Abstract
Purpose
Sodium/glucose cotransporter (SGLT) 1 and 2 expression in carcinoma cells was recently examined, but their association with the clinicopathological factors of the patients and their biological effects on breast carcinoma cells have remained remain virtually unknown. Therefore, in this study, we explored the expression status of SGLT1 and SGLT2 in breast cancer patients and examined the effects of SGLT1 inhibitors on breast carcinoma cells in vitro.
Methods
SGLT1 and SGLT2 were immunolocalized and we first correlated the findings with clinicopathological factors of the patients. We then administered mizagliflozin and KGA-2727, SGLT1 specific inhibitors to MCF-7 and MDA-MB-468 breast carcinoma cell lines, and their growth-inhibitory effects were examined. Protein arrays were then used to further explore their effects on the growth factors.
Results
The SGLT1 high group had significantly worse clinical outcome including both overall survival and disease-free survival than low group. SGLT2 status was not significantly correlated with clinical outcome of the patients. Both mizagliflozin and KGA-2727 inhibited the growth of breast cancer cell lines. Of particular interest, mizagliflozin inhibited the proliferation of MCF-7 cells, even under very low glucose conditions. Mizagliflozin downregulated vascular endothelial growth factor receptor 2 phosphorylation.
Conclusion
High SGLT1 expression turned out as an adverse clinical prognostic factor in breast cancer patient. This is the first study demonstrating that SGLT1 inhibitors suppressed breast carcinoma cell proliferation. These results indicated that SGLT1 inhibitors could be used as therapeutic agents for breast cancer patients with aggressive biological behaviors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer is the most commonly diagnosed malignancy globally in female [1]. Breast cancer is pathologically categorized according to the presence or absence of estrogen receptor (ER), progesterone receptor (PgR), and human epidermal growth factor receptor 2 (HER2) status [2] Each targeted therapy was reported to markedly improve clinical outcome of breast cancer patients but it is also true that the patients subsequently developed therapeutic resistance. Therefore, novel therapies based on biological markers unique to individual patients have been warranted. Invasive ductal carcinoma (IDC) is known as the most common histologic type of breast cancer. At least some ductal carcinoma in situ (DCIS) patients are known to develop into IDC but approximately 90% of DCIS patients do not progress to IDC [3]. The mechanistic aspects of those discrepancies among DCIS patients in terms of developing into invasive phenotypes has not been necessarily explored.
In general, the rapid cell proliferation of malignant cells have been well known to be due to accelerated metabolism as a result of increased glucose uptake and lactic acid fermentation even under aerobic conditions, which has been known as the “Warburg effect” [4]. Since positron emission tomography (PET) imaging has been used in clinical practice, glucose uptake has been demonstrated to be increased in cancer cells. Increased glucose uptake by malignant cells also depends on the relative abundance of cell membrane glucose transporters [5]. Glucose transporters are generally classified into two types: glucose transporters (GLUT) and sodium/glucose cotransporters (SGLT) [6]. GLUTs transport glucose with liberty in and out of the cell resulting in the equilibrium of glucose concentrations inside and outside the cell [7]. Both sodium/glucose cotransporter 1 (SGLT1) and sodium/glucose cotransporter 2 (SGLT2) are well known to mediate the active transport of glucose according to the concentration gradient of electrochemical sodium ions across the plasma membrane, regardless of the amounts of extracellular glucose concentration [8]. Of particular interest, GLUTs have been reported to be ubiquitously present in all the living organism ranging from bacteria to mammals, whereas SGLTs present only in a limited number of mammalian organs [8]. SGLT1 was reported to be mainly located in the human small intestine and absorb glucose from the diet [9]. In addition, SGLT1 has been reported to be widely expressed in the human body including the kidney, heart, skeletal muscle, trachea, and others [10]. In contrast, SGLT2 is located only in the renal cortex and plays a pivotal roles in glucose reabsorption [10, 11]. Several recent studies have demonstrated the overexpression of SGLT1 in some human malignancies including colorectal adenocarcinoma [12], and head and neck squamous cell carcinoma [13, 14]. In addition, SGLT2 was also detected in renal cell carcinoma [15], lung cancer[16], and others.
SGLT2 inhibitors have been reported to be widely used to treat the patients with type 2 diabetes mellitus at clinical practice and to exert potential anticancer effects in certain human malignancies expressing SGLT2 [17]. In addition, SGLT2 inhibitors were also reported to attenuate the cell proliferation of carcinoma cells in the cancer of pancreas [18], prostate [18], liver [19], colon [20], and lung [21]. Based on those clinical findings, several investigators have explored the possible correlation between SGLT2 status and therapeutic efficacy of its inhibitors in some human carcinoma cells. In breast cancer, SGLT2 inhibitors, dapagliflozin and canagliflozin, were reported to arrest the cell cycle in the G1/G0 phase and induce apoptosis in ER-positive, HER2-negative carcinoma cell lines [22]. The SGLT2 inhibitor ipragliflozin was also reported to suppress the cell proliferation of ER-positive, HER2-negative breast cancer cells in a dose-dependent manner via membrane hyperpolarization and mitochondrial membrane instability, and these effects were also reported to be completely reversed by the administration of SGLT2 [23]. In addition, SGLT2 immunoreactivity in breast cancer tissues is reported to be higher than that in normal breast tissue [22]. However, the correlation between the status of SGLT2 and clinicopathological variables has not yet been reported in breast cancer patients.
SGLT1-specific inhibitors have not been clinically applied as diabetic therapies at this juncture because of their relatively marked clinical side effects, such as diarrhea, which is caused by the inhibition of SGLT1 activities in the human gastrointestinal tract [24]. The association between the status of SGLT1 and the biological behavior of breast carcinoma cells was explored. Tissue microarray analysis of triple-negative breast cancer revealed that high SGLT1 expression was associated with a larger tumor size [25]. In HER2-positive breast cancer patients, SGLT1 abundance has been reported to be an adverse clinicopathological factor [26]. In addition, at in vitro levels, SGLT1 knockdown has been reported to inhibit the cell proliferation of triple-negative [25] and HER2-positive [26] breast carcinoma cell lines. High SGLT1 expression enhanced glucose uptake and lactic acid secretion, thereby promoting M2-like tumor-associated macrophage polarization and feedback activation of epidermal growth factor receptor (EGFR)/ phosphoinositide-3 kinase (PI3K)/Akt/SGLT1 signaling in tumor cells to enhance tamoxifen resistance [27]. However, the mechanistic aspects of those findings above have also remained unknown. Therefore, in this study, we firstly attempted to elucidate the status and function of SGLT1 and SGLT2 in breast cancer patients to explore their possible therapeutic manipulation.
Materials and methods
Patients
In total, 162 primary IDC patients and 26 DCIS patients without invasive foci who underwent surgery at Tohoku University Hospital (Sendai, Japan) between January 1998 and December 2013 were included in this study. Patients with stage IV disease and those who had received neoadjuvant therapy were excluded. Clinicopathological factors and prognosis of the patients were also retrieved. This study was approved by the Medical Ethics Committee of the Tohoku University Graduate School of Medicine (Reception No. 2020–1-942). The clinicopathological features of IDC and DCIS were summarized in Tables 1 and 2.
Immunohistochemistry
Immunohistochemical analyses were performed to determine SGLT1 and SGLT2 expression levels. The characteristics of the primary antibodies used in this study were summarized in Table 3. We used the modified H-score. We defined this score as the cutoff score for high and low expression, as described in a previous study [28]. (see supplementary material for details).
Immunoblotting
The characteristics of the primary antibodies used for immunoblotting were summarized in Table 3 (see supplementary material for further details). Anti-β-actin antibody (Sigma-Aldrich, A1978, St Louis, MO, USA) was used as an internal control.
Cell lines and cultures
Two human breast carcinoma cell lines were used. MCF-7 and MDA-MB-468 cells were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA) (see supplementary material for details).
Cell proliferation assay
The effects of glucose and SGLT-1 inhibitors on cell proliferation were evaluated in MCF-7 and MDA-MB 468 cells (see supplementary material for details).
Growth factor antibody array
To analyze functions of SGLT1 other than glucose uptake, an antibody array (ab134002; Abcam) was performed following the manufacturer’s protocol (see supplementary material for details).
Statistical analysis
Statistical analyses were performed using JMP Pro 16.2.0 (SAS Institute, Cary, NC, USA) (see supplementary material for details). Statistical significance was set at P < 0.05.
Results
SGLT1 and SGLT2 status in human breast cancer tissue
The representative immunoreactivity of SGLT1 and SGLT2 in IDC was illustrated in Fig. 1. Both SGLT1 and SGLT2 were detected in the cytoplasm of invasive cancer cells. SGLT1 and SGLT2 immunoreactivity in IDC cases (n = 162), based on the modified H-score analysis, was summarized in Table 4.
SGLT1 expression as a poor prognostic factor of breast cancer
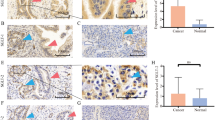
No significant correlation was detected between SGLT1-positive (n = 47) and negative (n = 115) cases (Table 5). However, SGLT1 positivity tended to be correlated with lymph node metastasis (P = 0.15) and higher pathological stage (P = 0.12), although the difference did not reach statistical significance. In both overall survival (OS) and disease-free survival (DFS) of the patients, the SGLT1 positive group demonstrated a significantly worse prognosis than that of the SGLT1 negative group (log-rank test OS, P = 0.03; DFS, P < 0.01) (Fig. 2A, B). In 44 cases, the non-pathological mammary ducts demonstrated significantly higher SGLT1 immunoreactivity than that in the breast carcinoma cells (Fig. 3A–D). The results of individual subtypes including Luminal, HER2 positive, and triple-negative breast cancer, were demonstrated in Fig. S1. We assessed 118 cases in which invasive lesions demonstrated more abundant SGLT1 immunoreactivity than that in non-pathological mammary ducts. However, there were no significant clinicopathological differences between SGLT1 positive and negative cases in those 118 cases (Table 6). The correlation between SGLT1 and prognosis in the 118 cases in which the invasive lesion was immunostained more intensely than that of the non-pathological mammary duct, and SGLT1 status was an unfavorable prognostic factor for both OS and DFS (log-rank test OS, P = 0.02; DFS, P < 0.01) (Fig. 3E, F). In addition, the number of non-pathological mammary ducts harboring more marked immunoreactivity was significantly higher in the patients younger than 50 years than in those older than 50 years in both SGLT1 (P = 0.03) and SGLT2 (P < 0.01) (Fig. S2).
Kaplan–Meier survival analysis for SGLT1 and SGLT2 status in all 162 patients with IDC. Association between SGLT1 expression and A OS and B DFS. The SGLT1-positive group had worse prognosis for both OS and DFS than those of the SGLT1-negative group. Association of SGLT2 status with C OS and D DFS. No significant differences were observed between the SGLT2-positive and SGLT2-negative groups in terms of clinical prognosis. OS overall survival, DFS disease-free survival, SGLT1 sodium/glucose cotransporter 1, SGLT2 sodium/glucose cotransporter 2, IDC invasive ductal carcinoma
Representative staining of the invasive region and non-pathological mammary ducts and Kaplan–Meier survival analysis. Two cases of IDC with different intensities of immunoactivity by SGLT1 are presented. Case 1: The immunoactivity of SGLT1 is weaker in the A invasive area than in the B non-pathological mammary ducts of the same specimen. Case 2: The immunoactivity of SGLT1 is stronger in the invasive area (C) than in the non-pathological mammary ducts (D). Kaplan–Meier survival analysis (log-rank test) for SGLT1 in a limited number of IDC patients (n = 118) whose invasive lesions showed stronger immunoactivity than that exhibited by non-pathological mammary ducts in the same specimen. Association of SGLT1 status with E OS and F DFS. IDC invasive ductal carcinoma, SGLT1 sodium/glucose cotransporter 1, SGLT2 sodium/glucose cotransporter 2
The correlation between SGLT2 and clinical outcome of breast cancer patients
There were no significant differences between SGLT2 positive (n = 36) and negative (n = 126) cases (Table 5). No significant correlation was detected between OS and DFS (log-rank test: OS, P = 0.26; DFS, P = 0.07) (Fig. 2C, D).
SGLT1 in DCIS components of IDC cases and pure DCIS cases
We then explored SGLT1 immunoreactivity in the DCIS components of IDC cases. Coexisting IDC, pure DCIS, ER-positive, and HER2-negative cases were examined. Representative findings of these DCIS components were illustrated in Fig. 4A–D. Similar to IDC cases, both SGLT1 and SGLT2 were immunolocalized in the cytoplasm of DCIS cells. As shown in Fig. 5, SGLT1 modified H-score of the DCIS component was higher in DCIS associated with IDC than in pure DCIS without invasion (P < 0.01). No significant differences were detected in the SGLT2 levels (P = 0.16).
Positive immunoactivity cases of DCIS components. A IDC with a DCIS component and B pure DCIS (without an IDC component) stained with SGLT1 (× 40 and × 100). C IDC with a DCIS component and D pure DCIS (without an IDC component) stained for SGLT2 (× 40 and × 100). IDC, invasive ductal carcinoma; DCIS, ductal carcinoma in situ; SGLT1 sodium/glucose cotransporter 1, SGLT2 sodium/glucose cotransporter 2
Comparison of modified H-score of DCIS component. SGLT1 expression was higher in DCIS associated with IDC than in pure DCIS without invasion (P < 0.01). No significant difference was found in the SGLT2 levels (P = 0.16). SGLT1 sodium/glucose cotransporter 1, DCIS ductal carcinoma in situ, IDC invasive ductal carcinoma, SGLT2 sodium/glucose cotransporter 2
SGLT1 and SGLT2 expression levels in breast carcinoma cell lines MCF-7 and MDA-MB-468
Protein expression levels of SGLT1 and SGLT2 in MCF-7 and MDA-MB-468 cells were demostrated in Fig. 6. Immunoblotting analysis revealed that both MCF-7 and MDA-MB-468 expressed SGLT1. Jurkat cells (positive controls) exhibited high SGLT2 expression. However, it was extremely low in the MCF-7 and MDA-MC-468 cells.
Immunoblotting analysis. Protein expression of SGLT1 and SGLT2. β-actin expression was used as a control. In both MCF-7 and MDA-MD-468 cells, SGLT1 was detected. SGLT2 expression in MCF-7 and MDA-MD-468 cells was significantly lower than that in positive control Jurkat cells. SGLT1 sodium/glucose cotransporter 1, SGLT2 sodium/glucose cotransporter 2
SGLT1 inhibitors suppressed cell proliferation of breast carcinoma cell lines
Based on the results of immunoblotting analysis, SGLT1 inhibitors were administered to the MCF-7 and MDA-MB-468 cells. In this study, we used two SGLT1 inhibitors: KGA-2727 and mizagliflozin. KGA-2727 demonstrated concentration-dependent anti-proliferative activity in MCF-7 cells at 48 h (Fig. 7A) and in MDA-MB-468 cells at 72 h (Fig. 7B). In addition, mizagliflozin inhibited MCF-7 cells (Fig. 7C) and MDA-MB-468 cells (Fig. 7D) growth at 1–100 μM concentration at 48 h. In order to further study whether the ability of mizagliflozin to suppress cell proliferation could depend on the inhibition of glucose uptake, we changed the medium to high glucose (4,500 mg/L) and glucose free (though in the presence of 10% FBS). In a high glucose medium, MCF-7 cell growth was inhibited by 1–10 μM of mizagliflozin at 48 h (Fig. 7E). Of particular interest, even under extremely low-glucose conditions, mizagliflozin decreased the number of MCF-7 cells after 72 h (Fig. 7F). These results all indicated that mizagliflozin had antiproliferative effects other than inhibition of glucose uptake.
Cell proliferation assay. Inhibition of proliferation by SGLT1 inhibitors in breast cancer cell lines was measured using the WST-8 assay. Cells were exposed to control (DMSO), KGA-2727, or mizagliflozin for 48 h. Both SGLT1 inhibitors inhibited the proliferation of MCF-7 and MDA-MB-468 cells in a volume-dependent manner. *P < 0.05, compared to the proliferation levels in the control. A MCF-7 for KGA-2727; 1 μM: P = 0.02, 10 μM: P < 0.01, 100 μM: P < 0.01. B MDA-MB-468 for KGA-2727; 1 μM: P = 0.03, 10 μM: P < 0.01, 100 μM: P < 0.01. C MCF-7 for mizagliflozin; 1 μM: P < 0.01, 10 μM: P = 0.02, 100 μM: P < 0.01. D MDA-MB-468 for mizagliflozin; 1 μM: P < 0.01, 10 μM: P < 0.01]. After 24 h after seeding the MCF-7 cells in a 96-well dish, the medium was replaced with E glucose-rich (4,500 mg/L glucose) or F glucose-free medium. The cells were then exposed to Control (DMSO) or mizagliflozin for 48 or 72 h. G Mizagliflozin attenuated the proliferation of MCF-7 after 48 h with a glucose-rich medium [1 μM: P = 0.02, 10 μM: P < 0.01]. F Mizagliflozin also attenuated the proliferation of MCF-7 after 72 h with a glucose-free medium [1 μM: P = 0.02, 10 μM: P = 0.03]. DMSO dimethyl sulfoxide, SGLT1 Sodium/glucose cotransporter 1
Inhibition of MCF-7 cells by mizagliflozin possibly through suppressing VEGFR-2 phosphate
We performed a growth factor antibody array analysis to further explore the mechanisms underlying the suppression of cell proliferation by mizagliflozin. Figure 8A and B demonstrated the results of the protein array analysis. Four vascular endothelial growth factor (VEGF) family proteins were significantly downregulated by 10 mM mizagliflozin treatment compared with their expression in DMSO treatment. Western blotting was subsequently performed (Fig. 8C–G), and the intensities of FMS-like tyrosine kinase-1 (Flt-1) and vascular endothelial growth factor receptor-2 (VEGFR-2) (230 kDa) expression were equivalent in the mizagliflozin-treated and control (DMSO) groups. VEGFR-2 (210 kDa) and phospho-vascular endothelial growth factor receptor-2 (pVEGFR-2) were downregulated in the mizagliflozin-treated group compared to the expression levels observed in the control group.
Results of growth factor antibody array and its validation. An antibody array (ab134002; Abcam, Cambridge, UK) was used. These proteins were downregulated following mizagliflozin treatment. Compared the expression levels in the A control (DMSO), B exposure to 10 µM mizagliflozin for 5 days suppressed VEGF-A, VEGF R-2, VEGF R-3, and VEGF-D expression in MCF-7 cells. C Immunoblotting analysis of VEGFR family members. β-actin expression was used as a control. D–G Normalized by β-actin (control = 100). The expression of pVEGFR-2 was markedly downregulated in the mizagliflozin-treated group compared with that in the control (DMSO) group. DMSO dimethyl sulfoxide, VEGF-A vascular endothelial growth factor-A, VEGFR-2 vascular endothelial growth factor receptor-2, VEGFR-3 Vascular endothelial growth factor receptor-3, VEGF-D vascular endothelial growth factor D, Flt1 FMS-like tyrosine kinase-1, pVEGFR-2 phospho-vascular endothelial growth factor receptor-2
Discussion
Results of our present study demonstrated that the SGLT1 status of carcinoma cells was an adverse clinical prognostic factor. SGLT1 status was not necessarily correlated with any of the clinicopathological factors assessed. Regardless of the immunointensity of SGLT1 in the adjacent non-pathological mammary ducts, SGLT1 abundance was significantly associated with worse clinical outcome of the patients, which indicated the biological importance of increased SGLT1 expression following malignant transformation. SGLT1 overexpression was previously reported to be correlated with poor prognosis in gastric cancer patients [29] and ovarian cancer patients [30]. SGLT1 status has been reported to be correlated with advanced clinical stages in colorectal cancer patients [12]. In oral squamous cell carcinoma, SGLT1 is associated with poor tumor differentiation [13].
In addition, in our present study, SGLT1 immunoreactivity in DCIS cells was higher in the DCIS components detected in IDC than in pure DCIS. However, the comparative status of SGLT1 and SGLT2 in IDC and DCIS remains unknown. The progression from DCIS to IDC has been recently studied based on two hypotheses: molecular and genetic changes in DCIS cells, and changes resulting from crosstalk between DCIS cells and their microenvironment [31]. Molecular profiling has identified the same genes in DCIS as those found in IDC [32, 33]. Therefore, it is reasonable to assume that invasive progression is induced by the loss of activity of suppressor gene activity rather than by the acquisition of additional oncogenic drivers, and there are in vitro reported that decreased Rap1Gap activity is responsible for invasive potential [34]. In addition, by using a 3D co-culture model with DCIS breast cancer cell lines along with human breast cancer-associated fibroblasts, myoblasts, and/or macrophages, the transition of DCIS to IDC can mimic the microenvironment of cancerous breast tissue [31]. In the human prostate, SGLT1-positive stromal cells around carcinoma cells are more abundant than those in the normal prostate or benign prostatic hyperplasia [35]. These findings indicated that SGLT1 could be related to the invasive properties of breast carcinoma cells; however, further investigation is required for clarification.
SGLT2 inhibitors have been reported to suppress the cell proliferation of breast carcinoma cells. SGLT2 expression was detected by western blotting [22] and quantitative reverse transcription-polymerase chain reaction [23] in MCF-7 cell line. SGLT2 specific inhibitors canagliflozin [22], dapagliflozin [17, 22] and ipragliflozin [23] been all reported to reduce the number of viable MCF-7 cells. In our present study, no significant correlations were detected between SGLT2 status and clinical outcomes of the breast cancer patients were detected but the SGLT2 positive groups tended to have better DFS (P = 0.07).
Overexpression of the SGLT2 gene has been considered to be glucose uptake facilitative, more proliferative, and associated with poor prognosis; therefore, the result was unpredictable. SGLT2 has been reported to be an unfavorable prognostic factor in clear cell renal carcinoma [15]. However, it is also true that SGLT1 overexpression was reported as a favorable prognostic factor in some human malignancies. Those findings were attributed to the improved therapeutic response in laryngeal [36] and cervical cancers [37] associated with MAP17-induced reactive oxygen species production. SGLT2 may also play a role in increasing treatment responsiveness in breast cancer patients; however, further investigations are required for confirmation.
In our present study, approximately one-third of the IDC cases had higher SGLT1 expression in the non-pathological mammary ducts than in the adjacent invasive carcinoma cells. Of particular interest, these patients were significantly younger with less than 50 years than those not. SGLT1 expression has been detected in lactating human mammary glands [38]. In our present study, regardless of SGLT1 expression in normal breast ducts, high SGLT1 expression in IDC was a poor prognostic factor. In our study, some patients showed higher SGLT2 expression in normal breast duct cells than in IDC cells. The sample size in this study was relatively small and similar to that of the SGLT1, the patients were younger. Low levels of SGLT2 mRNA have been detected in bovine mammary glands [39]. To the best of our knowledge, this has not been reported in human. These findings above suggested that expression of SGLT1 and SGLT2 in normal breast ducts could be influenced by sex hormone levels and lactation, but the exact mechanism is unclear.
Results of our present study demonstrated that SGLT1 inhibitors attenuated breast cancer cell proliferation, suggesting that SGLT1 also plays an important role in breast cancer cell proliferation. To the best of our knowledge, the growth-inhibitory effects of SGLT1 specific inhibitors have not been reported in any cancer cell line. KGA-2727 is the first selective SGLT1 inhibitor and has 140-fold higher specificity for human SGLT1 than for SGLT2 when evaluated by inhibition constant values (Ki) [40]. Mizagliflozin is a more specific SGLT1 inhibitor with 300-fold higher specificity for human SGLT1 than for SGLT2 [40]. In our study, both of the agents demonstrated different affinities for SGLT1 and inhibited breast carcinoma cell proliferation. We hypothesized that if SGLT1 inhibitors inhibit cell proliferation by blocking glucose uptake, their functions would be affected under high- and low-glucose conditions. Of particular interest, mizagliflozin attenuated MCF-7 cell growth under extremely low-glucose conditions. This also indicated that mizagliflozin inhibited MCF-7 cell proliferation through a mechanism other than glucose uptake inhibition.
Results of our present study also indicate that mizagliflozin inhibited MCF-7 cell proliferation by blocking VEGFR-2 phosphorylation. Protein array analysis also revealed the changes other than those in the VEGFR family, but VEGF was known to be expressed in MCF-7 cells and to be correlated with SGLT1 expression status [27]. Therefore, we tentatively focused on the VEGF family members. Angiogenesis provides nutrients, such as glucose and oxygen, for cancer cell growth and development [41]. VEGF is known as one of the pivotal factors of tumor angiogenesis. In addition, VEGF has been reported to maintain breast cancer survival independent of angiogenesis by stimulating breast cancer cell survival signals [42]. VEGFR-2 was expressed in MCF-7 cells, and when they acquire tamoxifen resistance, the VEGF/VEGFR-2 signaling loop is activated to increase cell proliferation and avoid apoptosis [43]. Tamoxifen-resistant MCF-7 cells have been reported to express SGLT1, resulting in enhanced glycolysis and lactic acid metabolism in tumor-associated macrophages and promoting their polarization via the hypoxia-inducible factor-1 alpha-STAT3 pathway [44]. VEGF has been known to be the most important tumor-promoting factor expressed by tumor-associated macrophages in breast cancer[45]. However, the correlation between SGLT1 and VEGF expression in breast cancer remains unclear. The mature form of VEGFR-2 had a molecular weight of 230 kDa, whereas the other two are non-glycosylated intermediate forms [46]. Since only the mature form of VEGFR-2 is involved in intracellular signaling [47], the upregulated 210 kDa immature VEGFR-2 may not participate in the regulatory function of cell growth. In our present study, VEGFR-2 phosphorylation was considered the main target of mizagliflozin-induced inhibition of MCF-7 cell proliferation. However, further investigations are required for confirmation.
Results of our present study also provided interesting results regarding the potential synergistic involvement of SGLT1 and VEGFR-2 in breast cancer. SGLT1 is a widely known glucose transporter, and when expressed in cancer cells, its primary function is considered to regulate glucose uptake. Signaling pathways stabilizing EGFR were also reported to be activated in oral squamous cell carcinoma [13], colorectal cancer [12], and triple-negative breast cancer [25]. SGLT1 activates the mTOR signaling pathway and promotes cell proliferation in HER2-positive breast cancer [26] and pancreatic carcinoma [48]. SGLT1 and SGLT2 transport glucose regardless of the extracellular glucose concentration. These results indicated that cancer cells in a hypoglycemic environment can express SGLTs to further increase the in situ availability of glucose and subsequently promote tumor angiogenesis by activating the VEGF pathway.
SGLT2 inhibitors have already been used in antidiabetic therapies and have demonstrated beneficial therapeutic effects in diabetes mellitus patients with heart or mild renal failure [49]. These drugs are generally considered to be anticancer agents, they have not been clinically used. Moreover, SGLT1 inhibitors are not currently used clinically in humans. Selective inhibition of SGLT1 is thought to result in adverse events such as hypoglycemia, severe diarrhea, and malabsorption due to the inhibition of glucose uptake in the intestines, such as glucose-galactose malabsorption caused by mutations in the SGLT1 gene. Novel SGLT1 selective inhibitors have been developed. In mouse experiments, KGA-2727 has been demonstrated to be relatively safe for diabetes therapy [50]. In addition, mizagliflozin has been safely administered as an oral treatment for chronic constipation in a phase 2 trial [39]. Therefore, we reasonably postulate that mizagliflozin could be safely used as an adjunct treatment for patients with breast cancer expressing SGLT1. In our present study, because VEGFR-2 phosphorylation was enhanced downstream of SGLT1, VEGFR-2 selective inhibitors may also have a similar effect. Lucitanib is a potent inhibitor of VEGFR 1–3, FGFR1–3, and platelet-derived growth factor receptor a/b, with promising antitumor activity in xenograft models [51]. However, at this stage, its efficacy in 76 patients with recurrent luminal breast cancer is limited (13% of the total cohort), and a small number of side effects such as hypertension (87%) and thyroid dysfunction (47%) are known to exist; therefore, SGLT1 inhibitors may be relatively safer.
Data availability
All data generated or analyzed during this study are included in this article and its supplementary information files.
Abbreviations
- ATCC:
-
American type culture collection
- DAB:
-
3,3′-Diaminobenzidine
- DFS:
-
Disease-free survival
- DMSO:
-
Dimethyl sulfoxide
- EGFR:
-
Epidermal growth factor receptor
- ER:
-
Estrogen receptor
- DCIS:
-
Ductal carcinoma in situ
- HER2:
-
Human epidermal growth factor receptor 2
- IDC:
-
Invasive ductal carcinoma
- GLUT:
-
Glucose transporters
- OS:
-
Overall survival
- PET:
-
Positron emission tomography
- PI3K:
-
Phosphoinositide-3 kinase
- PgR:
-
Progesterone receptor
- PVDF:
-
Polyvinylidene difluoride
- TBS-T:
-
Tris-buffered saline containing Tween-20
- SGLT:
-
Sodium/glucose cotransporters
- VEGFR-2:
-
Vascular endothelial growth factor receptor-2
References
Sung H, Ferlay J, Siegel RL et al (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71:209–249
Carey LA, Perou CM, Livasy CA et al (2006) Race, breast cancer subtypes, and survival in the Carolina breast cancer study. JAMA 295:2492–2502
Ryser MD, Weaver DL, Zhao F et al (2019) Cancer outcomes in DCIS patients without locoregional treatment. J Natl Cancer Inst 111:952–960
Warburg O (1956) On the origin of cancer cells. Science 123:309–314
Szablewski L (2013) Expression of glucose transporters in cancers. Biochim Biophys Acta 1835:164–169
Wright EM, Loo DDF, Hirayama BA (2011) Biology of human sodium glucose transporters. Physiol Rev 91:733–794
Mueckler M, Thorens B (2013) The SLC2 (GLUT) family of membrane transporters. Mol Aspects Med 34:121–138
Hediger MA, Rhoads DB (1994) Molecular physiology of sodium-glucose cotransporters. Physiol Rev 74:993–1026
Vrhovac I, Balen Eror D, Klessen D et al (2015) Localizations of Na(+)-D-glucose cotransporters SGLT1 and SGLT2 in human kidney and of SGLT1 in human small intestine, liver, lung, and heart. Pflugers Arch 467:1881–1898
Chen J, Williams S, Ho S et al (2010) Quantitative PCR tissue expression profiling of the human SGLT2 gene and related family members. Diabetes Ther 1:57–92
Kanai Y, Lee WS, You G et al (1994) The human kidney low affinity Na+/glucose cotransporter SGLT2. Delineation of the major renal reabsorptive mechanism for D-glucose. J Clin Invest 93:397–404
Guo GF, Cai YC, Zhang B et al (2011) Overexpression of SGLT1 and EGFR in colorectal cancer showing a correlation with the prognosis. Med Oncol. https://doi.org/10.1007/s12032-010-9696-8
Hanabata Y, Nakajima Y, Morita K-I et al (2012) Coexpression of SGLT1 and EGFR is associated with tumor differentiation in oral squamous cell carcinoma. Odontology 100:156–163
Helmke BM, Reisser C, Idzkoe M et al (2004) Expression of SGLT-1 in preneoplastic and neoplastic lesions of the head and neck. Oral Oncol 40:28–35
Kobayashi M, Uematsu T, Tokura Y et al (2019) Immunohistochemical expression of sodium-dependent glucose transporter - 2 (SGLT-2) in clear cell renal carcinoma: possible prognostic implications. Int Braz J Urol 45:169–178
Ishikawa N, Oguri T, Isobe T et al (2001) SGLT gene expression in primary lung cancers and their metastatic lesions. Jpn J Cancer Res 92:874–879
Villani LA, Smith BK, Marcinko K et al (2016) The diabetes medication Canagliflozin reduces cancer cell proliferation by inhibiting mitochondrial complex-I supported respiration. Mol Metabol 5:1048–1056
Scafoglio C, Hirayama BA, Kepe V et al (2015) Functional expression of sodium-glucose transporters in cancer. Proc Natl Acad Sci USA 112:E4111–E4119
Kaji K, Nishimura N, Seki K et al (2018) Sodium glucose cotransporter 2 inhibitor canagliflozin attenuates liver cancer cell growth and angiogenic activity by inhibiting glucose uptake. Int J Cancer 142:1712–1722
Saito T, Okada S, Yamada E et al (2015) Effect of dapagliflozin on colon cancer cell [rapid communication]. Endocr J 62:1133–1137
Yamamoto L, Yamashita S, Nomiyama T et al (2021) Sodium-glucose cotransporter 2 inhibitor canagliflozin attenuates lung cancer cell proliferation in vitro. Diabetol Int 12:389–398
Zhou J, Zhu J, Yu S-J et al (2020) Sodium-glucose co-transporter-2 (SGLT-2) inhibition reduces glucose uptake to induce breast cancer cell growth arrest through AMPK/mTOR pathway. Biomed Pharmacother 132:110821
Komatsu S, Nomiyama T, Numata T et al (2020) SGLT2 inhibitor ipragliflozin attenuates breast cancer cell proliferation. Endocr J 67:99–106
Song P, Onishi A, Koepsell H, Vallon V (2016) Sodium glucose cotransporter SGLT1 as a therapeutic target in diabetes mellitus. Expert Opin Ther Targets 20:1109–1125
Liu H, Ertay A, Peng P et al (2019) SGLT1 is required for the survival of triple-negative breast cancer cells via potentiation of EGFR activity. Mol Oncol 13:1874–1886
Wang J, Ji H, Niu X et al (2020) Sodium-dependent glucose transporter 1 (SGLT1) stabled by HER2 promotes breast cancer cell proliferation by activation of the PI3K/Akt/mTOR signaling pathway in HER2+ breast cancer. Dis Markers. https://doi.org/10.1155/2020/6103542
Niu X, Ma J, Li J et al (2021) Sodium/glucose cotransporter 1-dependent metabolic alterations induce tamoxifen resistance in breast cancer by promoting macrophage M2 polarization. Cell Death Dis 12:509
Zuguchi M, Miki Y, Onodera Y et al (2012) Estrogen receptor α and β in esophageal squamous cell carcinoma. Cancer Sci 103:1348–1355
Shi M, Wang C, Ji J et al (2022) CRISPR/Cas9-mediated knockout of SGLT1 inhibits proliferation and alters metabolism of gastric cancer cells. Cell Signal 90:110192
Lai B, Xiao Y, Pu H et al (2012) Overexpression of SGLT1 is correlated with tumor development and poor prognosis of ovarian carcinoma. Arch Gynecol Obstet 285:1455–1461
Brock EJ, Ji K, Shah S et al (2019) In vitro models for studying invasive transitions of ductal carcinoma in situ. J Mammary Gland Biol Neoplasia 24:1–15
Petridis C, Brook MN, Shah V et al (2016) Genetic predisposition to ductal carcinoma in situ of the breast. Breast Cancer Res 18:22
Schuetz CS, Bonin M, Clare SE et al (2006) Progression-specific genes identified by expression profiling of matched ductal carcinomas in situ and invasive breast tumors, combining laser capture microdissection and oligonucleotide microarray analysis. Cancer Res 66:5278–5286
Shah S, Brock EJ, Jackson RM et al (2018) Downregulation of Rap1Gap: a switch from DCIS to invasive breast carcinoma via ERK/MAPK activation. Neoplasia 20:951–963
Blessing Xu, Gao, et al (2012) Sodium/glucose co-transporter 1 expression increases in human diseased prostate. J Cancer Sci Ther. https://doi.org/10.4172/1948-5956.1000159
de Miguel-Luken M-J, Chaves-Conde M, de Miguel-Luken V et al (2015) MAP17 (PDZKIP1) as a novel prognostic biomarker for laryngeal cancer. Oncotarget 6:12625–12636
Perez M, Praena-Fernandez JM, Felipe-Abrio B et al (2013) MAP17 and SGLT1 protein expression levels as prognostic markers for cervical tumor patient survival. PLoS ONE 8:e56169
Obermeier S, Hüselweh B, Tinel H et al (2000) Expression of glucose transporters in lactating human mammary gland epithelial cells. Eur J Nutr 39:194–200
Inoue T, Takemura M, Fushimi N et al (2017) Mizagliflozin, a novel selective SGLT1 inhibitor, exhibits potential in the amelioration of chronic constipation. Eur J Pharmacol 806:25–31
Shibazaki T, Tomae M, Ishikawa-Takemura Y et al (2012) KGA-2727, a novel selective inhibitor of a high-affinity sodium glucose cotransporter (SGLT1), exhibits antidiabetic efficacy in rodent models. J Pharmacol Exp Ther 342:288–296
Carmeliet P (2005) VEGF as a key mediator of angiogenesis in cancer. Oncology 69(Suppl 3):4–10
Bachelder RE, Crago A, Chung J et al (2001) Vascular endothelial growth factor is an autocrine survival factor for neuropilin-expressing breast carcinoma cells. Cancer Res 61:5736–5740
Aesoy R, Sanchez BC, Norum JH et al (2008) An autocrine VEGF/VEGFR2 and p38 signaling loop confers resistance to 4-hydroxytamoxifen in MCF-7 breast cancer cells. Mol Cancer Res 6:1630–1638
Moon SY, Lee H, Kim S et al (2021) Inhibition of STAT3 enhances sensitivity to tamoxifen in tamoxifen-resistant breast cancer cells. BMC Cancer 21:931
Lewis JS, Landers RJ, Underwood JC et al (2000) Expression of vascular endothelial growth factor by macrophages is up-regulated in poorly vascularized areas of breast carcinomas. J Pathol 192:150–158
Wang X, Bove AM, Simone G, Ma B (2020) Molecular bases of VEGFR-2-mediated physiological function and pathological role. Front Cell Dev Biol 8:599281
Takahashi T, Shibuya M (1997) The 230 kDa mature form of KDR/Flk-1 (VEGF receptor-2) activates the PLC-gamma pathway and partially induces mitotic signals in NIH3T3 fibroblasts. Oncogene 14:2079–2089
Gao HF, Chen LY, Cheng CS et al (2019) SLC5A1 promotes growth and proliferation of pancreatic carcinoma via glucose-dependent AMPK/mTOR signaling. Cancer Manag Res 11:3171–3185
Zelniker TA, Wiviott SD, Raz I et al (2019) SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet 393:31–39
Dobbins RL, Greenway FL, Chen L et al (2015) Selective sodium-dependent glucose transporter 1 inhibitors block glucose absorption and impair glucose-dependent insulinotropic peptide release. Am J Physiol Gastrointest Liver Physiol 308:G946–G954
Hui R, Pearson A, Cortes J et al (2020) Lucitanib for the treatment of HR+/HER2- metastatic breast cancer: results from the multicohort phase II FINESSE study. Clin Cancer Res 26:354–363
Acknowledgements
We thank Akiko Morohashi and Katsuhiko Ono for the excellent technical assistance.
Funding
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Contributions
ST and YO performed the experiments. EI and YM designed the study. ST, AK, and TI interpreted the clinical data. ST wrote the manuscript. TS and HS reviewed and edited the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
This study was approved by the Medical Ethics Committee of Tohoku University Graduate School of Medicine (Reception No. 2020–1-942).
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tsunokake, S., Iwabuchi, E., Miki, Y. et al. SGLT1 as an adverse prognostic factor in invasive ductal carcinoma of the breast. Breast Cancer Res Treat 201, 499–513 (2023). https://doi.org/10.1007/s10549-023-07024-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-023-07024-9