Abstract
Fungal diseases are the major constraint on canola (Brassica napus) production in Australia and worldwide. Blackleg (caused by Leptosphaeria maculans) and Sclerotinia stem rot (Sclerotinia sclerotiorum) are the predominant diseases limiting production but, with increased intensification of production, other diseases previously considered of minor importance and sporadic may be increasing in prevalence. We report on the incidence and severity of four ‘minor’ diseases of canola in Australia: white leaf spot (caused by Pseudocercosporella capsellae), downy mildew (Peronospora parasitica), Alternaria leaf and pod spot (Alternaria brassicae) and powdery mildew (Erysiphe cruciferarum). Diseases were monitored at more than 30 sites across Australia from 2013 to 2015. Regions were identified in which specific diseases are a consistent problem, such as white leaf spot in Hamilton in Victoria. In these regions, control strategies to minimise disease may be required. Varietal differences were observed for some diseases suggesting that resistance to these pathogens is already present in Australian advanced breeding material. Lastly, fungicide applications were shown to control some diseases such as white leaf spot.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fungal diseases are a major constraint to crop production worldwide. Intensification of land use results in large areas of monoculture that can be conducive to fungal infection and subsequent yield-limiting disease. In Australia, canola (Brassica napus, oilseed rape) is the third largest grain crop after wheat and barley and is primarily grown as a break crop for wheat. Canola can be sown every three or 4 years in rotation with cereals, pulses and pastures, but is also commonly grown one in every 2 years with wheat (Van de Wouw et al. 2016). Since 1990, the canola cropping area has increased from 50,000 ha to 2.7 million ha in 2013–2014 and yield has increased from 78 kt to 3832 kt (ABARES 2015).
Blackleg disease, caused by the fungal pathogen Leptosphaeria maculans, is a major constraint on canola production and as a consequence has been the focus of much research in Australia and worldwide (Fitt et al. 2006; Van de Wouw et al. 2016; Khangura and Barbetti 2001). In Australia, it is estimated that blackleg disease causes 10–15 % yield losses annually with up to 90 % yield loss in extreme situations (Sprague et al. 2006). The impact of blackleg disease in Australia is minimised through rotation with other crops, avoidance of stubble, application of fungicides and rotation of cultivars that have been bred with different resistance genes (Marcroft et al. 2004; Marcroft et al. 2012b; Van de Wouw et al. 2016). In addition to L. maculans, Sclerotinia sclerotiorum is problematic in Australia with particular regions experiencing variable levels of disease due to this fungus each year (Hind et al. 2003). Reduced yields caused by Sclerotinia stem rot are as high as 24 % in some regions in some years (Kirkegaard et al. 2006; Khangura et al. 2014). Surveys of S. sclerotiorum inoculum on petals indicate widespread presence of the pathogen (Sprague and Stewart-Wade 2002; Hind et al. 2003). Sclerotinia stem rot is more complicated to control than blackleg as disease expression occurs under very specific environmental conditions, the pathogen has a very wide host range, a necrotrophic life cycle and forms sclerotia that can survive in the soil for up to 10 years (Bolton et al. 2006; Kabbage et al. 2015).
In Australia, information on the severity and prevalence of fungal diseases of canola other than blackleg and Sclerotinia stem rot is limited. It is not known what impact increased canola intensity and production is having on other fungal diseases. In the current study, we identify four other fungal diseases of canola of wide prevalence across Australia through the analysis of their incidence and severity over a 3 year period: caused by Pseudocercosporella capsellae (white leaf spot), Peronospora parasitica (downy mildew), Alternaria brassicae (Alternaria leaf/pod spot) and Erysiphe cruciferarum (powdery mildew). We identify potential areas of high risk (‘hot spots’) for these diseases as well as varietal differences and potential fungicide control strategies.
Materials and methods
To provide the first assessment of the less common fungal diseases affecting the Australian canola industry, a set of field sites in the major canola growing areas of Australia were established and monitored for disease. The same sets of cultivars were used with standard assessments for disease symptoms (Table 1) to ensure similar comparisons across the country, the trials were assessed twice to measure diseases at the early and late stages in canola growth, and the assessments were conducted over three growing seasons to establish long term patterns.
Monitoring sites
In conjunction with the National Variety Trial (NVT) sites across Australia, disease monitoring sites were established each year between 2013 and 2015 (Fig. 1). The NVT program is a national program funded by the Australian Grains Research and Development Corporation to compare cultivars and breeding lines with standardised trial management, data generation and collection across Australia (http://www.nvtonline.com.au/). The number of sites monitored for disease each year ranged from 16 to 33. These disease monitoring sites were established primarily to monitor blackleg disease and they contained seven cultivars/advanced breeding lines that represent different blackleg resistance groups (Marcroft et al. 2012a). The cultivars/lines varied each year due to seed availability and were sown in the absence of fungicide. The B. napus cultivars/lines used were ATR-Stingray (all years), CB Telfer (all years), CrusherTT (2013, 2014), Hyola444TT (2013, 2014), ThumperTT (2013, 2014), ATR-Marlin (2014, 2015), T28156 (2014, 2015), Surpass501TT (2013), Hyola650TT (2015), ATR-Gem (2015) and Hyola450TT (2015). A B. juncea advanced breeding line, JBOT800407, was also used in 2013. All cultivars were sown in three replicate plots (10 m × 1 m) with complete randomisation designed using DiGGer software. All sites were monitored for the incidence of canola diseases. Dates of sowing and harvest, monthly rainfall, minimum and maximum temperate are provided for the sites in Supplementary Table 1.
Location of disease monitoring sites across Australia. Numbers refer to locations listed in Table 3
Fungicide control trials
Three trials were established (one each in Victoria, New South Wales and Western Australia) to determine whether current fungicides used to control blackleg disease would also reduce disease severity for white leaf spot and downy mildew. These two diseases were chosen as they are common in most years and are present during the early vegetative growth when fungicides are applied in commercial fields for blackleg control. All trials were sown adjacent to the disease monitoring sites described above, in three replicate plots (10 m × 1 m in Victoria and NSW and 20 m × 1.4 m in WA), with complete randomisation designed using DiGGer software. Cultivars with triazine tolerance (TT) were used: CrusherTT, CB Telfer, ATR-Stingray, PioneerSturtTT and Hyola559TT. Three fungicide treatments were used; seed dressing (Jockey®, fluquinconazole) only, seed dressing plus foliar fungicide (Prosaro®, mixture of prothioconazole and tebuconazole) applied at the 4–5 leaf stage, or fungicide amended fertilizer (Impact®, flutriafol), as per label recommendations, plus an untreated control.
Data collection and analysis
The monitoring and fungicide trial sites were surveyed twice each year to detect diseases expressed at (1) the rosette vegetative growth stage (primarily for white leaf spot and downy mildew) and (2) adult plant stages (primarily for Alternaria leaf/pod spot and powdery mildew). General disease severity (designated ‘site severity’) was recorded as absent, present, minor, moderate and severe for each of the four diseases at the sites (Table 1). In addition, a selection of sites at which disease levels were severe was scored in more detail to determine potential genetic differences or efficacy of fungicides. At these sites, disease incidence and/or disease severity was determined. Disease incidence was determined by counting 20 consecutive plants in a plot and recording the number of plants that displayed disease symptoms. These data were collected from a single plot for each cultivar. Secondly, each of the three replicate plots per cultivar was scored for disease severity on a 0–4 scale (Table 1). Average disease severity for each cultivar was then determined from the three replicate plots. Significant differences in disease severity were identified by a one-way analysis of variance after square root transformation of the data. P-values less than 0.05 were considered statistically significant. All data were analysed using GenStat® 16th Edition.
Results
Canola diseases were assessed in trial sites across Australia over three growing seasons. While blackleg (caused by L. maculans) and Sclerotinia stem rot (caused by S. sclerotorium) were the primary diseases observed in the trials, other diseases were also present. Although some of these diseases were rare, localised or sporadic (such as hypocotyl rot, charcoal rot, club root and turnip yellows virus), four fungal diseases were consistently present in sites across Australia, and were therefore analysed in more detail.
General symptom descriptions
The symptoms of the four diseases are represented in Fig. 2. White leaf spot and downy mildew generally occur during the vegetative stages of crop growth, however, white leaf spot can spread up the canopy as plants elongate. White leaf spot lesions occur on the leaves and are greyish-white to brown often with brown margins. In severe situations, complete loss of leaf area resulting in crop failure can occur (Fig. 2a and b). Downy mildew lesions are characterised by yellowish-brown discolouration on the upper leaf surface with white mycelial masses on the under surface of the leaf (Fig. 2c). Symptoms of this disease are usually restricted to the oldest true leaves, which are in contact with the soil surface, and in severe situations reduce photosynthetic area resulting in potential yield loss (Fig. 2d). Alternaria pod and leaf spot symptoms include dark target-like lesions on the leaves (Fig. 2e) and black spots on the pods (Fig. 2f). Alternaria pod spot can lead to premature ripening and shattering of pods. Powdery mildew symptoms appear as white powdery spots on leaves (Fig. 2g) and stems (Fig. 2h) most commonly during pod fill.
Symptoms of four fungal pathogens of Brassica napus and Brassica juncea in Australia between 2013 to 2015 (excluding Leptosphaeria maculans and Sclerotinia sclerotorium). White leaf spot (caused by Pseudocercosporella capsellae) symptoms visible on the oldest leaf (a) and moving up the canopy of the plant (b). Hyphal growth of downy mildew (caused by Peronospora parasitica) on the underside of the oldest leaf (c) as well as yellowing of the oldest leaves across a plot (d). Symptoms caused by Alternaria brassicae on a leaf (e) and pods (f). Powdery mildew (caused by Erysiphe cruciferarum) symptoms on the stem (g) and leaves (h)
White leaf spot
White leaf spot was detected in most of the trial sites assessed in at least 1 year of the survey, and ranged from 56 % incidence in 2013 to 55 % in 2015 (Table 2). The highest levels of severity were detected in Victoria, and at one site, Hamilton, the level of disease was moderate to severe each year of the survey thus indicating a hot spot for this disease (Table 3).
In 2013, white leaf spot was detected at all sites in Victoria and disease incidence was determined for three genotypes, two B. napus cultivars and a B. juncea advanced breeding line. Disease incidence was significantly lower in the B. juncea cultivar (14 %) compared to the B. napus varieties (50 % for CrusherTT and 54 % for CB Telfer). In 2014, the severity of white leaf spot was determined at Hamilton for all B. napus cultivars to detect potential genotypic differences. The level of disease was similar (ranging from 3.7 to 4.0) in all cultivars with the exception of line T28156, which had significantly less disease (Table 4).
The application of fungicides significantly reduced the level of disease caused by white leaf spot but varied across experiments. At Diggora, Victoria, the application of a seed dressing (Jockey®) and foliar fungicide (Prosaro®) significantly reduced the level of white leaf spot on the three cultivars tested compared to the bare control (Fig. 3). At Grenfell, NSW, the combined application of a seed dressing (Jockey®) and fungicide amended fertilizer (Impact®) significantly reduced disease compared to Jockey® alone but the foliar fungicide (Prosaro®) did not significantly reduce disease compared to the seed treatment alone (Fig. 4). In addition, without the application of fungicides cultivar Pioneer SturtTT exhibited less disease symptoms than ATR-Gem and Hyola650TT.
Effect of fungicide application on the severity of white leaf spot (caused by Pseudocercosporella capsellae) at Diggora, Victoria, in 2013. The application of the seed-dressing fungicide, Jockey®, as well as the addition of a foliar fungicide, Prosaro®, significantly reduced disease severity compared to the nil control for all three B. napus cultivars screened. Plants were assessed at rosette vegetative growth stage. Data are average of three replicate plots. * Represents p-value less than 0.05
The effect of fungicide application on severity of white leaf spot (caused by Pseudocercosporella capsellae) at Grenfell, NSW, in 2015. Plants were assessed at the rosette vegetative growth stage. Values are the mean of 4 replicate plots. Mean infection scores followed by the same letter are not significantly different at p = 0.05
Downy mildew
Downy mildew was detected at most sites assessed, with the site incidence ranging from 100 % in 2013 to 52 % in 2015 (Tables 2 and 3). In 2013, the disease incidence for downy mildew was determined for two B. napus cultivars and a B. juncea advanced breeding line. As for white leaf spot, the average disease severity of downy mildew was significantly less in B. juncea (12 %) than the B. napus cultivars (64 % for CrusherTT and 59 % for CB Telfer) for the nine trial sites. Genotypic differences were evident in the B. napus cultivars at various sites in 2013. Cultivars ATR-Gem, CB Telfer and ATR-Stingray had consistently less disease than each of the other cultivars (Fig. 5). The impact of fungicides to control downy mildew was also determined. Unlike for white leaf spot, the application of the fungicides in these trials did not significantly reduce severity of downy mildew (Table 5).
Severity of downy mildew (caused by Peronospora parasitica) at six sites (1 VIC, 2 SA, 3 NSW) in 2013. Cultivars ATR-Gem, CB Telfer and ATR-Stingray had significantly less disease than the remaining three cultivars. Plants were assessed at the rosette vegetative growth stage. Data are average of three replicate plots across 6 sites. * Represents p-value less than 0.05
Alternaria leaf and pod spot
Alternaria leaf/pod spot was a sporadic disease in the 2013–2015 seasons, with site incidence ranging from 9 % in 2014 to 48 % in 2013 (Table 2) and moderate to severe infection only detected at four sites in 2013 (Table 3). In 2013, disease severity was lower in the B. juncea line than the B. napus cultivars at two Victorian sites, Hamilton and Streatham. Cultivars Surpass501TT and ThumperTT had less disease at Streatham but this was not consistent with the Hamilton site (Table 6).
Powdery mildew
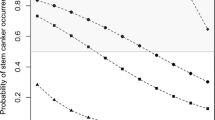
Powdery mildew was a sporadic disease with incidence ranging from 4 % in 2013 to 33 % in 2014 (Table 2), with only three sites showing moderate levels, all in 2014 (Tables 2 and 3). In WA, although powdery mildew was not detected at the blackleg monitoring sites, it was widely distributed in the northern and Central Agricultural regions in 2015 (data not shown). In 2014, severity was scored on the B. napus genotypes at five sites (1 VIC, 4 SA). Cultivars CrusherTT, ThumperTT and ATR-Marlin showed significantly less disease than the other cultivars (Fig. 6).
Severity of powdery mildew (caused by Erysiphe cruciferarum) at five sites (4 SA, 1 Vic) in 2014. Cultivars CrusherTT, ThumperTT and ATR-Marlin show significantly less disease than the other cultivars. Plants were assessed at maturity. Data are average of three replicate plots across 6 sites. * Represents p-value less than 0.05
Discussion
The canola industry in Australia has increased more than 50-fold since 1995, with 2.7 million hectares sown and 3.8 million tonnes production in 2013–2014 (ABARES 2015). A number of microbial diseases affect B. napus thereby reducing grain production, and while some diseases are ubiquitous and common, such as blackleg disease, others occur sporadically, are localised to certain regions, or occur in different parts of the landscape where the micro-climate is conducive.
There have been previous estimates of the potential impact of the less common canola diseases in Australia, such as charcoal rot caused by Macrophomina phaseolina in Western Australia (Khangura and Aberra 2009) or Sclerotinia stem rot in southern New South Wales (Kirkegaard et al. 2006) and Western Australia (Khangura et al. 2014) but these have been specific to individual regions. Diseases can be influenced by genetic background of the cultivar, climate, management practices and fungicide applications. While localised in reporting, the current distribution and the potential for diseases to spread geographically remains unclear and therefore requires a robust surveillance system. However, a national approach is required to be able to take a systematic analysis of disease incidence, define trends in disease prevalence that may pose a threat to the industry, and identify potential control mechanisms. In this study, these challenges were addressed such that a standard set of B. napus cultivars was monitored for disease at 33 sites in three growing seasons. The primary observations were that white leaf spot and downy mildew are common across all canola growing regions of Australia, however in most cases they are present at low levels and unlikely to affect yield.
Some diseases were problematic at specific locations, yet may be targeted by fungicide treatment. For instance, white leaf spot was moderate or severe at Hamilton each year with disease probably resulting in yield loss at this site due to damage to leaf tissue and therefore presumably photosynthesis capability. Data from two field experiments showed that control of white leaf spot can be achieved with fungicides currently used to control blackleg disease, viz. Jockey® (seed dressing), Impact® (fungicide amended fertilizer) and Prosaro® (foliar fungicide), although Prosaro® was not effective at Grenfel in 2015. Unlike white leaf spot, downy mildew was not controlled by Jockey®, Impact® or Prosaro®. These fungicides all belong to the azole class of fungicides that target the product of the Cyp51 gene, lanosterol 14α-demethlyase (Joseph-Horne and Hollomon 1997). This is consistent with the fact that downy mildew is an oomycete in the Order Peronosporales, a lineage that does not make ergosterol and lacks the Cyp51 target gene (Haas et al. 2009).
Genetic resistance in crop plants is one of the main strategies to control diseases. In the current survey, varietal differences were detected in all four diseases. An advanced breeding line of B. juncea showed significantly less white leaf spot, Alternaria leaf/pod spot and downy mildew than the B. napus cultivars. This is consistent with previous studies whereby accessions of B. juncea have shown differential reactions to isolates of P. parasitica (causing downy mildew), A. brassicae (causing Alternaria leaf/pod spot) and P. capsellae (causing white leaf spot) (Nashaat and Awasthi 1995; Vishwanath et al. 1999; Gunasinghe et al. 2014). Unfortunately, there is no longer any commercial B. juncea cultivars on the market in Australia. However, B. juncea has been used in breeding programs for blackleg resistance (Van de Wouw et al. 2016) so it is possible that some of these traits have also been introgressed. Furthermore, within the B. napus cultivars and lines tested here, varietal differences were observed for downy mildew, powdery mildew and to a lesser extent white leaf spot. Although more extensive experimental work is required, the differences in cultivars detected in just a small number of genotypes in this study indicate that there may be more substantial resistance sources within current advanced breeding material. Given the sporadic nature of some of these diseases and the low return on investment for breeding companies to develop resistant cultivars, knowledge that there is already some resistance in available germplasm is useful information as an incentive to consider these ‘minor’ diseases in cultivar selection.
Powdery mildew and Alternaria leaf/pod spot were sporadic diseases in Australia over the 3 years assessed, and at current levels are unlikely to warrant specific breeding directions or control strategies. However, extremely dry springs in 2014 and 2015 probably constrained the severity of these diseases that traditionally develop late in the season under wetter conditions. Alternaria leaf/pod spot was detected at the greatest number of sites in 2013 when rainfall was greater in the spring.
Canola provides significant break crop benefits to subsequent cereal crops, yet has also become an economically viable crop in its own right. Increased production leads to an environment in which pathogens, which may have been rare, can emerge as major threats to the entire industry across the country particularly if no resistant cultivars are known or the impact of fungicides is untested. Identification of these pathogens can be difficult due to their sporadic nature. This study sets a nation-wide benchmark for how to measure disease severity across a continent, defines the potential disease threats to the canola industry, and provides evidence that commercial fungicides are available that can reduce disease risk for some of these pathogens. While the discoveries provide insight into the diseases that currently reduce the full potential of the Australian canola industry, taking similar experimental approaches around the world would likely be able to provide greater insight into diseases affecting the oilseed industry.
References
ABARES (2015) Agricultural commodity statistics 2015. Australian Government Department of Agricultural and Water Resources, Canberra
Bolton MD, Thomma BPHJ, Nelson BD (2006) Sclerotinia sclerotiorum (Lib.) de Bary: biology and molecular traits of a cosmopolitan pathogen. Mol Plant Pathol 7:1–16
Fitt BDL, Brun H, Barbetti MJ, Rimmer SR (2006) World-wide importance of phoma stem canker (Leptosphaeria maculans and L. biglobosa) on oilseed rape (Brassica napus). Eur J Plant Pathol 114:3–15
Gunasinghe N, Pei You M, Banga SS, Barbetti MJ (2014) High level resistance to Pseudocercosporella capsellae offers new opportunities to deploy host resistance to effectively manage white leaf spot disease across major cruciferous crops. Eur J Plant Pathol 138:873–890
Haas BJ, Kamoun S, Zody MC, Jiang RHY, Handsaker RE, Cano LM, Grabherr M, Kodira CD, Raffaele S, Torto-Alalibo T, Bozkurt TO, Ah-Fong AMV, Alvarado L, Anderson VL, Armstrong MR, Avrova A, Baxter L, Beynon J, Boevink PC, Bollmann SR, Bos JIB, Bulone V, Cai G, Cakir C, Carrington JC, Chawner M, Conti L, Costanzo S, Ewan R, Fahlgren N, Fischbach MA, Fugelstad J, Gilroy EM, Gnerre S, Green PJ, Grenville-Briggs LJ, Griffith J, Grünwald NJ, Horn K, Horner NR, Hu CH, Huitema E, Jeong DH, Jones AME, Jones JDG, Jones RW, Karlsson EK, Kunjeti SG, Lamour K, Liu Z, Ma L, MacLean D, Chibucos MC, McDonald H, McWalters J, Meijer HJG, Morgan W, Morris PF, Munro CA, O'Neill K, Ospina-Giraldo M, Pinzón A, Pritchard L, Ramsahoye B, Ren Q, Restrepo S, Roy S, Sadanandom A, Savidor A, Schornack S, Schwartz DC, Schumann UD, Schwessinger B, Seyer L, Sharpe T, Silvar C, Song J, Studholme DJ, Sykes S, Thines M, van de Vondervoort PJI, Phuntumart V, Wawra S, Weide R, Win J, Young C, Zhou S, Fry W, Meyers BC, van West P, Ristaino J, Govers F, Birch PRJ, Whisson SC, Judelson HS, Nusbaum C (2009) Genome sequence and analysis of the Irish potato famine pathogen Phytophthora infestans. Nature 461:393–398
Hind TL, Ash GJ, Murray GM (2003) Prevalence of sclerotinia stem rot of canola in New South Wales. Aust J Exp Agric 43:1–6
Joseph-Horne T, Hollomon DW (1997) Molecular mechanisms of azole resistance in fungi. FEMS Microbiol Lett 149:141–149
Kabbage M, Yarden O, Dickman MB (2015) Pathogenic attributes of Sclerotinia sclerotiorum: switching from a biotrophic to necrotrophic lifestyle. Plant Sci 233:53–60
Khangura R, Aberra M (2009) First report of charcoal rot on canola caused by Macrophomina phaseolina in Western Australia. Plant Dis 93:666
Khangura RK, Barbetti MJ (2001) Prevalence of blackleg (Leptosphaeria maculans) of canola (Brassica napus) in Western Australia. Aust J Exp Agric 41:71–80
Khangura R, Van Burgel A, Salam M, Aberra M, MacLeod WJ (2014) Why Sclerotinia was so bad in 2013? Understanding the disease and management options. Proceedings of the 2014 Crop Updates, Perth, Western Australia
Kirkegaard JA, Robertson MJ, Hamblin P, Sprague SJ (2006) Effect of blackleg and sclerotinia stem rot on canola yield in the high rainfall zone of southern New South Wales, Australia. Aust J Agric Res 57:201–212
Marcroft SJ, Elliott VL, Cozijnsen AJ, Salisbury PA, Howlett BJ, Van de Wouw AP (2012a) Identifying resistance genes to Leptosphaeria maculans in Australian Brassica napus cultivars based on reactions to isolates with known avirulence genotypes. Crop Pasture Sci 63:338–350
Marcroft SJ, Sprague SJ, Pymer SJ, Salisbury PA, Howlett BJ (2004) Crop isolation, not extended rotation length, reduces blackleg (Leptosphaeria maculans) severity of canola (Brassica napus) in South-Eastern Australia. Aust J Exp Agric 44:601–606
Marcroft SJ, Van de Wouw AP, Salisbury PA, Potter TD, Howlett BJ (2012b) Rotation of canola (Brassica napus) cultivars with different complements of blackleg resistance genes decreases disease severity. Plant Pathol 61:934–944
Nashaat NI, Awasthi RP (1995) Evidence for differential resistance to Peronospora parasitica (downy mildew) in accessions of Brassica juncea (mustard) at the cotyledon stage. J Phytopathol 143:157–159
Sprague SJ, Marcroft SJ, Hayden HL, Howlett BJ (2006) Major gene resistance to blackleg in Brassica napus overcome within three years of commercial production in southeastern Australia. Plant Dis 90:190–198
Sprague SJ, Stewart-Wade S (2002) Sclerotinia in canola - results from petal and disease surveys across Victoria in 2001. In: Grains research and development corporation research update - southern region, Australia. Grains Research and Development Corporation, Victoria, p. 78
Van de Wouw AP, Marcroft SJ, Howlett BJ (2016) Blackleg disease of canola in Australia. Crop Pasture Sci 67:273–283
Vishwanath SJ, Kolte SJ, Singh MP, Awasthi RP (1999) Induction of resistance in mustard (Brassica juncea) against Alternaria black spot with an avirulent Alternaria brassicae isolate-D. Eur J Plant Pathol 105:217–220
Acknowledgments
We thank the Grains Research and Development Corporation for funding, and Advanta Seeds, Nuseed, Seednet, DuPont Pioneer and Canola Breeders Australia for providing seed.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Supplementary Table 1
Dates of sowing and harvest, monthly rainfall, minimum and maximum temperate data for all disease monitoring sites (PDF 60 kb)
Rights and permissions
About this article
Cite this article
Van de Wouw, A.P., Idnurm, A., Davidson, J.A. et al. Fungal diseases of canola in Australia: identification of trends, threats and potential therapies. Australasian Plant Pathol. 45, 415–423 (2016). https://doi.org/10.1007/s13313-016-0428-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13313-016-0428-1