanism of action. At least, Cry11Aa and Cry11Ba may share their specific receptors, and competitive interaction for binding in the midgut of C. pipiens larva may be induced. However, since the synergistic effect values observed in the Cry11Aa-Cry11Ba mixtures were also around 1, which could be explained by a single additive model (Fig. 4b), the competitive interaction for receptor binding did not restrict the LC50 values, at least in the combination of Cry11Aa and Cry11Ba. The mechanisms of synergistic toxicity observed in toxin mixtures remain controversial, particularly because the mechanisms of toxicity in Cry toxins are very complicated and affected by multiple factors. Consequently, synergism between multiple toxins may also be affected by multiple factors.
In conclusion, the recombinant Cry11Ba produced in this study showed very high toxicity against C. pipiens larvae. While a further increase in toxicity was achieved by mixing Cry11Ba with Cry4Aa, no such synergism was observed with Cry11Aa. The higher toxicity obtained by combining Cry4Aa and Cry11Ba is considered to be very valuable in terms of mosquito control. It would also be of interest to examine whether the combination of Cry4Aa and Cry11Ba is capable of delaying the development of resistance in insects.
References
Abdullah MA, Alzate O, Mohammad M, McNall RJ, Adang MJ, Dean DH (2003) Introduction of Culex toxicity into Bacillus thuringiensis Cry4Ba by protein engineering. Appl Environ Microbiol 69:5343–5353. doi:10.1128/AEM.69.9.5343-5353.2003
Abdullah MA, Valaitis AP, Dean DH (2006) Identification of a Bacillus thuringiensis Cry11Ba toxin-binding aminopeptidase from the mosquito, Anopheles quadrimaculatus. BMC Biochem 7:16. doi:10.1186/1471-2091-7-16
Ben-Dov E (2014) Bacillus thuringiensis subsp. israelensis and its dipteran-specific toxins. Toxins 6:1222–1243. doi:10.3390/toxins6041222
Boonserm P, Min M, Angsuthanasombat C, Lescar J (2006) Structure of the functional form of the mosquito-larvicidal Cry4Aa toxin from Bacillus thuringiensis at a 2.80-angstorm resolution. J Bacteriol 188:3391–3401. doi:10.1128/JB.188.9.3391-3401.2006
Breman JG, Alilio MS, Mills A (2004) Conquering the intolerable burden of malaria: what’s new, what’s needed: a summary. Am J Trop Med Hyg 71:1–15
Chen J, Aimanova KG, Pan S, Gill SS (2009) Identification and characterization of Aedes aegypti aminopeptidase N as a putative receptor of Bacillus thuringiensis Cry11A toxin. Insect Biochem Mol Biol 9:688–696. doi:10.1016/j.ibmb.2009.08.003
Cheong H, Gill SS (1997) Cloning and characterization of a cytolytic and mosquitocidal delta-endotoxin from Bacillus thuringiensis subsp. jegathesan. Appl Environ Microbiol 63:3254–3260
Crickmore N, Bone EJ, Williams JA, Ellar DJ (1995) Contribution of the individual components of the δ-endotoxin crystal to the mosquitocidal activity of Bacillus thuringiensis subsp. israelensis. FEMS Microbiol Lett 131:249–254
Delécluse A, Poncet S, Klier A, Rapoport G (1993) Expression of cryIVA and cryIVB genes, independently or in combination, in a crystal-negative strain of Bacillus thuringiensis subsp. israelensis. Appl Environ Microbiol 59:3922–3927
Delécluse A, Rosso ML, Ragni A (1995) Cloning and expression of a novel toxin gene from Bacillus thuringiensis subsp. jegathesan encoding a highly mosquitocidal protein. Appl Environ Microbiol 61:4230–4235
Fernandez LE, Aimanova KG, Gill SS, Bravo A, Soberón M (2006) A GPI-anchored alkaline phosphatase is a functional midgut receptor of Cry11Aa toxin in Aedes aegypti larvae. Biochem J 394:77–84
Fernandez-Luna MT, Lanz-Mendoza H, Gill SS, Bravo A, Soberon M, Miranda-Rios J (2010) An alpha-amylase is a novel receptor for Bacillus thuringiensis ssp. israelensis Cry4Ba and Cry11Aa toxins in the malaria vector mosquito Anopheles albimanus (Diptera: Culicidae). Environ Microbiol 12:746–757. doi:10.1111/j.1462-2920.2009.02117.x
Finney DJ (1971) Probit analysis, 3rd edn. Cambridge University Press, London
Georghiou GP, Wirth MC (1997) Influence of exposure to single versus multiple toxins of Bacillus thuringiensis subsp. israelensis on development of resistance in the mosquito Culex quinquefasciatus (Diptera: Culicidae). Appl Environ Microbiol 63:1095–1101
Hayakawa T, Howlader MT, Yamagiwa M, Sakai H (2008) Design and construction of a synthetic Bacillus thuringiensis Cry4Aa gene-Hyperexpression in Escherichia coli. Appl Microbiol Biotechnol 80:1033–1037. doi:10.1007/s00253-008-1560-9
Howlader MT, Kagawa Y, Sakai H, Hayakawa T (2009) Biological properties of loop-replaced mutants of Bacillus thuringiensis mosquitocidal Cry4Aa. J Biosci Bioeng 108:179–183. doi:10.1016/j.jbiosc.2009.03.016
Howlader MT, Kagawa Y, Miyakawa A, Yamamoto A, Taniguchi T, Hayakawa T, Sakai H (2010) Alanine scanning analyses of the three major loops in domain II of Bacillus thuringiensis mosquitocidal toxin Cry4Aa. Appl Environ Microbiol 76:860–865. doi:10.1128/AEM.02175-09
Kawalek MD (1998) Cloning and characterization of mosquitocidal genes from Bacillus thuringiensis subsp. jegathesan. Ph.D. dissertation, University of California, Riverside
Otieno-Ayayo ZN, Zaritsky A, Wirth MC, Manasherob R, Khasdan V, Cahan R, Ben-Dov E (2008) Variations in the mosquito larvicidal activities of toxins from Bacillus thuringiensis ssp. israelensis. Environ Microbiol 10:2191–2199. doi:10.1111/j.1462-2920.2008.01696.x
Pérez C, Fernandez LE, Sun J, Folch JL, Gill SS, Soberón M, Bravo A (2005) Bacillus thuringiensis subsp. israelensis Cyt1Aa synergizes Cry11Aa toxin by functioning as a membrane-bound receptor. Proc Natl Acad Sci USA 102:18303–18308. doi:10.1073/pnas.0505494102
Pérez C, Muñoz-Garay C, Portugal LC, Sánchez J, Gill SS, Soberón M, Bravo A (2007) Bacillus thuringiensis ssp. israelensis Cyt1Aa enhances activity of Cry11Aa toxin by facilitating the formation of a pre-pore oligomeric structure. Cell Microbiol 9:2931–2937. doi:10.1111/j.1462-5822.2007.01007.x
Poncet S, Delécluse A, Klier A, Rapoport G (1995) Evaluation of synergistic interactions among the CryIVA, CryIVB, and CryIVD toxic components of B. thuringiensis subsp. israelensis crystals. J Invertebr Pathol 66:131–135
Rosso ML, Delécluse A (1997) Contribution of the 65-kilodalton protein encoded by the cloned gene cry19A to the mosquitocidal activity of Bacillus thuringiensis subsp. jegathesan. Appl Environ Microbiol 63:4449–4455
Servant P, Rosso ML, Hamon S, Poncet S, Delécluse A, Rapoport G (1999) Production of Cry11A and Cry11Ba toxins in Bacillus sphaericus confers toxicity towards Aedes aegypti and resistant Culex populations. Appl Environ Microbiol 65:3021–3026
Sun Y, Zhao Q, Xia L, Ding X, Hu Q, Federici BA, Park HW (2013) Identification and characterization of three previously undescribed crystal proteins from Bacillus thuringiensis subsp. jegathesan. Appl Environ Microbiol 79:3364–3370. doi:10.1128/AEM.00078-13
Tabashnik BE (1992) Evaluation of synergism among Bacillus thuringiensis toxins. Appl Environ Microbiol 58:3343–3346
Wirth MC, Georghiou GP, Federici BA (1997) CytA enables CryIV endotoxins of Bacillus thuringiensis to overcome high levels of CryIV resistance in the mosquito, Culex quinquefasciatus. Proc Natl Acad Sci USA 94:10536–10540. doi:10.1073/pnas.94.20.10536
Wirth MC, Yang Y, Walton WE, Federici BA, Berry C (2007) Mtx toxins synergize Bacillus sphaericus and Cry11Aa against susceptible and insecticide-resistant Culex quinquefasciatus larvae. Appl Environ Microbiol 73:6066–6071. doi:10.1128/AEM.00654-07
Wirth MC, Walton WE, Federici BA (2012) Inheritance, stability, and dominance of cry resistance in Culex quinquefasciatus (Diptera: Culicidae) selected with the three cry toxins of Bacillus thuringiensis subsp. israelensis. J Med Entomol 49:886–894. doi:10.1603/ME11192
Wu D, Johnson JJ, Federici BA (1994) Synergism of mosquitocidal toxicity between CytA and CryIVD proteins using inclusions produced from cloned genes of Bacillus thuringiensis. Mol Microbiol 13:965–972. doi:10.1111/j.1365-2958.1994.tb00488.x
Yamagiwa M, Sakagawa K, Sakai H (2004) Functional analysis of two processed fragments of Bacillus thuringiensis Cry11A toxin. Biosci Biotechnol Biochem 68:523–528. doi:10.1271/bbb.68.523
Zhang R, Hua G, Andacht TM, Adang MJ (2008) A 106-kDa aminopeptidase is a putative receptor for Bacillus thuringiensis Cry11Ba toxin in the mosquito Anopheles gambiae. Biochemistry 47:11263–11272. doi:10.1021/bi801181g
Zhang Q, Hua G, Bayyareddy K, Adang MJ (2013) Analyses of α-amylase and α-glucosidase in the malaria vector mosquito, Anopheles gambiae, as receptors of Cry11Ba toxin of Bacillus thuringiensis subsp. jegathesan. Insect Biochem Mol Biol 43:907–915. doi:10.1016/j.ibmb.2013.07.003
Acknowledgments
C. pipiens eggs were kindly supplied by the Research and Development Laboratory at Dainihon Jochugiku, Osaka. This work was supported by the Japan Society for the Promotion of Science KAKENHI (Grant Nos. JP24380034 and JP26660268).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
13355_2016_454_MOESM1_ESM.pptx
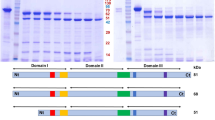
Supplemental Fig. 1 Alignment of the native and the synthetic cry11Ba gene. The amino acid sequence is shown above the nucleotide sequence. The nucleotide residues modified in accordance with the codon preference of E. coli gene are indicated. Nucleotide and amino acid numbers are shown on the right. Supplemental Fig. 2 Schematic structure of Cry protoxins and the recombinant Cry toxins used in this study. Insecticidal toxin regions are shaded. Arrow heads Internal cleavage site located in the insecticidal toxin region. After ingestion by susceptible mosquito larvae, Cry toxins are processed by residential proteases in the midgut, and the protease-resistant segments which form a heterodimer as an insecticidal toxin are generated (PPTX 182 kb)
Rights and permissions
About this article
Cite this article
Hayakawa, T., Yoneda, N., Okada, K. et al. Bacillus thuringiensis Cry11Ba works synergistically with Cry4Aa but not with Cry11Aa for toxicity against mosquito Culex pipiens (Diptera: Culicidae) larvae. Appl Entomol Zool 52, 61–68 (2017). https://doi.org/10.1007/s13355-016-0454-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13355-016-0454-z