Abstract
Here, we present the current challenges in women’s reproductive health and the current state-of-the-art treatment and prevention options for STI prevention, contraception, and treatment of infections. We discuss how the versatile platform of electrospun fibers can be applied to each challenge, and postulate at how these technologies could be improved. The void of approved electrospun fiber-based products yields the potential to apply this useful technology to a number of medical applications, many of which are relevant to women’s reproductive health. Given the ability to tune drug delivery characteristics and three-dimensional geometry, there are many opportunities to pursue new product designs and routes of administration for electrospun fibers. For each application, we provide an overview of the versatility of electrospun fibers as a novel dosage form and summarize their advantages in clinical applications. We also provide a perspective on why electrospun fibers are well-suited for a variety of applications within women’s reproductive health and identify areas that could greatly benefit from innovations with electrospun fiber-based approaches.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Current challenges in women’s reproductive health
Reproductive health is a crucial part of general well-being and is particularly important for women, especially during their reproductive years. According to the World Health Organization (WHO), one third of health issues for women between the ages of 15 and 44 years are associated with sexual and reproductive health. Contraception, infertility, menopause, gynecologic cancers, and sexually transmitted infections (STIs) including human immunodeficiency virus (HIV) are the most common reproductive health concerns for women [1,2,3,4]. In particular, STIs and HIV/AIDS have disproportionally affected women worldwide. More than one million STIs are acquired every day, and there are an estimated ∼500 million new infections of curable STIs (chlamydia, gonorrhea, syphilis, and trichomoniasis) each year [5,6,7]. Over 500 million people are living with incurable genital herpes simplex virus (HSV) infection [8], and approximately 300 million women are infected with human papillomavirus (HPV) [6]. STIs can also cause serious consequences for women’s health including adverse birth outcomes, cervical cancer, and infertility [6, 9]. Women are also at greater risk of being infected with HIV during sex. Worldwide, nearly half of all individuals living with HIV are now women. In sub-Saharan Africa in 2014, women comprised 59% of the adults living with HIV [2]. Women bear the greatest burden of reproductive health problems; thus, empowering women through education and tools to control their reproductive health are an essential element to improve their lives.
Electrospun fibers for medical applications
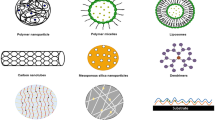
In this section, we provide an overview of the versatility of electrospun fibers as a novel dosage form and summarize their advantages in clinical applications. We also provide a perspective on why electrospun fibers are well-suited for a variety of applications within women’s reproductive health and identify areas that could greatly benefit from innovations with electrospun fiber-based approaches (Fig. 1).
Electrospun fibers: a versatile, solid dosage form for biomedical applications
The process of using an electric field to pull a polymer solution into fibers dates back to the 1930s [10] and was later described as “electrospinning” by Reneker et al. [11]. Electrospinning has since been widely explored for biomedical applications including drug delivery and tissue engineering. A number of electrospun fiber-based products are in clinical trials and include a hemostatic wound dressing [12], a transdermal patch for treatment of leishmaniasis ulcers [13], and a patch for treatment of diabetic ulcers [14]. However, there is not yet an electrospun fiber-based product that has been approved by the FDA. Several reviews focus on electrospun fibers for biomedical applications beyond the depth of this review [15, 16].
The wide variety of polymers that can be electrospun allow for a broad design space. Many of the commonly used polymers are generally recognized as safe (GRAS) by the FDA, which could potentially ease progression into pre-clinical and clinical studies [17]. Fibers can be tailored to dissolve in seconds [18], or biodegrade over the course of months [19]. In addition, fibers have been shown to encapsulate an array of small molecules [20], nucleic acids [21, 22], and protein drugs [23, 24] that greatly expand their applications for treatment and prevention of disease [25]. Electrospinning is a proven way to achieve high drug loading of therapeutics into a solid dosage form. Furthermore, it is possible to control the architecture at both the fiber and macroscopic levels such as fabricating core-sheath morphologies [26], multilayered composites of drugs and polymers [27, 28], and various three-dimensional geometries [29]. Finally, the scale-up production of electrospun fibers has been accelerated by developments made in industries such as filtration technologies, and there are commercially available instruments that produce fibers at rates up to three orders of magnitude higher than laboratory scale electrospinning rigs [30]. The versatility of the fiber platform provides opportunities to formulate dosage forms that can be effectively used in diverse biomedical applications.
How do fibers fill a gap within women’s reproductive health?
The void of approved electrospun fiber-based products yields the potential to apply this useful technology to a number of medical applications, many of which are relevant to women’s reproductive health. Given the ability to tune drug delivery characteristics and three-dimensional geometry, there are many opportunities to pursue new product designs and routes of administration for electrospun fibers. For example, electrospun fibers have been used as coatings for tissue engineering implants to reduce inflammation and calcification on vascular grafts [31] and prevent formation of biofilms [32]. Similar coating approaches of implants commonly used in the realm of women’s reproductive health such as intrauterine devices or subcutaneous contraceptive implants could augment their functionality and efficacy. Next, we discuss the three main categories of women’s reproductive health that we identified as most conducive to innovations with electrospun fibers: prevention of sexually transmitted infections, contraception, and treatment of infections. For each category, we discuss the current state-of-the-art, including what products, if any, are approved and available. Then, we discuss how electrospun fibers have been applied to certain challenges within each category. Finally, we draw on parallel, relevant examples of applications of electrospun fibers, and postulate on how these ideas and findings might be applied to challenges in women’s reproductive health.
Electrospun fibers for female reproductive challenges: current state-of-the-art, applications, and future directions
Prevention of sexually transmitted infections
Current state of STI prevention
STIs, including HIV, have a significant impact on women’s sexual and reproductive health worldwide. More effective approaches are urgently needed for the prevention and control of STIs. The current STIs and HIV prevention strategies include education [33], abstinence [34], mutual monogamy [35], vaccination (hepatitis B, HPV) [36, 37], barrier methods (condom) [38], male circumcision [39], and pre-exposure prophylaxis (PrEP) for HIV [40, 41] (Table 1) . Although correct and consistent use of condom is highly effective in reducing STDs transmission, it is a male-controlled prevention method [42, 43]. Due to social, gender, and economical inequities, female-controlled STI/HIV prevention strategies such as topical microbicides are desirable alternatives to protect women [44]. Microbicides will fill an important gap in current prevention strategies by empowering women with a discreet tool against STIs/HIV [3, 45, 46].
Electrospun fibers for STI prevention
To date, the use of fibers as a modality for preventing sexually transmitted infections has primarily focused on intravaginal delivery of small molecule drugs and proteins for HIV-1 and HSV-2 inhibition. Ball et al. formulated fibers from poly-L-lactic acid (PLLA) and polyethylene oxide (PEO) to independently deliver maraviroc (MVC), a CCR-5-mediated entry inhibitor of HIV, 3′-azido-3′-deoxythymidine (AZT), a reverse transcriptase inhibitor of HIV, and acyclovir (ACV), which has been shown to have activity against HSV-2 [20]. These formulations achieved both burst and sustained release of all three compounds in vitro, and MVC and AZT eluted from the fibers were found to maintain antiviral activity against HIV-1 BaL, while activity of eluted acyclovir was not tested. However, Aniagyei et al. have shown release of ACV from poly(lactic-co-glycolic) acid (PLGA) and poly(lactide-co-caprolactone) (PLCL) over the course of 28 days and observed complete viral inhibition with eluted doses of 1–200 μg/mL [48]. Carson et al. showed programmable release of tenofovir (TFV), AZT, MVC, and raltegravir (RAL) from fibers composed of blends of PLGA and polycaprolactone (PCL) [49]. Huang et al. developed pH-responsive electrospun fibers composed of cellulose acetate phthalate (CAP) and loaded with etravirine (ETR). These fibers maintained their integrity at normal vaginal pH of 4.5, but dissolved and release drug upon exposure to semen (pH 7.4–8.4) [50]. Blakney et al. found that release of TFV from polyvinyl alcohol (PVA) fibers could be hindered by increasing fiber mat thickness or incorporating another hydrophobic small molecule drug into the fiber matrix [27]. Krogstad et al. explored the scale-up of TFV-loaded PVA fibers from a laboratory-scale instrument to a production-scale instrument and found that fibers could be loaded with up to 60 wt.% TFV when the drug was solubilized in the polymer solution by adding sodium hydroxide [30]. Non-commercially available PLGA-based polyurethane polymers have also been electrospun with TFV for sustained release in vitro [51]. MVC has been formulated into both coaxial electrospun fibers with an ethylcellulose core and polyvinylpyrrolidone (PVP) shell [26], as well as amorphous solid dispersions in PVP and PEO [52]. Grooms et al. investigated the effect of incorporating griffithsin (GRFT), a protein that has been shown to have potent activity against both HIV-1 and HSV-2, by grafting the biologic onto the surface of electrospun fibers, as opposed to encapsulating GRFT within the fiber [53]. As opposed to using drug-eluting fibers to prevent infection, Huang et al. coated polystyrene fibers with poly(allylamine hydrochloride) (PAH) or dextran sulfate sodium (DSS) and observed inactivation of HIV-1 and a reduction of CD4+ TZMbl cells in the presence of replication competent HIV-1 [54]. Despite a multitude of formulations that have been investigated for prevention of HIV-1 and HSV-2, in vivo efficacy studies have yet to be reported. Overall, these studies illustrate the versatility of electrospun fibers to encapsulate both physicochemically diverse small molecule drugs, as well as biologics, for preventative drug delivery, and warrant further studies of these formulations.
Future directions of STI prevention
While electrospun fibers have been widely investigated for intended use as vaginal drug delivery systems, it may also be possible to incorporate drug-loaded fibers into implantable or injectable devices for long-acting prevention of STIs. Recently, two devices for delivery of tenofovir alafenamide fumarate (TAF) have been developed as a dermal implant for long-term HIV prevention. Schlesinger et al. achieved a 90-day linear release of TAF from an implantable with a TAF core surrounded by a thin-film PCL membrane [55]. Similarly, Gunawardana fabricated a silicone implant with a TAF core and PVA membrane and observed continuous release over 40 days in a pharmacokinetic study in beagles [56]. Electrospun fibers can be fabricated to exhibit similar mechanical characteristics as thin films but can typically incorporate a higher drug loading and combinations of physicochemically diverse drugs unachievable by films and could potentially be formulated to achieve long-acting release rates upon incorporation into an implant. The wide array of antiretroviral drugs that have been formulated in electrospun fibers could potentially be applied to an implant for long-acting prevention of HIV-1, HSV-2, or other sexually transmitted infections that can be prevented by small molecule drugs or biologics.
Contraception
Current state of contraception
Effective contraception prevents unintended pregnancy and reduces the need for abortion. According to the WHO, 67% of women who practice contraception currently use non-permanent methods such as hormonal contraceptives (the pill, patch, implant, injectable, and vaginal ring), intrauterine devices (IUD), and condoms [57] (Table 2). However, as of 2014, an estimated 225 million women in developing countries were not using any method of contraception due to either lack of resources or other socioeconomic reasons [58,59,60,61]. Every year, 52 million unintended pregnancies in developing regions could be averted if all unmet needs for discreet and female-controlled methods of contraception were met [61, 62]. Safe and long-acting reversible contraception may be the ideal first option for most women.
Electrospun fibers for contraception
Electrospun fibers have been investigated for contraceptive indications with formulations for vaginal drug delivery as both physical and chemical barriers. Ball et al. used a combination of PLLA and PEO loaded with 1 or 10 wt.% glycerol monolaurate (GML), a surfactant that is known to inactivate lipid-coated viruses but was also found to inhibit sperm motility in a dose-dependent manner [20]. Furthermore, electrospun fibers without any loaded drug were found to act as a physical barrier to sperm when fabricated at a thickness of >150 μm. Although fiber-based fabrics are porous with pores on the size of >3 μm diameter, the sperm were unable to penetrate through the materials likely due to the tortuous path created by the intermingling network of fibers. Blakney et al. loaded levonorgestrel (LNG), a hormonal contraceptive used in oral contraceptives, intrauterine devices, implants, and intravaginal rings, into both PVA and PLGA-based polyurethane fibers [27, 51]. The PVA fibers were found to release in vitro doses of LNG up to 50 mg within 1 h, rendering this formulation more suitable for an on-demand contraceptive option. On the contrary, the PLGA-polyurethane fibers loaded with LNG were found to sustain release for at least 3 days, with a total release predicted to occur after 14 days. These studies show the capacity of electrospun fibers to prevent unintended pregnancy by a variety of mechanisms, including chemical sperm inhibition, acting as a physical barrier, and delivery of small molecule hormonal contraceptives.
Future directions of contraception
There are many diverse dosage forms currently available for contraception including oral pills, intrauterine devices, intravaginal rings, injectables, implants, and spermicides. The electrospun fibers that have been developed to date with an indication for contraception have either been proof-of-concept or intended for vaginal delivery. However, the versatility of fibers lends this technology to a variety of dosage forms and routes of administration. For example, injectable fibers have been developed for applications in tissue engineering as a cell scaffold [68, 69]. The aforementioned PLGA-based polyurethane fibers may be particularly well-suited to be used as an implant as they could be tailored to release LNG over the course of many months but may improve the current dosage form by eradicating the need for removal of the implant, as the polyurethane polymers are biodegradable [51]. There is also a void in permanent contraception options for women who no longer wish to conceive. While there have been numerous studies using tablets and intrauterine devices to deliver sclerosing agents to the uterotubal junctions for tubal occlusions, none of these devices have achieved reproducible efficacy with just a single administration [70, 71]. One of the main challenges with this method is that there is often inadequate exposure of the sclerosing agents at the uterotubal junction. This could potentially be overcome by pairing electrospun fibers, which have been previously loaded with known sclerosing agents such as doxycycline [72] and tetracycline [73], with an IUD frame to achieve a longer, more consistent exposure of the agent at the uterotubal junction to improve overall efficacy. However, the combination of electrospun fibers with other technologies, such as an IUD or IVR, may alter drug release rate from the fibers or require special safety evaluation for the combined dosage form. While there is currently a diverse array of female contraceptive options available, electrospun fibers may enhance the convenience and functionality of current dosage forms.
Treatment of reproductive tract infections
Current state of treatment of reproductive tract infections
Common bacterial and parasitic STIs such as gonorrhea, chlamydia, syphilis, bacterial vaginosis, and trichomoniasis can be treated with antibiotics given either orally or by intramuscular injection. Vaginal yeast infection can also be treated by non-prescription creams [74]. Although there is no cure for viral STIs including HSV, HPV, hepatitis B virus (HBV), and HIV, these viral infections can be controlled by antiviral drugs [75]. Infection of HPV and HBV can be prevented by vaccination [76], and HIV can be treated and controlled by highly active antiretroviral therapy (HAART). HAART has significantly improved the quality of life of people living with HIV and reduced the risk of transmission [77].
Electrospun fibers for treatment of infections
Electrospun fibers have been formulated for the treatment of two common infections in the context of women’s reproductive health: bacterial vaginosis (BV) and yeast infections (candidiasis). Fibers offer considerable advantages compared to the currently available dosage forms for both BV and yeast infections, which are typically treated with oral tablets/gels, including localized drug delivery, potentially limiting side effects, and less leakage from the vaginal tract upon administration. Sharma et al. formulated mucoadhesive PVA nanofibers with fluconazole, an antifungal small molecule drug, which were shown to have activity against Candida albicans while having no effect on Lactobacillus acidophilus cultures, which are important for maintaining normal vaginal pH and health [78]. Other formulations have also been fabricated for oral candidiasis, which could also potentially be used as a vaginal dosage form [79]. While not intended specifically for bacterial vaginosis treatment, a number of fiber formulations of the two most common small molecule antibiotics used for treatment of BV, metronidazole [80,81,82,83], and clindamycin [84, 85] have been fabricated. The fibers were specifically formulated for periodontitis, but similarities between the oral cavity and vaginal reproductive tract may facilitate the use of these formulations for a more effective treatment of BV. These studies exhibit the advantages of fiber systems for local treatment of vaginal infections including BV and candidiasis, which may minimize the required drug dose and limit systemic exposure and potential side effects.
Future directions of infection treatment
In proof-of-concept studies, electrospun fibers have been formulated for vaginal delivery of antibacterial or antifungal agents intended for treatment of BV or candidiasis. Recurring BV can be a formidable clinical challenge to clear, often requiring repeated doses of antibiotics. An alternative treatment that has gained traction is the administration of beneficial strains of bacteria to equilibrate the vaginal microbiome back to its normal state. Reid et al. showed that oral administration of L. rhamnosus and L. fermentum probiotics eradicated BV in seven out of 11 patients [86]. Hallen et al. administered L. acidophilus vaginally and found that 16 of 28 women had normal test results following treatment, but noticed that this strand of bacteria did not adhere to the vaginal walls [87]. This treatment could be improved by using a mucoadhesive fiber matrix to increase the residence time of the administered probiotics within the vaginal cavity. Probiotic bacteria have previously been encapsulated into electrospun fibers [88,89,90,91,92], with viability immediately after the electrospinning process and up to 130 days when stored at 4 °C. Nagy et al. incorporated L. acidophilus bacteria into PVA and PVP fibers, which showed long-term storage stability of the encapsulated bacteria and postulated that their biohybrid nanowebs could at as a dosage for treatment of BV, but did not test the efficacy of the fibers [93]. Incorporating bacteria post-electrospinning is another technique that could be used to produce bacteria-laden fiber doses for treatment of infections, which may offer a greater bacterial viability than encapsulation [94]. This strategy could be used to treat BV and also potentially reduce the risk of infections that are known to have increased risk with a disrupted vaginal microbiome such as HIV [95], gonorrhea [96], or chlamydia [96]. Overall, encapsulation of small molecule drugs and/or probiotics into electrospun fibers may improve the treatment of infections in women, including BV and related opportunistic infections.
Perspective
Electrospun fibers have many advantages as a drug delivery system, including tunable drug release and the ability to encapsulate a wide variety of therapeutic agents and are poised to impact the challenges currently faced in women’s reproductive health. This versatility may also enable the development of multipurpose prevention technologies (MPTs). The intention for these devices is to prevent multiple indications in a single dosage, including unintended pregnancy, HIV, and/or other STIs. MPTs are currently being investigated in the form of intravaginal rings [97], gels [98], and fibers [27] and have the potential to provide a new convenient prevention option for women in both developed and developing countries worldwide. MPTs are a particularly promising platform for progress in women’s reproductive health, and as an adaptable drug delivery system, electrospun fibers may enable the development of products that improve the health of women worldwide. Though electrospun fibers have enormous potential as a drug delivery platform, they have yet to be approved as a commercially available dosage form. Current electrospinning technology must be augmented to meet the production capabilities needed for a commercial product, and these materials must be evaluated for safety and efficacy through clinical trials in order to fill voids in female reproductive health.
References
Lewis DA, Latif AS, Ndowa F. WHO global strategy for the prevention and control of sexually transmitted infections: time for action. Sex Transm Infect. 2007;83(7):508-9. doi:10.1136/sti.2007.028142.
UNAIDS. UNAIDS. Global AIDS Update. 2016;2016:2016.
Ortayli N, Ringheim K, Collins L, Sladden T. Sexually transmitted infections: progress and challenges since the 1994 International Conference on Population and Development (ICPD). Contraception. 2014;90(6, Supplement):S22-2S31. doi: 10.1016/j.contraception.2014.06.024.
Satterwhite CL, Torrone E, Meites E, Dunne EF, Mahajan R, Ocfemia MCB, et al. Sexually Transmitted Infections Among US Women and Men: Prevalence and Incidence Estimates, 2008. Sex Transm Dis. 2013;40(3):187-93. doi:10.1097/OLQ.0b013e318286bb53.
Lanfranco OA, Alangaden GJ. Genitourinary Tract Infections. Microbiology Spectrum. 2016;4(4). doi: 10.1128/microbiolspec.DMIH2-0019-2015.
CDC. 2015 Sexually Transmitted Diseases Surveillance. 2015.
Wijesooriya NS, Rochat RW, Kamb ML, Turlapati P, Temmerman M, Broutet N, et al. Global burden of maternal and congenital syphilis in 2008 and 2012: a health systems modeling study. Lancet Glob Health. 4(8):e525-e33. doi:10.1016/S2214-109X(16)30135-8.
Bradley H, Markowitz LE, Gibson T, McQuillan GM. Seroprevalence of Herpes Simplex Virus Types 1 and 2—United States, 1999-2010. J Infect Dis. 2013; doi:10.1093/infdis/jit458.
WHO. Sexually transmitted infections(STIs) key facts. http://www.who.int/mediacentre/factsheets/fs110/en/. 2016.
Tucker N, Stanger J, Staiger MP, Razzaq H, Hofman K. The History of the Science and Technology of Electrospinning from 1600 to 1995. J Eng Fabr Fibers. 2012;7(3):63-73.
Doshi J, Reneker DH. Electrospinning process and applications of electrospun fibers. IEEE. 1993;1698
Rothwell SW, Reid TJ, Dorsey J, Flournoy WS, Bodo M, Janmey PA, et al. A Salmon Thrombin-Fibrin Bandage Controls Arterial Bleeding in a Swine Aortotomy Model. J Trauma Acute Care Surg. 2005;59(1):143. doi:10.1097/00005373-200,507,000-00023.
López-Jaramillo P, Rincón MY, García RG, Silva SY, Smith E, Kampeerapappun P, et al. A controlled, randomized-blinded clinical trial to assess the efficacy of a nitric oxide releasing patch in the treatment of cutaneous leishmaniasis by Leishmania (V.) panamensis. AmJTrop Med Hyg. 2010;83(1):97-101. doi:10.4269/ajtmh.2010.09-0287.
Silva SY, Rueda LC, Márquez GA, López M, Smith DJ, Calderón CA, et al. Double blind, randomized, placebo controlled clinical trial for the treatment of diabetic foot ulcers, using a nitric oxide releasing patch: PATHON. Trials. 2007;8:26. doi:10.1186/1745-6215-8-26.
Liang D, Hsiao BS, Chu B. Functional electrospun nanofibrous scaffolds for biomedical applications. Adv Drug Deliv Rev. 2007;59(14):1392-412. doi:10.1016/j.addr.2007.04.021.
Agarwal S, Wendorff JH, Greiner A. Use of electrospinning technique for biomedical applications. Polymer. 2008;49:5603-21.
Administration FaD. Generally Recognized As Safe (GRAS). 2016. http://www.fda.gov/Food/IngredientsPackagingLabeling/GRAS/.
Yu D-G, Shen X-X, Branford-White C, White K, Zhu L-M, Bligh ASW. Oral fast-dissolving drug delivery membranes prepared from electrospun polyvinylpyrrolidone ultrafine fibers. Nanotechnology. 2009;20(5). doi: 10.1088/0957-4484/20/5/055104.
Kim K, Yu M, Zong X, Chiu J, Fang D, Seo YS. Control of degradation rate and hydrophilicity in electrospun non-woven poly (D, L-lactide) nanofiber scaffolds for biomedical applications. Biomaterials. 2003;24:4977-85.
Ball C, Krogstad E, Chaowanachan T, Woodrow K. Drug-Eluting Fibers for HIV-1 Inhibition and Contraception. PloS one. 2012;7(11). doi: 10.1371/journal.pone.0049792.
Luu YK, Kim K, Hsiao BS, Chu B, Hadjiargyrou M. Development of a nanostructured DNA delivery scaffold via electrospinning of PLGA and PLA-PEG block copolymers. J Control Release: Off J Control Release Soc. 2003;89(2):341-53. doi:10.1016/S0168-3659(03)00097-X.
Cao H, Jiang X, Chai C, Chew S. RNA interference by nanofiber-based siRNA delivery system. J Control Release: Off J Control Release Soc. 2010;144(2):203-12. doi:10.1016/j.jconrel.2010.02.003.
Chew S, Wen J, Yim E, Leong K. Sustained release of proteins from electrospun biodegradable fibers. Biomacromolecules. 2005;6(4):2017-24. doi:10.1021/bm0501149.
Zeng J, Aigner A, Czubayko F, Kissel T, Wendorff J, Greiner A. Poly(vinyl alcohol) nanofibers by electrospinning as a protein delivery system and the retardation of enzyme release by additional polymer coatings. Biomacromolecules. 2005;6(3):1484-8. doi:10.1021/bm0492576.
Sill TJ, von Recum HA. Electrospinning: applications in drug delivery and tissue engineering. Biomaterials. 2008;29(13):1989-2006.
Ball C, Chou S-F, Jiang Y, Woodrow KA. Coaxially electrospun fiber-based microbicides facilitate broadly tunable release of maraviroc. Mater Sci Eng C. 2016;63:117-24. doi:10.1016/j.msec.2016.02.018.
Blakney AK, Krogstad EA, Jiang YH, Woodrow KA. Delivery of multipurpose prevention drug combinations from electrospun nanofibers using composite microarchitectures. Int J Nanomedicine. 2014;9:2967-78. doi:10.2147/IJN.S61664.
Hong Y, Fujimoto K, Hashizume R, Guan J, Stankus JJ, Tobita K, et al. Generating elastic, biodegradable polyurethane/poly(lactide-co-glycolide) fibrous sheets with controlled antibiotic release via two-stream electrospinning. Biomacromolecules. 2008;9(4):1200-7. doi:10.1021/bm701201w.
Falde EJ, Freedman JD, Herrera VLM, Yohe ST, Colson YL, Grinstaff MW. Layered superhydrophobic meshes for controlled drug release. J Control Release. 2015;214:23-9. doi:10.1016/j.jconrel.2015.06.042.
Krogstad EA, Woodrow KA. Manufacturing scale-up of electrospun poly(vinyl alcohol) fibers containing tenofovir for vaginal drug delivery. Int J Pharm. 2014;475(1-2):282-91. doi:10.1016/j.ijpharm.2014.08.039.
Tara S, Kurobe H, Rocco KA, Maxfield MW, Best CA, Yi T, et al. Well-organized neointima of large-pore poly(l-lactic acid) vascular graft coated with poly(l-lactic-co-ε-caprolactone) prevents calcific deposition compared to small-pore electrospun poly(l-lactic acid) graft in a mouse aortic implantation model. Atherosclerosis. 2014;237(2):684-91. doi:10.1016/j.atherosclerosis.2014.09.030.
Zhang Z, Tang J, Wang H, Xia Q, Xu S, Han CC. Controlled Antibiotics Release System through Simple Blended Electrospun Fibers for Sustained Antibacterial Effects. ACS Appl Mater Interfaces. 2015;7(48):26,400-4. doi:10.1021/acsami.5b09820.
Cui W, Li X, Zhu X, Yu G, Zhou S, Weng J. Investigation of drug release and matrix degradation of electrospun poly(DL-lactide) fibers with paracetanol inoculation. Biomacromolecules. 2006;7(5):1623-9. doi:10.1021/bm060057z.
Stanger-Hall KF, Hall DW. Abstinence-Only Education and Teen Pregnancy Rates: Why We Need Comprehensive Sex Education in the U.S. PLoS One. 2011;6(10):e24658. doi:10.1371/journal.pone.0024658.
Conley TD, Matsick JL, Moors AC, Ziegler A, Rubin JD. Re-examining the effectiveness of monogamy as an STI-preventive strategy. Prev Med. 2015;78:23-8. doi:10.1016/j.ypmed.2015.06.006.
CDC. Vaccines: The Pink Book. Hepatitis B 2012.
CDC. Vaccine Information Staement I HPV Cervarix I VIS CDC. 2015.
Ahmed S, Lutalo T, Wawer M, Serwadda D, Sewankambo NK, Nalugoda F, et al. HIV incidence and sexually transmitted disease prevalence associated with condom use: a population study in Rakai, Uganda. AIDS. 2001;15(16):2171-9.
Cook LS, Koutsky LA, Holmes KK. Circumcision and sexually transmitted diseases. Am J Public Health. 1994;84(2):197-201.
Naswa S, Marfatia YS. Pre-exposure prophylaxis of HIV. Indian J Sex Transm Dis. 2011;32(1):1-8. doi:10.4103/0253-7184.81246.
Hankins CA, Dybul MR. The promise of pre-exposure prophylaxis with antiretroviral drugs to prevent HIV transmission: a review. Curr Opin HIV AIDS. 2013;8(1):50-8. doi:10.1097/COH.0b013e32835b809d.
Izugbara Chimaraoke O, Ochako R, Izugbara C. Gender scripts and unwanted pregnancy among urban Kenyan women. Cult Health Sex. 2011;13 doi:10.1080/13691058.2011.598947.
Kabiru Caroline W, Orpinas P. Correlates of Condom Use Among Male High School Students in Nairobi, Kenya. J Sch Health. 2009;79 doi:10.1111/j.1746-1561.2009.00430.x.
Raphael M-C. Microbicides are promoted as offering a ‘female-controlled’ HIV prevention method: so can they revolutionize the HIV crisis of young women in Kenya? Journal of Public Health. 2012; doi:10.1093/pubmed/fds049.
Omar RF, Bergeron MG. The future of microbicides. Int J Infect Dis. 2011;15(10):e656-e60. doi:10.1016/j.ijid.2011.05.001.
Madan RP, Keller MJ, Herold BC. Prioritizing prevention of HIV and sexually transmitted infections: first-generation vaginal microbicides. Curr Opin Infect Dis. 2006;19(1):49-54.
Jr DA. Challenges of sexually transmitted disease prevention and control: no magic bullet, but some bullets would still be appreciated. Clin Infect Dis. 2005;41(6):804-7.
Aniagyei SE, Sims LB, Malik DA, Tyo KM, Curry KC, Kim W, et al. Evaluation of poly(lactic-co-glycolic acid) and poly(dl-lactide-co-ε-caprolactone) electrospun fibers for the treatment of HSV-2 infection. Mater Sci Eng C. 2017;72:238-51. doi:10.1016/j.msec.2016.11.029.
Carson D, Jiang Y, Woodrow KA. Tunable Release of Multiclass Anti-HIV Drugs that are Water-Soluble and Loaded at High Drug Content in Polyester Blended Electrospun Fibers. Pharm Res. 2015; doi:10.1007/s11095-015-1769-0.
Huang C, Soenen S, van Gulck E, Vanham G, Rejman J, Van Calenbergh S, et al. Electrospun cellulose acetate phthalate fibers for semen induced anti-HIV vaginal drug delivery. Biomaterials. 2012;33(3):962-9. doi:10.1016/j.biomaterials.2011.10.004.
Blakney AK, Simonovsky F, Suydam IT, Ratner BD, Woodrow KA. Rapidly Biodegrading PLGA-Polyurethane Fibers for Sustained Release of Physicochemically Diverse Drugs. ACS Biomaterials Science & Engineering. 2016; doi:10.1021/acsbiomaterials.6b00346.
Ball C, Woodrow KA. Electrospun solid dispersions of maraviroc for rapid intravaginal preexposure prophylaxis of HIV. Antimicrobial agents and chemotherapy. 2014;58(8):4855-4865. doi: 10.1128/AAC.02564-14.
Grooms TN, Vuong HR, Tyo KM, Malik DA, Sims LB, Whittington CP et al. Griffithsin-Modified Electrospun Fibers as a Delivery Scaffold to Prevent HIV Infection. Antimicrobial agents and chemotherapy. 2016;60(10).
Huang C, Soenen SJ, Gulck E, Rejman J, Vanham G, Lucas B, et al. Electrospun polystyrene fibers for HIV entrapment. Polym Adv Technol. 2014;25:827-34. doi:10.1002/pat.3310.
Schlesinger E, Johengen D, Luecke E, Rothrock G, McGowan I, van der Straten A, et al. A Tunable, Biodegradable, Thin-Film Polymer Device as a Long-Acting Implant Delivering Tenofovir Alafenamide Fumarate for HIV Pre-exposure Prophylaxis. Pharm Res. 2016;33(7):1649-56. doi:10.1007/s11095-016-1904-6.
Gunawardana M, Remedios-Chan M, Miller CS, Fanter R, Yang F, Marzinke MA, et al. Pharmacokinetics of long-acting tenofovir alafenamide (GS-7340) subdermal implant for HIV prophylaxis. Antimicrob Agents Chemother. 2015;59(7):3913. doi:10.1128/AAC.00656-15.
Rodríguez I, Say L, Temmerman M. Family planning versus contraception: what’s in a name? Lancet Glob Health. 2014;2 doi:10.1016/s2214-109x(13)70177-3.
Remare E, Catherine K. Physical access to health facilities and contraceptive use in Kenya: Evidence from the 2008-2009 Kenya Demographic and Health Survey. Afr J Reprod Health. 2012:16.
Tavrow P, Karei Eunice M, Obbuyi A, Omollo V. Community Norms About Youth Condom Use in Western Kenya: Is Transition Occurring? Afr J Reprod Health. 2012;16
Ochako R, Mbondo M, Aloo S, Kaimenyi S, Thompson R, Temmerman M, et al. Barriers to modern contraceptive methods uptake among young women in Kenya: a qualitative study. BMC Public Health. 2015;15(1):118. doi:10.1186/s12889-015-1483-1.
Rozina M, Uzma A, Haleema HA. Contraceptive Knowledge, Attitude and Practice Among Rural Women. J Coll Physicians Surg Pak. 2008;18
Babar ST. Unmet need for family planning in Pakistan - PDHS 2006-2007: it’s time to re-examine déjà vu. Open Access J Contracept. 2010:1. doi:10.2147/oajc.s13715.
Kahlenborn C, Modugno F, Potter DM, Severs WB. Oral Contraceptive Use as a Risk Factor for Premenopausal Breast Cancer: A Meta-analysis. Mayo Clin Proc. 2006;81(10):1290-302. doi:10.4065/81.10.1290.
Smith JS, Green J, de Gonzalez AB, Appleby P, Peto J, Plummer M, et al. Cervical cancer and use of hormonal contraceptives: a systematic review. Lancet. 2003;361(9364):1159-67. doi:10.1016/S0140-6736(03)12949-2.
Tanis BC, van den Bosch MAAJ, Kemmeren JM, Cats VM, Helmerhorst FM, Algra A, et al. Oral Contraceptives and the Risk of Myocardial Infarction. N Engl J Med. 2001;345(25):1787-93. doi:10.1056/NEJMoa003216.
Baillargeon J-P, McClish DK, Essah PA, Nestler JE. Association between the Current Use of Low-Dose Oral Contraceptives and Cardiovascular Arterial Disease: A Meta-Analysis. J Clin Endocrinol Metab. 2005;90(7):3863-70. doi:10.1210/jc.2004-1958.
Marnach ML, Long ME, Casey PM. Current Issues in Contraception. Mayo Clin Proc. 2013;88(3):295-9. doi:10.1016/j.mayocp.2013.01.007.
Rajeswari R, Jayarama Reddy V, Subramanian S, Shayanti M, Radhakrishnan S, Seeram R. Minimally invasive injectable short nanofibers of poly(glycerol sebacate) for cardiac tissue engineering. Nanotechnology. 2012;23(38):385,102.
Lee S, Yun S, Park KI, Jang J-H. Sliding Fibers: Slidable, Injectable, and Gel-like Electrospun Nanofibers as Versatile Cell Carriers. ACS Nano. 2016;10(3):3282-94. doi:10.1021/acsnano.5b06605.
Bairagy NR, Mullick BC. Use of erythromycin for nonsurgical female sterilization in West Bengal, India: a study of 790 cases. Contraception. 2004;69(1):47-9. doi:10.1016/j.contraception.2003.07.005.
Sokal DC, Zipper J, King T. Transcervical quinacrine sterilization: clinical experience. Int J Gynecol Obstet. 1995;51:S57-69. doi:10.1016/0020-7292(95)90370-4.
Chaturvedi TP, Srivastava R, Srivastava AK, Gupta V, Verma PK. Doxycycline poly e-caprolactone nanofibers in patients with chronic periodontitis-a clinical evaluation. J Clin Diagn Res. 2013;7(10):2339-42.
Kenawy E-R-R, Bowlin GL, Mansfield K, Layman J, Simpson DG, Sanders EH, et al. Release of tetracycline hydrochloride from electrospun poly(ethylene-co-vinylacetate), poly(lactic acid), and a blend. J Control Release: Off J Control Release Soc. 2002;(1-2):81, 57-64. doi:10.1016/S0168-3659(02)00041-X.
Guidelines ST. STD Treatment Guidelines. 2015:2015.
Steen R, Wi TE, Kamali A, Ndowa F. Control of sexually transmitted infections and prevention of HIV transmission: mending a fractured paradigm. Bull World Health Organ. 2009;87(11):858-65. doi:10.2471/BLT.08.059212.
CDC. CDC 2015 sexually transmitted diseases (STDs) treatment guidlines. 2015.
WHO/UNAIDS/UNICEF. WHO/UNAIDS/UNICEF. Global update on HIV treatment 2013; June 2013.2013.
Sharma R, Garg T, Goyal AK, Rath G. Development, optimization and evaluation of polymeric electrospun nanofiber: A tool for local delivery of fluconazole for management of vaginal candidiasis. Artif Cells, Nanomedicine Biotechnol. 2016;44:524-31. doi:10.3109/21691401.2014.966194.
Tonglairoum P, Ngawhirunpat T, Rojanarata T, Kaomongkolgit R, Opanasopit P. Fabrication of a novel scaffold of clotrimazole-microemulsion-containing nanofibers using an electrospinning process for oral candidiasis applications. Colloids Surf B: Biointerfaces. 2015;126:18-25. doi:10.1016/j.colsurfb.2014.12.009.
Reise M, Wyrwa R, Müller U, Zylinski M, Völpel A, Schnabelrauch M, et al. Release of metronidazole from electrospun poly(l-lactide-co-d/l-lactide) fibers for local periodontitis treatment. Dent Mater. 2012;28(2):179-88. doi:10.1016/j.dental.2011.12.006.
Chaturvedi TP, Srivastava R, Srivastava AK, Gupta V, Verma PK. Evaluation of metronidazole nanofibers in patients with chronic periodontitis: A clinical study. Int J Pharm Investig. 2012;2(4):213-7. doi:10.4103/2230-973X.107007.
Reise M, Wyrwa R, Müller U, Zylinski M, Völpel A, Schnabelrauch M, et al. Release of metronidazole from electrospun poly(<span class = “small” > l</span> − lactide-co- < span class = “small” > d</span>/<span class = “small” > l</span> − lactide) fibers for local periodontitis treatment. Dent Mater. 28(2):179-88. doi:10.1016/j.dental.2011.12.006.
Schkarpetkin D, Reise M, Wyrwa R, Völpel A, Berg A, Schweder M, et al. Development of novel electrospun dual-drug fiber mats loaded with a combination of ampicillin and metronidazole. Dent Mater. 32(8):951-60. doi:10.1016/j.dental.2016.05.002.
Xu X, Zhong W, Zhou S, Trajtman A, Alfa M. Electrospun PEG-PLA nanofibrous membrane for sustained release of hydrophilic antibiotics. J Appl Polym Sci. 2010;118(1):588-95. doi:10.1002/app.32415.
Bölgen N, Vargel İ, Korkusuz P, Menceloğlu YZ, Pişkin E. In vivo performance of antibiotic embedded electrospun PCL membranes for prevention of abdominal adhesions. J Biomed Mater Res B Appl Biomater. 2007;81B(2):530-43. doi:10.1002/jbm.b.30694.
Reid G, Beuerman D, Heinemann C, Bruce AW. Probiotic Lactobacillus dose required to restore and maintain a normal vaginal flora. FEMS Immunol Med Microbiol. 2001;32:37-41. doi:10.1111/j.1574-695X.2001.tb00531.x.
Hallen A, Jarstrand C, Pahlson C. Treatment of bacterial vaginosis with lactobacilli. Sex Transm Infect. 1992;19(3):146.
Salalha W, Kuhn J, Dror Y, Zussman E. Encapsulation of bacteria and viruses in electrospun nanofibres. Nanotechnology. 2006;17:4675-81. doi:10.1088/0957-4484/17/18/025.
López-Rubio A, Sanchez E, Sanz Y, Lagaron JM. Encapsulation of living bifidobacteria in ultrathin PVOH electrospun fibers. Biomacromolecules. 2009;10(10):2823-9. doi:10.1021/bm900660b.
Amna T, Hassan MS, Pandeya DR, Khil M-S, Hwang IH. Classy non-wovens based on animate L. gasseri-inanimate poly(vinyl alcohol): upstream application in food engineering. Appl Microbiol Biotechnol. 2013;97(10):4523-31. doi:10.1007/s00253-012-4666-z.
Liu Y, Rafailovich MH, Malal R, Cohn D, Chidambaram D. Engineering of bio-hybrid materials by electrospinning polymer-microbe fibers. Proc Natl Acad Sci. 2009;106(34):14,201-6. doi:10.1073/pnas.0903238106.
Zussman E. Encapsulation of cells within electrospun fibers. Polym Adv Technol. 2011;22(3):366-71. doi:10.1002/pat.1812.
Nagy ZK, Wagner I, Suhajda Á, Tobak T, Harasztos AH, Vigh T, et al. Nanofibrous solid dosage form of living bacteria prepared by electrospinning. Express Polym Lett. 2014;8(5):352-61.
Abrigo M, Kingshott P, McArthur SL. Electrospun Polystyrene Fiber Diameter Influencing Bacterial Attachment, Proliferation, and Growth. ACS Appl Mater Interfaces. 2015;7(14):7644-52. doi:10.1021/acsami.5b00453.
Thurman AR, Doncel GF. Innate immunity and inflammatory response to Trichomonas vaginalis and bacterial vaginosis: relationship to HIV acquisition. Am J Reprod Immunol. 2011;65:89-98. doi:10.1111/j.1600-0897.2010.00902.x.
Brotman RM, Klebanoff MA, Nansel TR, YU KF, Andrews WW, Zhang J, et al. Bacterial vaginosis assessed by gram stain and diminished colonization resistance to incident gonococcal, chlamydial, and trichomonal genital infection. J Infect Dis. 2010;202(12):1907-15.
Thurman A, Clark M, Hurlburt J, Doncel G. Intravaginal rings as delivery systems for microbicides and multipurpose prevention technologies. Int J Women’s Health. 2013;5:695-708. doi:10.2147/IJWH.S34030.
Kizima L, Rodríguez A, Kenney J, Derby N, Mizenina O, Menon R, et al. A potent combination microbicide that targets SHIV-RT, HSV-2 and HPV. PLoS One. 2014;9(5):e94547. doi:10.1371/journal.pone.0094547.
Acknowledgements
This work is supported by funding from the NIH/NIAID (AI112002) and the Bill and Melinda Gates Foundation (OPP1067729, OPP1110945).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Blakney, A.K., Jiang, Y. & Woodrow, K.A. Application of electrospun fibers for female reproductive health. Drug Deliv. and Transl. Res. 7, 796–804 (2017). https://doi.org/10.1007/s13346-017-0386-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-017-0386-3