Abstract
Aims
The effects of ipragliflozin, the first sodium-glucose co-transporter 2 inhibitors (SGLT2i) launched in Japan in 2014, and with dipeptidyl peptidase-4 inhibitors (DPP-4i) on glycemic control and metabolic changes were investigated comprehensively on various conditioned type 2 diabetes (T2DM) by evaluating various clinical parameters in a real-world setting.
Materials and methods
A total of 101 patients with T2DM aged 20–80 years with 7.0% ≤ HbA1c < 10.0% were followed in this 52-week, open-label, prospective, real-world, multicenter study.
Results
HbA1c decreased significantly in all groups. In ipragliflozin using groups, body weight, waist circumference, blood pressure, HOMA-IR, AST, ALT, γ-GTP, uric acid and leptin levels decreased, in contrast, HDL-cholesterol, total ketone bodies, blood urea nitrogen, creatinine, RBC, hemoglobin and hematocrit levels increased, however, in DPP-4i sole group, no significant trends were observed in these parameters. Change in leptin positively correlated with insulin, while change in total ketone bodies inversely correlated with ALT in ipragliflozin using groups. Fasting active gastric inhibitory polypeptide levels decreased in ipragliflozin sole group. Glucagon showed no changes. No significant safety concerns were observed in this study.
Conclusions
Ipragliflozin is useful and safe, showing some contrastive effects on several clinical parameters which are not shown with DPP-4i, resulting several clinical benefits. The co-administration of ipragliflozin and a DPP-4i has a better clinical outcome than either single-agent therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diet therapy is essential for the treatment of diabetes, however, it is somewhat difficult to perform it properly for patients with diabetes, especially with obese type 2 diabetes (T2DM). Sodium-glucose co-transporter 2 inhibitors (SGLT2i) have emerged as novel anti-hyperglycemic agents that reduce body weight by initial dehydration and successive activation of lipid metabolism, followed by glucose over-excretion in urine, suppressing tubular reabsorption of glucose with SGLT2 inhibition. Reducing body weight leads to improving insulin resistance and glycemic control in diabetic patients [1]. Because of this unique pharmacological mechanism, it was expected that SGLT2i might be able to overcome overeating obese patients with diabetes [2].
Dipeptidyl peptidase-4 inhibitors (DPP-4i) prolong physiological incretin levels by inhibiting DPP-4, benefiting patients with T2DM [3], especially Asian [4, 5]. Owing to the different pharmacological mechanisms of each agent, observing their individual and combined effects, a synergistic or offsetting interaction between the two agents and additional benefits of their use are expected in treating diabetes.
In Japan, ipragliflozin was the first SGLT2i to receive approval for use in 2014. Just after launched this agent, we planned and started a real-world multicenter trial: study about glucose and lipid metabolism of dipeptidyl peptidase-4 inhibitor versus ipragliflozin in inadequately controlled type 2 diabetes (LEVELUP trial) for evaluating the safety and efficacy of this agent.
As the influence of SGLT2i on adipocytokine, incretin, and glucagon is also interesting, we examined additionally, besides common clinical parameters, changes in the levels of adiponectin, leptin, active glucagon-like peptide 1 (GLP-1), active gastric inhibitory polypeptide (GIP), glucagon and total ketone bodies, expecting some novel interesting findings might be obtained in a real-world clinical setting.
Materials and methods
Trial design
This was an open-label, prospective, real-world multicenter clinical intervention study conducted at 11 facilities in Japan. A total of 101 patients among 112 were enrolled between October 2014 and September 2016, provided written informed consent, were allocated to receive DPP-4i (Group D), ipragliflozin (Group I), or DPP-4i and ipragliflozin (Group D + I), and were followed for 52 weeks (w).
To elucidate the effects of ipragliflozin on glycemic control and metabolic changes, we examined the plasma glucose levels and other various clinical parameters related to metabolic change, and adipocytokine, incretin, and glucagon levels in a real-world clinical setting.
This study was performed in compliance with the Declaration of Helsinki and the ethical guidelines for medical and health research involving human subjects and was conducted with approval (the approval number 326, dated on August 28, 2014) from the Ethics Committee, Hiroshima Red Cross Hospital & Atomic-bomb Survivors Hospital (UMIN-CTR: UMIN000015372).
Participants
Inclusionary criteria were: (1) aged 20–80 years, (2) T2DM with inadequate control despite treatment for at least 12 weeks (3) on dietary and exercise therapy only or on antidiabetic agents other than SGLT2i for more than 12 weeks and (4) 7.0% ≤ HbA1c < 10.0%.
Patients with a history of treatment with a consistent DPP-4i for less than 6 consecutive months were enrolled in Group D, while DPP-4i-naive patients were enrolled in Group I. Patients with insufficient efficacy of treatment with DPP-4i for more than 6 months were enrolled in Group D + I. Paying attention to dehydration or sarcopenia for elders was appealed at that time, in consequence, patients enrolled in Group I were obese and younger than other groups.
The patients with (1) type 1 diabetes mellitus, (2) severe ketosis, diabetic coma, (3) severe infection, recent or pending surgery, or serious trauma, (4) history of hypersensitivity to the agents used in the study, (5) renal dysfunction (serum creatinine: male ≥ 1.5 mg/dL, female ≥ 1.3 mg/dL, or eGFR < 30 mL/min/1.73 m2), (6) serious liver dysfunction, (7) serious cardiovascular complication, (8) on SGLT2i, insulin, or GLP-1 receptor agonists, and (9) pregnant or lactating women were excluded.
Treatment and endpoints
DPP-4i (any of those chosen by attending doctors) and/or ipragliflozin 50 mg was administered orally once a day for 52 weeks Changes in the HbA1c levels at 52 w were considered the primary endpoint. Secondary endpoints included changes in the levels of plasma glucose (fasting or postprandial), body weight, waist circumference, systolic blood pressure (SBP), diastolic blood pressure (DBP), fasting serum insulin, Homeostatic Model Assessment of Insulin Resistance and of β-cell function (HOMA-IR, HOMA-β), low-density lipoprotein cholesterol (LDL-C), triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), non-HDL-C, additional parameters (high molecular weight [HMW] adiponectin, leptin, active GLP-1, active GIP, glucagon and total ketone bodies [acetoacetate + beta-hydroxybutyrate + acetone]). Aspartate transaminase (AST), alanine transaminase (ALT), and gamma-glutamyl transferase (γ GTP) for liver function, blood urea nitrogen (BUN), creatinine (Cr) and eGFR for renal function, uric acid (UA), creatinine phosphokinase (CK), red blood cell (RBC), hemoglobin (Hb), hematocrit (Ht), white blood cell (WBC). platelet (Plt) and mineral balance (Na, K, Cl) were also examined. Adverse events (AEs) were recorded during the study period.
Because additional examinations were optional, not all patients underwent them. Measurement methods included the latex immunoturbidimetric assay for HMW adiponectin (LSI Medience Corp., Tokyo, Japan), double-antibody radioimmunoassay for leptin (Millipore, Burlington, MA, USA), and enzyme-linked immunosorbent assay (ELISA) for active GLP-1 (Millipore) and active GIP (Immuno-Biological Laboratories Co, Ltd, Gumma, Japan). Glucagon was obtained at fasting and measured by the sandwich ELISA (Mercodia Inc, Winston-Salem, NC, USA). Adipocytokine and incretin were obtained also at fasting because the sampling was performed simultaneously for glucagon.
During the study, other oral antidiabetic agents were available to add to or change patients’ regimen at the discretion of investigators only, in case of efficacy or safety concerns. Patients were allowed to continue daily use of concomitant drugs (e.g., antiplatelets, antihypertensives, or antihyperlipidemics), however, they were advised not to discontinue or change these medications, and not to start any new therapy or consume any supplementary food during the study.
Statistical analyses
In this study, we examined using changes from baseline in all clinical parameters. The demographic and baseline data for analysis were obtained from patients treated with a study agent at least once. SAS 9.4® software (SAS Institute Inc., Cary, NC, USA) was used for the analysis. P < 0.05 was adopted as statistically significant. The mixed effect models for repeated measures [6] was used to compare changes over time within each group (baseline vs 12 w, 24 w, 36 w, 52 w), and multiplicity was adjusted with the Dunnett method [7]. The analysis of covariance [8] with adjustment was used for comparing changes from baseline between groups, whereas multiplicity was adjusted with the Tukey method [9]. Because of the different baseline patient characteristics between 3 groups, the obtained data were adjusted at baseline for age, body weight and HbA1c. The relationship between changes in clinical parameters was assessed using Pearson’s correlation coefficient.
Results
Patient demographics
A total of 101 patients were treated in Group D (n = 28), Group I (n = 23), or Group D + I (n = 50) (Fig. 1). Of the patients analyzed, 26 discontinued the study treatment because of withdrawal of consent, moving or transferring hospitals, or use of prohibited agents owing to worsening of symptoms.
Patient dispositions. Group D, treated with DPP-4 inhibitor, included patients with less than 6 consecutive months of DPP-4i treatment and no history of change in DPP-4i at the time of enrollment; Group I, treated with ipragliflozin, included patients with no history of treatment with DPP-4i at the time of enrollment; Group D + I, treated with DPP-4i and ipragliflozin, included patients with histories of treatment with DPP-4i for more than 6 months at the time of enrollment
Patient demographics of each group are shown in Table 1. At baseline, body mass index (BMI) of patients in each group was more than 25.0 kg/m2 and patients in Group I were the most obese (BMI = 29.0 ± 4.6) and younger than the other groups (p < 0.001 vs D, p = 0.021 vs D + I). In body weight and BMI, significant difference was observed between Group D and I (p = 0.023, p = 0.007 for each). The most common concomitant medications used during the study included biguanides (56.4% of patients), followed by sulfonylureas (31.7%), α-glucosidase inhibitors (15.8%), glinides (8.9%), antihyperlipidemic agents (65.3%), and antihypertensive agents (49.5%).
Efficacy
Laboratory tests at baseline and changes from baseline (12 weeks, 24 weeks, 36 weeks, 52 weeks) are shown in Table 2.
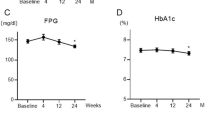
In HbA1c levels, statistically significant reductions were observed from 12 w until 52 w continuously in all groups (Fig. 2). In body weight, significant reductions in Groups I and D + I were statistically greater than those in Group D (Fig. 3a). Likewise, in waist circumference, significant reductions were observed from 12 weeks until 52 weeks continuously in Groups I and in Group D + I at 24 w and 52 w (Fig. 3b). SBP levels significantly reduced at 24 w in Group I, and from 12 w until 52 w continuously in Group D + I, and the reduction was significantly greater than in Group D (Fig. 3c). DBP levels significantly reduced at 24 w and 36 w in Group D, at 24 w and 52 w in Group D + I (Fig. 3d). HOMA-IR reduced significantly at 12 w and 52 w in Group I, at 24 w, 36 w and 52 w in Group D + I (Fig. 3e), and HOMA-β significantly increased at 52 w in Group D + I (Fig. 3f). HDL-C was significantly increased in Group I at 36 w (Fig. 5a). Non-HDL-C, LDL-C and TG levels showed no significant changes in any group throughout the study period. Although significant baseline difference were observed in AST and ALT between Group D and I (p < 0.05 for each) and between Group I and D + I (p < 0.05 for each), AST increased at 36 w in Group D, but decreased significantly from 12 w until 52 w continuously in Groups I and in Group D + I at 24 w, 36 w, 52 w, and statistically significant differences were observed in both groups at 52 w compared with Group D (Fig. 4a). ALT decreased significantly from 12 w until 52 w continuously in Group I, and in Group D + I at 24 w, 36 w, 52 w, and significant differences were observed compared with Group D (Fig. 4b). γ GTP decreased significantly in Groups I at 12 w, 24 w, 36 w, and in Group D + I throughout the study period, but showed no significant changes in Group D (Fig. 4c). BUN increased significantly in Group I at 24 weeks and in Group D + I throughout the study period, but showed no significant changes in Group D (Fig. 4d). Serum creatinine increased significantly in Group I at 12 w and in Group D + I at 24 w (Fig. 4e). eGFR decreased significantly only in Group D + I at 24 w (p < 0.0001). UA significantly decreased only in Group I at 52 w (Fig. 4f). RBC, Hb, and Ht increased significantly from 12 w until 52 w continuously in Groups I and D + I, and statistically significant differences compared with Group D were observed in Group D + I (Fig. 5b, c). Change in Hb inversely correlated with γ GTP at 52 w in Group I (Fig. 7a), while in Group D, positively correlated (Fig. 7b) and in Group D + I, offsetting an inverse correlation in Group I, a positive correlation between changes in RBC and ALT was observed (r = 0.749, p = 0.033). WBC, Plt, CK and mineral balances showed no distinct changes in any group throughout the study period.
Changes in body weight (kg) (a), and waist circumference (cm) (b), systolic blood pressure (mmHg) (c), diastolic blood pressure (mmHg) (d), HOMA-IR (e), HOMA-β (f). Data are mean ± SD. P values denote differences between baseline data versus week 12, 24, 36, and 52 data (***p < 0.001; **p < 0.01; *p < 0.05) and between DPP-4 inhibitor versus ipragliflozin or DPP-4 inhibitor and ipragliflozin (###p < 0.001; #p < 0.05)
Changes in alanine aminotransferase (IU/L) (a), aspartate aminotransferase (IU/L) (b), γ glutamyl transpeptidase (IU/L) (c), blood urea nitrogen (mg/dL) (d), serum creatinine (mg/dL) (e), uric acid (mg/dL) (f). Data are mean ± SD. P-values denote differences between baseline data versus week 12, 24, 36, and 52 data (***p < 0.001; **p < 0.01; *p < 0.05) and between DPP-4 inhibitor versus ipragliflozin or DPP-4 inhibitor and ipragliflozin (###p < 0.001; ##p < 0.01; #p < 0.05)
Changes in high-density lipoprotein cholesterol (mg/dL) (a), red blood cell (104/µL) (b), hemoglobin (mg/dL) (c), hematocrit (%) (d), white blood cell (/µL) (e), platelet (10^4/µL) (f). Data are mean ± SD. P-values denote differences between baseline data versus week 12, 24, 36, and 52 data (***p < 0.001; **p < 0.01; *p < 0.05) and between DPP-4 inhibitor versus ipragliflozin or DPP-4 inhibitor and ipragliflozin (##p < 0.01; #p < 0.05)
The data of additional examination and insulin along the clinical course are shown in Table 3. HMW adiponectin decreased significantly from 12 w until 52 w continuously in Group D, while in Group I and in Group D + I, tended to increase not significantly (Fig. 6a). Leptin decreased significantly in Group I at 12 and 24 w, and in Group D + I at 12 w, while increased significantly in Group D at 36 w (Fig. 6b). Significant positive correlation was observed between changes in leptin and insulin at 36 w in Group D + I (Fig. 7c), however, in Group I, no significant correlation was observed. For incretin, fasting active GLP-1 showed no changes in any group (Fig. 6c). Despite the fasting condition, active GIP decreased significantly in Group I at 24 w and 52 w (Fig. 6d). Insulin level at baseline in Group I was higher than that in Group D, but there was no significant difference. Significant difference in change from baseline was observed only in Group I at 52 w (Table 3). Glucagon showed no significant changes in any group (Fig. 6e). Total ketone bodies significantly increased in Group I at 36 w, whereas no significant changes were observed in Group D (Fig. 6f). Significant inverse correlation between changes in total ketone bodies and ALT was observed in Group I from 12 w until 52 w (Fig. 7d), and similarly inverse correlation between total ketone bodies and AST was observed in Group I at 36 and 52 w (r = -0.742, p = 0.035, r = -0.987, p < 0.001 for each point). No other significant correlation between changes in each additional examination data was observed.
Effects of DPP-4 inhibitor, ipragliflozin, or DPP-4 inhibitor and ipragliflozin on the time course of additional examinations. Changes from baseline data in high molecular weight adiponectin (μg/mL) (a) and leptin (mg/mL) (b), glucagon-like peptide-1 (pmol/L) (c) and gastric inhibitory polypeptide (pmol/L) (d), glucagon (pg/mL) (e) and total ketone bodies (μmol/L) (f). Data are mean ± SD. P-values denote differences between baseline data versus week 12, 24, 36, and 52 data (**p < 0.01; *p < 0.05)
Correlations between change in γ glutamyl transpeptidase (IU/L) and change in hemoglobin (g/dL) in Group I (a), and in Group D (b), and between change in leptin (mg/mL) and change in insulin (μU/mL) in Group D + I (c), and between change in total ketone bodies (μmol/L) and change in alanine aminotransferase (IU/L) in Group I (d). The relationship between the changes was assessed using Pearson’s correlation coefficient
Safety
AEs occurred in 17.9% (n = 5) of patients in Group D, 8.7% (n = 2) in Group I, and 20.0% (n = 10) in Group D + I, although none of the events was related to the agents used in this study.
Discussion
Among lifestyle modification, proper diet therapy is essential for the treatment of diabetes, especially for T2DM. In addition, the responsiveness to antidiabetic agents including DPP-4i considerably varies in the patients with T2DM, although Japanese people are generally better responders to DPP-4i compared with the population of European ancestry [10]. The BMI was higher in the patients in Group I, and they were presumably associated with insufficient lifestyle modification including proper diet therapy. Since the patients demonstrating insufficient efficacy of DPP-4i were preferentially recruited in Group D + I according to the allocation criteria, they were supposed to be poor responders to DPP-4i. In this study, HbA1c levels were significantly improved in all groups in a real-world setting. These results suggest that ipragliflozin could improve the glycemic control in the patients with obesity and insufficient lifestyle changes. Although the patients in Group D + I failed to achieve adequate improvement in HbA1c levels with preceding treatment with DPP-4i, they exhibited comparable reduction of HbA1c levels like Group D. Moreover, ipragliflozin showed the additional beneficial efficacy on several clinical parameters, which DPP-4i did not. Taken together, ipragliflozin could be one of the next additional medication strategies in the patients with insufficient glycemic control with DPP-4i, and the combination therapy of ipragliflozin and DPP-4i is useful. Significant positive correlation between changes in leptin and insulin was observed only in the patients with combination treatment (Group D + I), suggesting merit of the combination therapy.
As for lipid metabolism, ipragliflozin (Group I) improved significantly only HDL-C levels, but showed significant decrease in AST, ALT andγGTP. Moreover, ipragliflozin (Group I and Group D + I) significantly decreased body weight, waist circumference, blood pressure, HOMA-IR, uric acid and leptin levels, suggesting that ipragliflozin could reduce insulin resistance according to the metabolic cascade initiated from the energy deprivation followed by glucose over-excretion in urine, inhibiting SGLT2 and lead to the improvement not only in glycemic control but also in lipid metabolism including the amelioration of hepatic steatosis. In contrast, ipragliflozin (Group I and Group D + I) increased BUN, Cr, RBC, Hb, Ht and total ketone bodies levels. In addition, a significant inverse correlation was found between changes in liver function tests and changes in total ketone bodies and Hb in the patients treated with ipragliflozin. These results suggest that the elevation of these data supposed to be some other beneficial effects of ipragliflozin, possibly endorsing clinically several studies on kidney [11,12,13,14,15,16,17] and hematopoietic function [18]. In the combination therapy, above-mentioned inverse correlation changed to positive one. It might be offsetting effect of both agents.
Significant decrease of fasting active GIP levels with ipragliflozin (Group I) is interesting. In obese people, especially in obese patient with T2DM, the fasting insulin levels and the fasting GIP levels are a little higher compared with ordinary people [19]. Ipragliflozin improved obesity related parameters in obese patients, reducing the elevated fasting insulin levels, which might be the reason of this results.
No changes in glucagon were noted in this study. Similar to this study, reports of canagliflozin [20] and tofogliflozin [21] have noted no increases in glucagon levels, but other previous studies have described increased glucagon levels after treatment with dapagliflozin [22], empagliflozin [23] and luseogliflozin [24]. Both ipragliflozin and canagliflozin are five-membered rings with low selectivity for SGLT2. It has also been reported that glucagon secretion from alpha cells, in which no SGLT2 is expressed, of the pancreatic islets are suppressed via the SGLT1 inhibition effect [25]. However, some studies have reported increased glucagon levels with canagliflozin [26], further examination of changes in glucagon levels after treatment with SGLT2i are still warranted.
There is a possibility that the metabolic cascade initiated from the energy deprivation followed by inhibiting renal reabsorption of glucose by SGLT2i extends to central nervous system (CNS) like “entero-insular-central axis”, but no clinical data in this trial could elucidate this hypothesis, although ipragliflozin made some influence on leptin, which is known concerned with CNS [27].
The limitation of this study comes from small number of the patients and a real-world setting, however, the latter brought out several interesting and important clinical information.
In conclusion, ipragliflozin, an SGLT2i, is safe and effective in the treatment of T2DM, at least through improving insulin resistance, resulting several clinical benefits. Moreover, the combination treatment of ipragliflozin with a DPP-4i is useful in the patients with insufficient glycemic control with single agent, owing to synergistic, offsetting and complementary effects, and should be indicated from the early stage of medication therapy.
References
DeFronzo RA, Davidson JA, Del Prato S. The role of the kidneys in glucose homeostasis: a new path towards normalizing glycaemia. Diabetes Obes Metab. 2012;14:5–12.
Yokono M, Takasu T, Hayashizaki Y, Mitsuoka K, Kihara R, Muramatsu Y, Miyoshi S, Tahara A, Kurosaki E, Li Q, Tomiyama H, Sasamata M, Shibasaki M, Uchiyama Y. SGLT2 selective inhibitor ipragliflozin reduces body fat mass by increasing fatty acid oxidation in high-fat diet-induced obese rats. Eur J Pharmacol. 2014;727:66–74.
Mulvihill EE, Drucker DJ. Pharmacology, physiology, and mechanisms of action of dipeptidyl peptidase-4 inhibitors. Endocr Rev. 2014;35:992–1019.
Kim YG, Hahn S, Oh TJ, Kwak SH, Park KS, Cho YM. Diabetologia. 2013;56(4):696–708.
Yabe D, Seino Y, Fukushima M, Seino S. β Cell Dysfunction versus insulin resistance in the pathogenesis of type 2 diabetes in East Asians. Curr Diab Rep. 2015;15:36.
Mallinckrodt CH, Clark WS, David SR. Accounting for dropout bias using mixed-effects models. J Biopharm Stat. 2001;11:9–21.
Dunnett WC. A multiple comparison procedure for comparing several treatments with a control. J Am Stat Assoc. 1955;50:1096–121.
Cochran WG. Analysis of covariance: its nature and uses. Biometrics. 1957;13:261–81.
Tukey JW. Comparing individual means in the analysis of variance. Biometrics. 1949;5:99–114.
Suzuki K, Akiyama M, Ishigaki K, Kanai M, Hosoe J, Shojima N, Hozawa A, Kadota A, Kuriki K, Naito M, Tanno K, Ishigaki Y, Hirata M, Matsuda K, Iwata N, Ikeda M, Sawada N, Yamaji T, Iwasaki M, Ikegawa S, Maeda S, Murakami Y, Wakai K, Tsugane S, Sasaki M, Yamamoto M, Okada Y, Kubo M, Kamatani Y, Horikoshi M, Yamauchi T, Kadowaki T. Identification of 28 new susceptibility loci for type 2 diabetes in the Japanese population. Nat Genet. 2019;51:379–86.
Ito D, Inoue K, Sumita T, Hamaguchi K, Kaneko K, Yanagisawa M, Inukai K, Inoue I, Noda M, Shimada A. Long-term effects of ipragliflozin on diabetic nephropathy and blood pressure in patients with type 2 diabetes: 104-week follow-up of an open-label study. J Clin Med Res. 2018;10:679–87.
Kamezaki M, Kusaba T, Komaki K, Fushimura Y, Watanabe N, Ikeda K, Kitani T, Yamashita N, Uehara M, Kirita Y, Shiotsu Y, Sakai R, Fukuda T, Yamazaki M, Fukui M, Matoba S, Tamagaki K. Comprehensive renoprotective effects of ipragliflozin on early diabetic nephropathy in mice. Sci Rep. 2018;8(4029):1–14.
Wanner C, Inzucchi SE, Lachin JM, Fitchett D, Eynatten M, Mattheus M, Biomath D, Johansen OE, Woerle HJ, Broedl UC, Zimman B. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2015;375:323–34.
Toyama T, Neuen BL, Jun M, Ohkuma T, Neal B, Jardine MJ, Heerspink HL, Wong MG, Ninomiya T, Wada T, Perkovic V. Effect of SGLT2 inhibitors on cardiovascular, renal and safety outcomes in patients with type 2 diabetes mellitus and chronic kidney disease. Diabetes Obes Metab. 2019;21:1237–50.
Pollock C, Stefansson B, Reyner D, Rossing P, Sjöström CD, Wheeler DC, Langkilde AM, Heerspink HJL. Albumiuria-lowering effect of dapagliflozin alone and in combination with saxagliptin and effect of dapagliflozin and saxagliptin on glycemic control in patients with type 2 diabetes and chronic kidney disease (DELIGHT):a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2019;7:429–41.
Heerspink HJL, Karasik A, Thresson M, Melzer-Cohen C, Chodick G, Khunti K, Wilding JPH, Rodriguez LAG, Cea-Soriano L, Kohsaka S, Nicolucci A, Lucisano G, Lin FJ, Wang CY, Wittbrodt E, Fenici P, Koshiborod M. Lancet Diabetes Endocrinol. 2020;8:27–35.
Tomita I, Kume S, Sugahara S, Osawa N, Yamahara K, Yasuda-Yamahara M, Takeda N, Chin-Kanasaki M, Kaneko T, Mayoux E, Mark M, Yanagita M, Ogita H, Araki S, Maegawa H. Cell Metab. 2020;32:404–19.
Sano M, Takei M, Shiraishi Y, Suzuki Y. Increased hematocrit during sodium-glucose cotransporter 2 inhibitor therapy indicates recovery of tubulointerstitial function in diabetic kidneys. J Clin Med Res. 2016;8:844–7.
Creutzfeldt W, Ebert R, Willms B, Frerichs H, Brown JC. Gastric inhibitory polypeptide (GIP) and insulin in obesity: Increased response to stimulation and defective feedback control of serum levels. Diabetologia. 1978;14:15–24.
Ueta K, O’Brien TP, McCoy GA, Kim K, Healey EC, Farmer TD, Donahue P, Condren AB, Printz RL, Shiota M. Glucotoxicity targets hepatic glucokinase in Zucker diabetic fatty rats, a model of type 2 diabetes associated with obesity. Am J Physiol Endocrinol Metab. 2014;306:E1225–38.
Kaku K, Suganami H. Effects of a selective SGLT2 inhibitor tofogliflozin on postprandial glucose level. Blood glucose related hormones. Med Drug J. 2014;50:2274–85.
Bonner C, Kerr-Conte J, Gmyr V, Queniat G, Moerman E, Thevenet J, Beaucamps C, Delalleau N, Popescu I, Malaisse WJ, Sener A, Deprez B, Abderrahmani A, Staels B, Pattou F. Inhibition of the glucose transporter SGLT2 with dapagliflozin in pancreatic alpha cells triggers glucagon secretion. Nat Med. 2015;21:512–7.
Ferrannini E, Muscelli E, Frascerra S, Baldi S, Mari A, Heise T, Broedl UC, Woerle HJ. Metabolic response to sodium-glucose cotransporter 2 inhibition in type 2 diabetic patients. J Clin Invest. 2014;124:499–508.
Seino Y, Yabe D, Sasaki T, Fukatsu A, Imazeki H, Ochiai H, Sakai S. Sodium-glucose cotransporter-2 inhibitor luseogliflozin added to glucagon-like peptide 1 receptor agonist liraglutide improves glycemic control with bodyweight and fat mass reductions in Japanese patients with type 2 diabetes: A 52-week, open-label, single-arm study. J Diabetes Investig. 2018;9:332–40.
Suga T, Kikuchi O, Kobayashi M, Matsui S, Yokota-Hashimoto H, Wada E, Kohno D, Sasaki T, Takeuchi K, Kakizaki S, Yamada M, Kitamura T. SGLT1 in pancreatic α cells regulates glucagon secretion in mice, possibly explaining the distinct effects of SGLT2 inhibitors on plasma glucagon levels. Mol Metab. 2019;19:1–12.
Okahata S, Sakamoto K, Mitsumatsu T, Kondo Y, Tanaka S, Shiba T. Mechanistic insights from sequential combination therapy with a sodium glucose co-transporter-2 inhibitor and a dipeptidyl peptidase-4 inhibitor: Results from the CANARIS Trial using canagliflozin and teneligliptin. Diabetes Obes Metab. 2019;21:388–92.
Wang B, Cheng KKY. Hypothalamic AMPK as a mediator of hormonal regulation of energy balance. Int J Mol Sci. 2018;19(3552):1–19.
Acknowledgements
The authors are grateful to all the patients and staff at the hospitals and clinics who participated in this study. The authors also thank the staff of DOT WORLD Co., Ltd. for their assistance in data collection and statistical analysis. This study was supported by Astellas Pharma Inc., Tokyo, Japan, who were not involved in the study design.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
FS received research funding from Astellas Pharma Inc. NK received honoraria from Sumitomo Dainippon Pharma. SN received honoraria from Ono Pharmaceutical and Sanofi. The other authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Sawano, F., Kamei, N., Miyahara, M. et al. Comprehensive efficacy of ipragliflozin on various conditioned type 2 diabetes compared with dipeptidyl peptidase-4 inhibitors and with both agents, based on a real-world multicenter trial. Diabetol Int 12, 364–378 (2021). https://doi.org/10.1007/s13340-021-00492-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13340-021-00492-8