Abstract
Cancer is a major cause of death in patients with type 2 diabetes mellitus (T2DM) and lung cancer is one of the most prevalent cancers in patients with T2DM. In the present study, we examined the anti-cancer effect of the Sodium-glucose cotransporter 2 (SGLT2) inhibitor, canagliflozin, using a lung cancer model. In lung cancer tissues from non-T2DM human subjects, SGLT2 was detected by immunohistochemistry. SGLT2 mRNA and protein were also detected in A549, H1975 and H520 lung cancer cell lines by RT-PCR and immunohistochemistry, respectively. Canagliflozin at 1–50 µM significantly suppressed the growth of A549 cells in a dose-dependent manner. In BrdU assays, canagliflozin attenuated the proliferation of A549 cells, but did not induce apoptosis. In cell cycle analysis, S phase entry was attenuated by canagliflozin in A549 cells. In in vivo experiments, a xenograft model of athymic mice implanted with A549 lung cancer cells was treated with low and high dose oral canagliflozin. Despite the results of the in vitro experiments, tumor weight was not decreased by canagliflozin. In addition, the serum insulin level, but not body weight or blood glucose level, was decreased by canagliflozin. The number of cells positive for Ki67 was slightly decreased by canagliflozin, but this was not statistically significant. In conclusion, SGLT2 is expressed in human lung cancer tissue and cell lines, and the SGLT2 inhibitor, canagliflozin, attenuated proliferation of A549 lung cancer cells by inhibiting cell cycle progression in vitro but not in vivo.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recently, cancer has emerged as a major cause of death in patients with diabetes mellitus (DM) [1]. This is particularly true in Japan where cancer is the leading cause of death in patients with type 2 diabetes mellitus (T2DM). Accordingly, the Japan Diabetes Society and the Japan Cancer Association have issued a warning regarding increased cancer risk in patients with diabetes mellitus [2]. Notably, T2DM and metabolic syndrome caused by obesity have been suggested to be associated with a higher risk of many cancers [3]. This evidence suggests the need for a T2DM therapeutic strategy that decreases not only the glucose level but also the risk and progression of cancer. In our previous study, we investigated the anti-cancer effect of a glucagon-like peptide-1 (GLP-1) receptor agonist in prostate cancer [4] and breast cancer models [5]. We observed a further reduction of prostate cancer growth upon combined therapy with metformin and the GLP-1 receptor agonist [6].
Sodium-glucose cotransporter 2 (SGLT2) inhibitors are anti-diabetic agents that are currently approved for clinical application. Because of their unique glucose-lowering mechanism and cardiovascular protective effect, they have received substantial attention. We previously reported that the SGLT2 inhibitor, ipragliflozin, increased adiponectin and HDL cholesterol levels, and decreased glycated hemoglobin and serum C-peptide levels, body mass index, and blood pressure in Japanese patients with T2DM [7]. Indeed, obesity, hyperinsulinemia, hyperglycemia and lower serum adiponectin concentration are all possible mechanisms by which T2DM increases cancer risk [2]. In addition, glucose uptake through SGLT is a main source of energy for cancer cells. Furthermore, evidence from the CANVAS (Canagliflozin Cardiovascular Assessment Study) program, suggests that the SGLT2 inhibitor, canagliflozin, reduces cardiovascular events in patients with T2DM [8]. Interestingly, SGLT2 inhibitor canagliflozin decreased death from any cause by 2.2 participants per 1000 patients-year compared with placebo (17.3 vs. 19.5 participants per 1000 patients-year), while death from cardiovascular causes was decreased by 1.2 participants per 1000 patients-year compared with placebo (11.6 vs. 2.8 participants per 1000 patients-year) in CANVAS program [8]. This suggests that canagliflozin might be able to decrease mortality due to not only cardiovascular diseases but also unknown other critical diseases in patients with T2DM. In the present study, we examined the anti-cancer effect of canagliflozin in lung cancer cells.
Materials and methods
Human tissues
Human lung cancer tissues were obtained from two non-diabetic patients with lung cancer (both 72 years old) after lung segmental resection at Fukuoka University Hospital. The tissues were embedded in paraffin, fixed in formalin, and cut into 4-µm sections for immunofluorescence staining. One section was cut and stained from each of two independent lung cancers from the two independent patients. The study protocol was approved by the Ethics Committees of Fukuoka University Hospital (date of approval 4th/July/2018, approval number 2017M059) and the participating hospitals, and complied with the principles in the Declaration of Helsinki (2013). Patients provided written informed consent prior to all procedures.
Cell culture and proliferation assays
A549 human lung adenocarcinoma, H1975 human lung adenocarcinoma, and H520 human squamous cell carcinoma cell lines were purchased from the American Type Culture Collection (Manassas, VA, USA). A549 cells were maintained in Ham’s F-12K, and H1975 and H520 cells were maintained in RPMI-1640. Both media were supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin. Cell proliferation assays were performed as described previously [4,5,6] with minor modifications. Briefly, A549 cells were seeded in 12-well culture plates and maintained in complete medium with or without 1–50 μM canagliflozin (kindly provided by Mitsubishi Tanabe Pharma., Osaka, Japan). Cell proliferation was analyzed after 0–3 days by cell counting using a hemocytometer.
Animals
Male athymic CAnN.Cg-Foxn1nu/CrlCrlj mice were purchased from Charles River Laboratories Kanagawa, Japan, Inc. and housed in specific-pathogen-free barrier facilities at Fukuoka University. The mice were treated with either saline (control, n = 10), 10 mg/kg/day canagliflozin (low dose, n = 10), or 50 mg/kg/day canagliflozin (high dose, n = 10) in freely accessible drinking water for 8 weeks. When the mice reached 6 weeks of age, 5 × 105 A549 cells (passage 4–8) were mixed with 250 μl Matrigel (Becton Dickinson Labware, Bedford, MA, USA) and implanted subcutaneously in the flank region. When the mice reached 14 weeks of age, blood samples were collected, and the mice were euthanized. All procedures involving animals were reviewed and approved by the Institutional Animal Care Subcommittee at Fukuoka University Hospital. All institute and national guidelines for the care and use of laboratory animals were followed.
Immunohistochemistry
Paraffin-embedded sections were incubated with anti-SGLT2 (ab37296; Abcam, Cambridge, UK) or anti-Ki67 (ab66155; Abcam) antibodies and subsequently incubated with Alexa Fluor 488 goat anti-rabbit IgG (A-11008; Thermo Fisher Scientific, Rockford, IL, USA). Sections were counterstained with 4′,6-diamidino-2-phenylindole (DAPI) and visualized by confocal microscopy.
Reverse transcription and quantitative real-time PCR
Reverse transcription (RT) and quantitative real-time polymerase chain reaction (PCR) were performed as described previously [4, 6]. The primer sequences were as follows: human TATA box-binding protein (TBP), 5′-TGCTGCGGTAATCATGAGGATA-3′ (forward), 5′-TGAAGTCCAAGAACTTAGCTGGAA-3′ (reverse); human SGLT2, 5′-TGCATCTGATTGGCAGTCAC-3′ (forward), 5′-TTTTTGGACAGGGGAAAGGC-3′ (reverse).
Bromodeoxyuridine (BrdU) assays
To evaluate the proliferation of lung cancer cells, a BrdU incorporation assay was performed using a Cell Proliferation ELISA kit (1647229; Roche Applied Science, Mannheim, Germany), as described previously [4,5,6].
Apoptosis assays
To label nuclei of apoptotic cells, 1.2 × 105 lung cancer cells were plated on glass coverslips in Lab-Tek Chamber Slides (177380; Nunc, Thermo Scientific, Waltham, MA, USA) and fixed in 4% paraformaldehyde for 25 min. Terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) staining was performed using a DeadEnd Fluorometric TUNEL System (Promega, Madison, WI, USA), according to the manufacturer’s protocol. During the final 24 h, the cells were incubated with 10 μM canagliflozin. Cells treated with 1 U/100 μl RQ1 RNase-Free DNase (M6101; Promega) for 24 h were used as a positive control.
Cell cycle analysis by flow cytometry
Cell cycle analysis by flow cytometry was conducted as reported previously [9].
Western blot analysis
Western blotting was performed as described previously [4,5,6]. The following primary antibodies were used: phospho-ERK (Thr-202/Tyr-204) (#9101, Cell Signaling, Danvers, MA) and ERK (#9102, Cell Signaling). Protein expression was examined in A549 cells incubated in medium containing 10% FBS and stimulated with or without 10 μM canagliflozin for 24, or 48 h.
Insulin measurements
Insulin concentrations in mouse sera were measured using an Ultra Sensitive Mouse Insulin ELISA Kit (Morinaga Institute of Biological Science, Inc. Kanagawa, Japan), according to the manufacturer’s protocol.
Statistical analysis
One-way, two-way ANOVA, and ANOVA (mix-effect analysis) or the unpaired t-test for repeated measures were performed for statistical analysis as appropriate. P-values of < 0.05 were considered as statistically significant. Results are expressed as the mean ± SEM.
Results
SGLT2 is expressed in human lung cancer tissue and cell lines
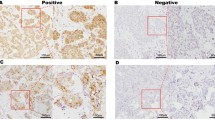
To detect SGLT2 expression in human lung cancer tissue extracted by lung segmental resection, we performed immunohistochemical analysis. As depicted in Fig. 1a, SGLT2 was expressed on the membrane of lung cancer cells in two independent cases. Furthermore, SGLT2 expression was detected in three human lung cancer cell lines, A549, H1975, and H520 (Fig. 1b). SGLT2 gene expression was confirmed by quantitative PCR (Fig. 1c) and significantly higher SGLT2 expression was detected in H1975 cells compared with the other two cell lines. Unfortunately, we could not perform immunohistochemical analysis of normal human lung tissue because it was not in our protocol submitted to the ethical committee. According to the NCBI Gene database, SGLT2 is abundantly expressed only in kidney and is undetectable in normal human lung tissue (https://www.ncbi.nlm.nih.ov/gene/6524). Therefore, we suggest that expression of SGLT2 in lung tissue is a cancer-specific phenotype.
SGLT2 is expressed in human lung cancer tissue and cell lines and SGLT2 inhibitor, canagliflozin, attenuates lung cancer cell proliferation. Immunohistochemistry was performed to examine SGLT2 expression in human lung cancer tissues (a) and cell lines (b). All samples were counterstained with DAPI (magnification, 400 ×). c Quantitative RT-PCR was performed using a set of primers targeting a 94-bp coding region of SGLT2. TBP expression was used for normalization. One-way ANOVA was performed to calculate statistical significance. **P < 0.01 vs. A549 cells and H520 cells (n = 3). d A549 cells were maintained in media supplemented with 10% fetal bovine serum (FBS) and DMSO or canagliflozin (1–50 μM). After 0, 24, 48, 72, and 96 h, the cells were harvested and cell proliferation was analyzed by cell counting using a hemocytometer. Two-way ANOVA was performed to calculate statistical significance. *P < 0.05, **P < 0.01 vs. control (n = 3). e A549 cells were seeded at a density of 5000 cells/well in 96-well plates in media supplemented with 10% FBS and incubated with canagliflozin (0–50 μM) for 24, 48 and 72 h. A BrdU solution was added during the last 2 h, and the cells were harvested to measure DNA synthesis using a microplate reader at 450–620 nm. Mean data are expressed as the relative ratio to the proliferation of control (untreated) cells. Two-way ANOVA was performed to calculate statistical significance. *P < 0.05, **P < 0.01 vs. control (n = 3)
SGLT2 inhibitor, canagliflozin, attenuates lung cancer cell proliferation
We next treated the three lung cancer cell lines with 0–50 μM canagliflozin and constructed growth curves. Adenocarcinoma is the most common lung cancer in Japan and the lung cancer tissues in Fig. 1a were both adenocarcinomas. Also, one of the most frequently used lung adenocarcinoma cell lines is A549; therefore, the following experiments were mainly performed using A549 cells. As shown in Fig. 1d, canagliflozin decreased the number of A549 cells in a dose-dependent manner. A549 cell proliferation was completely abolished with 50 μM canagliflozin. Furthermore, BrdU assays revealed that canagliflozin significantly inhibited DNA synthesis in A549 cells in a dose-dependent manner (Fig. 1e). Similar significant anti-proliferative effects by canagliflozin were also observed in H1975 and H520 cells (data no shown).
Canagliflozin does not induce apoptosis, but attenuates cell cycle progression
Apoptosis of lung cancer cells was not induced by 10 μM canagliflozin (Fig. 2a). To examine the mechanism by which canagliflozin attenuated lung cancer cell proliferation, we conducted cell cycle distribution analysis by flow cytometry. As shown in Fig. 2b, 1–50 μM canagliflozin significantly increased the number of cells in G0/G1 phase and decreased the number of S phase cells in a dose-dependent manner, suggesting that canagliflozin induced G1 arrest in lung cancer cells. To further elucidate the mechanism and cell signaling by which canagliflozin attenuated lung cancer cell proliferation, we employed western blotting. Although no significant changes were detected in Akt phosphorylation or cell cycle regulators (data not shown), ERK and MAPK phosphorylation was significantly decreased by treatment with canagliflozin for 24 h (Fig. 2c).
Canagliflozin does not induce apoptosis, but attenuates cell cycle progression. a A549 cells were seeded on glass coverslips in six-well plates. After incubation with 10 nM Ex-4 or 1 U/100 μl RQ1 DNase for 24 h, apoptotic cells were detected by terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining. Images are representative of three independent experiments. b Flow cytometric analysis was performed to determine the cell cycle distribution of A549 cells with or without canagliflozin treatment. Data are represented as the ratios of cells distributed in each phase to the total cells. Two-way ANOVA was performed to calculate statistical significance. **P < 0.01 vs. control (n = 3). c Western blotting of phosphorylated ERK and MAPK. Densitometry was conducted by comparison against GAPDH. Data are represented as relative expression to the control [Cana (−), 0 h]. The unpaired t-test was performed to calculate statistical significance. *P < 0.05 vs. control (n = 3)
Canagliflozin did not decrease lung cancer cell proliferation in vivo
Finally, we performed in vivo experiments using a xenograft model that we have reported previously [4,5,6]. A549 cells were implanted into athymic mice and treated with low and high dose canagliflozin. Tumor size (Fig. 3a), body weight (Fig. 3b) and blood glucose level (Fig. 3c) were not decreased by canagliflozin. The serum insulin level was decreased by canagliflozin (Fig. 3d); however, this was not statistically significant. The weight of resected tumors was not changed by canagliflozin treatment (Fig. 3e), and cells positive for Ki67, a proliferation marker, were slightly decreased by canagliflozin (Fig. 3f). Immunohistochemistry of tumor sections confirmed SGLT2 expression in tumors with or without canagliflozin treatment (Fig. 4).
Canagliflozin does not decrease lung cancer cell proliferation in vivo. a Athymic CAnN.Cg-Foxn1nu/CrlCrlj mice (6 weeks of age) were implanted with 5 × 105 A549 cells (passage 4–8) and treated with either saline (control, n = 10), 10 mg/kg/day canagliflozin (low dose, n = 10), or 50 mg/kg/day canagliflozin (high dose, n = 10) in freely accessible drinking water for 8 weeks. Body weight (b), casual plasma glucose in ad libitum-fed mice (mg/dL) (c), and plasma insulin (ng/mL) (d) are shown. Tumors were resected and weighed (e) when the mice reached 14 weeks of age. f Sections (5 µm thick) were subjected to immunohistochemistry for Ki67 and counterstained with 4′,6-diamidino-2-phenylindole (DAPI; magnification, 400 ×). Ki67-positive cells were quantified by analyzing the fraction of stained cells in the tumor relative to the total number of nuclei. Values are expressed as the percentage of positive cells. The unpaired t-test was performed to calculate statistical significance
SGLT2 is expressed in mouse cancer tissues. Sections (5 µm thick) were subjected to immunohistochemistry for SGLT2. All samples were counterstained with DAPI (magnification, 400 ×). SGLT2-positive cells were quantified by analyzing the fraction of stained cells in the tumor relative to the total number of nuclei. The unpaired t-test was performed to calculate statistical significance
Discussion
In the present study, we detected the expression of SGLT2 in human lung cancer tissue and determined the anti-proliferative effect of the SGLT2 inhibitor, canagliflozin, in A549 lung cancer cells through inhibition of cell cycle progression. SGLT2 inhibitors are currently used as anti-diabetic agents and have received much attention for their glucose-lowering effects without body weight gain or hypoglycemia, as well as for their clinical cardiovascular and renal protective effects. Notably, recent data from basic experimental studies indicate that SGLT2 inhibitors may exert effects against cancers, such as pancreatic, prostate [10], liver [11], and colon cancers [12]. We are currently investigating anti-breast cancer effects of the SGLT2 inhibitor, ipragliflozin, via membrane hyperpolarization and mitochondrial membrane instability [13]. Cancer is emerging as a leading cause of death in Japanese patients with DM, and lung cancer is the leading cause of cancer death in Japanese patients with DM [14]. Accordingly, glycemic control while inhibiting lung cancer progression is particularly important for DM patients in Japan.
In the present study, we investigated SGLT2 expression in human lung cancer tissue from non-diabetic subjects. SGLT2 is not expressed in normal lung tissue. Therefore, SGLT2 could be a diagnostic and therapeutic target for lung cancer, especially early-stage lung adenocarcinoma, as previously suggested [15]. The two lung cancer cases in Fig. 1a were both adenocarcinomas. In our in vitro experiment, we detected SGLT2 expression not only in adenocarcinoma A549 and H1975 cell lines, but also in the squamous cell carcinoma H520 cell line. In future investigations, SGLT2 expression in squamous cell carcinoma and other types of lung cancer should be determined and the anti-lung cancer effect of SGLT2 inhibitors in patients should be explored.
Our findings revealed that the SGLT2 inhibitor, canagliflozin, may attenuate lung cancer cell proliferation and DNA synthesis by inhibiting cell cycle progression. Anti-cancer effects have been reported for several of SGLT2 inhibitors, such as ipragliflozin [13] and dapagliflozin [12]. However, the most frequently used SGLT2 inhibitor to demonstrate anti-cancer effects is canagliflozin [11, 16,17,18,19]. Furthermore, a systematic review and meta-analysis revealed that canagliflozin decreases gastrointestinal cancer risk in T2DM patients compared with placebos [20]. Interestingly, the mechanisms by which canagliflozin attenuates cancer growth are slightly different among several reports depending on the cancer type and the experiments conducted. In previous reports by other groups, canagliflozin inhibited mitochondrial complex-I in prostate and lung cancer cells [16]. It also inhibited hepatocellular carcinogenesis and growth in model mice including the non-alcoholic steatohepatitis-related hepatocarcinogenesis model [11, 17,18,19]. In the present study, we did not observe that canagliflozin significantly reduced lung cancer growth in vivo, probably because our animal model differed from those used in previous reports [11, 19], and the dose of canagliflozin was much lower than in previous reports [17, 18]. There are many reasons why in vivo results do not reflect in vitro efficacy. In our previous report, we demonstrated an anti-cancer effect of a GLP-1 receptor agonist using the same mouse model [4, 5]. The GLP-1 receptor agonist was injected subcutaneously, while canagliflozin was administered orally; therefore, the certainty of treatment might be different. In addition, in the present study, mice were fed ad libitum and we did not measure food intake during canagliflozin treatment. One possibility for the non-compatible in vivo and in vitro results is compensatory over-eating that might mask the effect of canagliflozin. Accordingly, blood glucose levels were not decreased by canagliflozin. Further study incorporating food restriction and measurement of food intake should be performed. Attention should also be paid to interpreting differences between in vitro and in vivo pharmacology. The optimal treatment settings as well as dose regimens must be refined using lung cancer models. However, canagliflozin significantly attenuated lung cancer cell proliferation in vitro. As a mechanism by which canagliflozin attenuates lung cancer cell proliferation, we identified cell cycle blockade from G1 to S entry, but not apoptosis, which is consistent with previous reports [17, 19]. Taken together, canagliflozin might play an important role in cytostasis of lung cancer cells, but has no cytotoxic activity. Cell cycle regulation may underlie the anti-cancer effects of SGLT2 inhibitors and these drugs have potential for chemoprevention of lung cancer.
In conclusion, we showed expression of SGLT2 in human lung cancer tissue and the anti-cancer effect of the SGLT2 inhibitor, canagliflozin, against a lung cancer cell line through inhibition of cell cycle progression.
References
Emerging Risk Factors Collaboration, Seshasai SR, Kaptoqe S, Thompson A, Di Angelantonio E, Gao P, Sarwar N, Whincup PH, Mukamal KJ, Gillum RF, Holm I, Njolstad I, Fletcher A, Nilsson LS, Collins R, Gudnason V, Thompson SG, Sattar N, Selvin E, Hu FB, Danesh J. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med. 2011;364:829–41.
Kasuga M, Ueki K, Tajima N, Noda M, Ohashi K, Noto H, Goto A, Ogawa W, Sakai R, Tsugane S, Hamajima N, Nakagawa H, Tajima K, Miyazono K, Imai K. Report of the JDS/JCA joint committee on diabetes and cancer. Diabetol Int. 2013;4:81–96.
Esposito K, Chiodini P, Colao A, Lenzi A, Giugliano D. Metabolic syndrome and risk of cancer: a systemic review and meta-analysis. Diabetes Care. 2012;35:2402–11.
Nomiyama T, Kawanami T, Irie S, Hamaguchi Y, Terawaki Y, Murase K, Tsutsumi Y, Nagaishi R, Tanabe M, Morinaga H, Tanaka T, Mizoguchi M, Nabeshima K, Tanaka M, Yanase T. Exendin-4, a glicagon-like peptide-1 receptor agonist, attenuates prostate cancer growth. Diabetes. 2014;63:3891–905.
Iwaya C, Nomiyama T, Komatsu S, Kawanami T, Hamaguchi Y, Yoshinaga Y, Yamashita S, Tanaka T, Terawaki Y, Tanabe M, Nabeshima K, Iwasaki A, Yanase T. Exendin-4, a glucagonlike peptide-1 receptor agonist, attenuates breast cancer growth by inhibiting NF-kB activation. Endocrinology. 2017;158:4218–32.
Tsutsumi Y, Nomiyama T, Kawanami T, Hamagichi Y, Terawaki Y, Tanaka T, Murase K, Motonaga R, Tanabe M, Yanase T. Combined treatment with Exendin-4 and metformin attenuates prostate cancer growth. PLoS ONE. 2015;10:e0139709.
Nomiyama T, Shimono D, Horikawa T, Fujimura Y, Ohsako T, Terawaki Y, Fukuda T, Motonaga R, Tanabe M, Yanase T. Efficacy and safety of sodium-glucose cotransporter 2 inhibitor ipragliflozin on glycemic control and cardiovascular parameters in Japanese patients with type 2 diabetes mellitus; Fukuoka Study of Ipragliflozin (FUSION). Endocr J. 2018;65:859–67.
Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher E, Erondu N, Shaw W, Law G, Desai M, Matthews DR, CANVAS Program Collaborative Group. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644–57.
Takahashi H, Nomiyama T, Terawaki Y, Kawanami T, Hamaguchi Y, Tanaka T, Tanabe M, Bruemmer D, Yanase T. Glucagon-like peptide-1 receptor agonist exendin-4 attenuates neuron- derived orphan receptor 1 expression in vascular smooth muscle cells. J Atheroscler Thromb. 2019;26:183–97.
Scafoglio C, Hirayama BA, Kepe V, Liu J, Ghezzi C, Satyamurthy N, Moatamed NA, Huang J, Koepsell H, Barrio JR, Wright EM. Functional expression of sodium-glucose transporters in cancer. Proc Natl Acad Sci USA. 2015;112:E4111-4119.
Shiba K, Tsuchiya K, Komiya C, Miyachi Y, Mori K, Shimazu N, Yamaguchi S, Ogasawara N, Katoh M, Itoh M, Suganami T, Ogawa Y. Canagliflozin, an SGLT2 inhibitor, attenuates the development of hepatocellular carcinoma in a mouse model of human NASH. Sci Rep. 2018;8:2362.
Saito T, Okada S, Yamada E, Shimoda Y, Osaki A, Tagaya Y, Shibusawa R, Okada J, Yamada M. Effect of dapagliflozin on colon cancer cell. Endocr J. 2015;62:1133–7.
Komatsu S, Nomiyama T, Numata T, Kawanami T, Hamaguchi Y, Iwaya C, Horikawa T, Tanaka YF, Hamanoue N, Motonaga R, Tanabe M, Inoue R, Yanase T, Kawanami D. SGLT2 inhibitor ipragliflozin attenuates breast cancer cell proliferation. Endocr J. 2020;67:99–106.
Nakamura J, Kamiya H, Haneda M, Inagaki N, Tanizawa Y, Araki E, Ueki K, Nakayama T. Cause of death in Japanese patients with diabetes based on the resultd of survey of 45,708 cases during 2001–2010: report from the committee on the cause of death in diabetes mellitus. Diabetol Int. 2017;8:117–36.
Scafoglio CR, Villegas B, Abdelhady G, Bailey ST, Liu J, Shirali AS, Wallace WD, Magyar CE, Groan TR, Elashoff D, Walser T, Yanagawa J, Aberle DR, Barrio JR, Dubinett SM, Shackelford DB. Sodium-glucose transporter 2 is a diagnostic and therapeutic target for early-stage lung adenocarcinoma. Sci Transl Med. 2018;10:eaat5933. https://doi.org/10.1126/scitranslmed.aat5933.
Villani LA, Smith BK, Marcinko K, Ford RJ, Broadfield LA, Green AE, Houde VP, Muti P, Tsakiridis T, Steinberg GR. The diabetes medication canagliflozin reduces cancer cell proliferation by inhibition mitochondrial complex-I supported respiration. Mol Metab. 2016;5:1048–56.
Kaji K, Nishimura N, Seki K, Sato S, Saikawa S, Nakanishi K, Furukawa M, Kawaratani H, Kitade M, Moriyama K, Namisaki T, Yoshiji H. Sodium glucose cotransporter 2 inhibitor canagliflozin attenuates liver cancer cell growth and angiogenic activity by inhibiting glucose uptake. Int J Cancer. 2018;142:1712–22.
Hung MH, Chen YL, Chen LJ, Chu PY, Hsieh FS, Tsai MH, Shih CT, Chao TI, Huang CY, Chen KF. Canagliflozin inhibits growth of hepatocellular carcinoma via blocking glucose-influx-induced b-catenin activation. Cell Death Dis. 2019;10:420.
Jojima T, Wakamatsu S, Kase M, Iijima T, Maejima Y, Shimomura K, Kogai T, Tomaru T, Usui I, Aso Y. The SGLT2 inhibitor canagliflozin prevents carcinogenesis in a mouse model of diabetes and non-alcoholic steatohepatitis-related hepatocarcinogenesis: association with SGLT2 expression in hepatocellular carcinoma. Int Mol Sci. 2019;20:5237.
Tang H, Dai Q, Shi W, Zhai S, Song Y, Han J. SGLT2 inhibitors and risk of cancer in type 2 diabetes: a systematic review and meta-analysis of randomized controlled trials. Diabetologia. 2017;60:1862–72.
Acknowledgments
We thank Mitchell Arico from Edanz (https://en-author-services.edanz.com/ac) for editing the English text of a draft of this manuscript.
Funding
T.N. received lecture fees from MDS, Sumitomo Dainippon Pharma and Ono Pharmaceutical, and research grants from Sumitomo Dainippon Pharma and Mitsubishi Tanabe Pharma. T.Y. received research grants from MSD, Eli Lilly Japan, Takeda Pharmaceutical and Nippon Boehringer Ingelheim. D.K. received lecture fees from Eli Lilly Japan, Novo Nordisc Pharma and Takeda Pharmaceutical and Novartis Pharma, and research grants from Nippon Boehringer Ingelheim, Takeda Pharmaceutical, Sumitomo Dainippon Pharma and Ono Pharmaceutical.
Author information
Authors and Affiliations
Contributions
L. Y., T. K. and Y. H. performed experiments and data analysis. T. N. and S. Y. wrote the manuscript and conceived the research hypothesis and design. T. S., T. H., Y. F-T., T. Y., D. K., and A. I. reviewed the manuscript.
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Yamamoto, L., Yamashita, S., Nomiyama, T. et al. Sodium-glucose cotransporter 2 inhibitor canagliflozin attenuates lung cancer cell proliferation in vitro. Diabetol Int 12, 389–398 (2021). https://doi.org/10.1007/s13340-021-00494-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13340-021-00494-6