Abstract
New disease symptoms were observed on canola (Brassica napus) crops late in the 2019 and 2020 growing seasons in Western Australia. Cladosporium macrocarpum was isolated from infected material, and the fungus responsible for the symptoms was demonstrated by fulfilling Koch’s postulates. One strain exhibited relatively high tolerance to prothioconazole and tebuconazole fungicides compared to other canola pathogens Sclerotinia sclerotiorum and Leptosphaeria maculans. In a 2019 field trial in a commercial canola crop, symptom incidence caused by Cladosporium was only significantly reduced by a double application of a foliar fungicide (active ingredients prothioconazole and tebuconazole) registered in canola for other diseases, while single applications gave no significant response. This new disease, caused by a ubiquitous fungal species, may be a consequence of changes to farm management strategies to reduce other fungal diseases of canola.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diseases impact the production of the oilseed crop canola (or rapeseed; Brassica napus) wherever it is cultivated around the world. In Australia, the most destructive disease is blackleg, caused primarily by the fungus Leptosphaeria maculans or a lesser extent by Leptosphaeria biglobosa (Van de Wouw et al. 2016b). Other diseases are also present in Australia of variable levels of severity depending on geographic location and seasonal conditions (Van de Wouw et al. 2016a). One trend has been the increase in diseases of the aerial parts of mature plants, such as Sclerotinia stem rot caused by Sclerotinia sclerotiorum or upper canopy infection caused by L. maculans (Derbyshire and Denton-Giles 2016; Sprague et al. 2017).
In the 2019 and 2020 growing seasons, growers and agronomists in Western Australia (WA) noticed lesion symptoms on stems, branches and pods that were inconsistent with known canola diseases in Australia. The current study aimed to identify the cause.
Materials and methods
Fungal isolation and culturing
To isolate the causative organism on diseased canola, infected tissues were surface sterilized in diluted bleach (1.2% available chlorine; LabCo®) for 5 min, washed in sterile water, then excised with a scalpel and plated onto potato dextrose agar (PDA) supplemented with antibacterial chemicals cefotaxime (100 µg/ml) and chloramphenicol (30 µg/ml). Alternatively, diseased tissue was washed in sterile water to release spores, and then plated onto PDA supplemented with antibiotics. Emergent fungal colonies were subcultured onto the same medium by streaking spores with a metal loop, and isolating colonies derived from single spores.
One strain (UoM19-17) was tested for its ability to grow in the presence of two demethylation inhibitor (azole) fungicides, which target ergosterol biosynthesis, and are registered for use against Sclerotinia stem rot and commonly used to control upper canopy infection caused by L. maculans. The concentrations of tebuconazole and prothioconazole to inhibit growth on PDA were tested over a range of two-fold dilutions from 0.125 to 32 µg/ml. Colony diameters were measured and the data fit with a symmetrical sigmoidal model in MyCurveFit.com to estimate the effective concentration for inhibition of growth by 50% (EC50).
For competition with other fungi, strain UoM19-17 was inoculated on PDA ~ 0.5 cm away from L. maculans strain M1 (alternative names for this strain are IBCN18 or D5).
Plant inoculations
The strains were cultured on PDA, and the surface scraped with a scalpel and placed in 0.5% Tween 20 solution, then spores filtered through miracloth. Drops of 10 µl containing 500 spores were placed at different intervals, from the base to below the start of flower production on stems of B. napus cv. Westar at the flowering stage of development and allowed to run downwards. Canola cotyledons (two-week old seedlings), and leaves and seed pods on mature plants were also inoculated. Wounding of the plant tissues was not used, and relative humidity was not altered. The negative control was 0.5% Tween 20 solution.
Strain UoM19-17 was used primarily for disease testing. Three weeks after inoculation of a stem with this strain, the wounded site was excised, surface sterilised as described above, and plated onto PDA containing antibiotics to reisolate the fungus.
Light and scanning electron microscopy
Spores were examined by light and scanning electron microscopy.
Infected plant samples were rinsed with ethanol or bleach solution then incubated overnight with a wet paper towel, and transferred with sticky tape into lactic glycerol and a coverslip. Spores were examined with a Leica DM3000 compound microscope. Alternatively, spores were washed from actively growing cultures, filtered through miracloth, concentrated by centrifugation, and then mounted on a glass slide and examined with a Leica DM6000 compound microscope.
Strain UoM19-17 was cultured on nitrocellulose membrane placed on PDA, then transferred to a stub, sputter coated with gold using a Quorum 150 T ES plus machine, and examined in a Hitachi TM4000Plus scanning electron microscope.
DNA sequence generation and analysis
The strains were cultured in potato dextrose broth, then freeze-dried mycelia disrupted with glass beads and genomic DNA extracted (Pitkin et al. 1996). The internal transcribed spacer (ITS) regions were amplified with primers ITS1 (5´-TCCGTAGGTGAACCTGCGG-3´) and ITS4 (5´-TCCTCCGCTTATTGATATGC-3´) (White et al. 1990), and the amplicons sequenced, as this information is a common approach for the efficient identification of fungal species (Schoch et al. 2012).
To generate additional DNA markers, a low level of coverage of the genome of strain UoM19-17 was obtained as 150 nucleotide paired-end reads using an Illumina NovaSeq 6000 instrument from genomic DNA processed by the Murdoch Children's Research Institute (Melbourne, Victoria). Reads were analysed using Geneious version 8.1.9 (Kearse et al. 2012) by alignment and reiterative assemblies to four gene regions used most commonly for Cladosporium phylogenetic comparisons (i.e. fragments of genes encoding actin, translation elongation factor 1, histone H3 and calmodulin). Sequences were submitted to GenBank, with the accessions provided in Table 1.
DNA sequences from strains in the Cladosporium herbarum species complex were obtained from GenBank (as in Table 2). Cladosporium versiforme was used in initial alignments and analyses, but subsequently excluded due to the availability of sequences for only two gene regions, and evidence that the sequences of the Western Australian strains did not match this species. The four strains of C. macrocarpum and C. herbarum were selected to represent nucleotide diversity within these species, as defined by Schubert et al. (2007). The new and previously obtained sequences were aligned using ClustalW, the alignments adjusted manually, then concatenated and phylogenetic trees produced in MEGA 7 (Kumar et al. 2016).
The TEF1 region features multiple polymorphisms that distinguish C. macrocarpum and C. herbarum, and therefore a region of this gene was amplified with primers MAI0760 (5´-GCACATCCGAACACAATGAC-3´) and MAI0761 (5´-CTTGACACCGAGAGTGTAGG-3´) from the four other strains and sequencing using Sanger chemistry (GenBank accessions are provided in Table 1).
Field trials
The first focus on disease development was at Alma (near Northampton, Western Australia) in 2019 in a field trial conducted in a commercial canola crop designed to explore the management of foliar diseases. The paddock of Pioneer® 43Y23 RR was sown by the grower at 1.4 kg/ha with an emergence of an average of 30 plants/m2. Trial plots were 20 m × 1.8 m, with a buffer area of crop in between plots, and the trial had a randomized complete block design. Treatments in the trial were a range of timings of application of a foliar fungicide registered in canola at the time for Sclerotinia stem rot. Prosaro® (prothioconazole 210 g/L, tebuconazole 210 g/L) at 450 mL/ha was applied in 100 L/ha water volume with a boom spray at 0.5 m above crop height. Fungicide treatments were applied at: 20% bloom stage of crop flower development (single application), 50% bloom stage (single application), and at both 20% and 50% bloom stages (i.e. a double application of fungicides). There were four replicates of each treatment, and eight replicates of the untreated plots. Disease incidence was assessed in the trial during pod ripening just prior to crop maturity by assessing forty plants (ten random plants at four sites) per plot for presence of the dark lesions on the plants. Symptom severity was not scored. At maturity, plots were harvested using a small plot harvester, yield per plot recorded and a 1 kg sample of grain from each plot retained to assess grain quality measures (percentage moisture, oil, and 1000 grain weight). Yield, oil content and 1000 grain weight were standardized to 6% moisture content. Results were analysed with Genstat 19th Edition statistical software (VSN Intl. Ltd).
A similar field trial was conducted at Dale (south east of Perth, Western Australia) in 2020 in a commercial canola crop of Nuseed® ATR Bonito. Treatments in the trial were plus and minus fungicide application, with Prosaro® applied at 450 mL/ha at 20% bloom. Fungicide application methods, replicates, harvest methods, grain and data analysis were the same as at the Alma site. The plot sizes at Dale were reduced to 15 m in length and disease assessments performed on twenty plants (five plants at four sites) per plot during pod ripening for the presence of pod damage (no stem or branch lesions were noted at the site).
Results and discussion
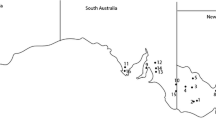
In 2019 and 2020 dark lesions were observed on the main stems, branches and pods of B. napus crops in Western Australia that did not appear to be due to L. maculans or other common pathogenic fungi of canola (Fig. 1a). The disease symptoms were found in multiple canola varieties at several locations across the canola growing area of Western Australia, with these locations spaced over more than 900 km apart. Symptoms were observed in November, 2019 at Alma (north of Geraldton), and in September–October, 2020 at Greenough and Mingenew (south of Geraldton), Dale (south east of Perth) and Wittenoom Hills (north of Esperance) (Fig. 1b, Table 1).
Canola (Brassica napus) disease symptoms and locations of strain isolation in Western Australia. a Symptoms across different plant stages, from the top of the plant on the left to the bottom on the right, observed on plants collected from Wittenoom Hills in 2020. Scale bar = 2 cm. b Map of southern Western Australia and sites and years from which strains were obtained, relative to the rest of Australia (inset; shaded regions indicate canola cultivation areas as defined by production over 500 tonnes between 2010–2014, redrawn from the Australian Bureau of Statistics)
Diseased materials were surface sterilized or spores liberated, then plated onto PDA to isolate potential pathogens. In addition to species in the genus Alternaria, which are commonly found in Australian canola fields (Al-Lami et al. 2019), one uncommon fungus was isolated from diseased material from five locations (Fig. 1b, Fig. 2a). Details of the origins of the five strains isolated by this approach are provided in Table 1.
Isolation of Cladosporium macrocarpum and reproduction of disease symptoms. a Colony of strain UoM19-17 growing on potato dextrose agar. b Lesions on B. napus cv. Westar stems at 12 (left) and 26 (right) days after inoculation. Scale bar = 1 cm. c Colony from a strain re-isolated from an inoculated plant, fulfilling Koch’s postulates. Petri dishes are 6 cm in diameter; 14 days growth
The ability of the fungal isolates to cause disease was tested by inoculating B. napus cv. Westar grown in a glasshouse. Lesion development was clearest on stems, in which dark lesions appeared within eight days (Fig. 2b). The diseased material arising after inoculation with strain UoM19-17 was excised, surface sterilised, and the fungus re-isolated onto PDA, showing the same colony form and spores as strain UoM19-17 (Fig. 2c), and thus fulfilling Koch’s postulates that the strain causes the symptoms. The UoM19-17 strain was also tested on stems of wheat (Triticum aestivum cv. Gladius) and Indian mustard (Brassica juncea cv. Aurea), where no lesions developed.
Morphological features of the colonies, hyphae and spores were consistent with a species of Cladosporium (Fig. 2a, Fig. 3). Strains produced colonies with dark brown pigments, including the production of asexual spores, but no sexual structures were observed. Under light microscopy, the conidia formed in alternations off hyphae, sometimes as short chains. The spores varied in size, and were usually, but not always, without a septum, and when formed, were primarily uniseptate (Fig. 3a, b). Strain UoM19-17 was examined by scanning electron microscopy (Fig. 3c). This level of resolution revealed that the spores of the strain were muricate, in contrast to undecorated hyphae, and consistent in appearance with strains in the C. herbarum species complex.
Morphology of conidia of C. macrocarpum. Light microscopy images of conidia, illustrating variation in spore size and morphology: a directly from a field sample from Dale, and b from in vitro culture of strain UoM19-17. Panel c shows spore ultrastructure as resolved by scanning electron microscopy for UoM19-17. Scale bars = 10 µm
Identification of Cladosporium strains to the species level is challenging when there are few morphological differences between species, such as those within the C. herbarum species complex (Bensch et al. 2012, 2015; David 1997), and hence DNA sequence information was obtained to refine species identification. The ITS sequences for the five strains were identical. When compared by BLAST to the GenBank nr database, the top hits (100% identity) from the strains were all to species in the C. herbarum species complex. This complex includes a number of distinctive species that cannot be resolved with ITS sequences (Schubert et al. 2007).
Four other gene region sequences were obtained from strain UoM19-17, aligned with those from other Cladosporium strains and used to construct phylogenetic trees. The phylogenies consistently placed strain UoM19-17 within C. macrocarpum (Fig. 4). As an example of this affinity, comparison with five gene regions revealed that strain UoM19-17 had only a single nucleotide difference to the same regions of C. macrocarpum strain CBS 299.67, which was isolated from wheat in Turkey. The TEF1 region was then amplified and sequenced from the other four strains, showing identical sequences for strains UoM20-4, UoM20-10 and UoM21-1, and one single nucleotide polymorphism for UoM20-5. These sequences support the identification of the other four strains as being C. macrocarpum, and thereby the wide distribution of this species on canola in Western Australia.
Phylogenetic analysis of sequences from C. macrocarpum strain UoM19-17 compared with other Cladosporium species, using C. iridis as the outgroup. Sequences from four gene regions (Table 2) were concantenated, aligned and compared using neighbour-joining. Numbers indicate bootstrap support (% from 100 reiterations)
The analysis of the strains isolated from diseased canola is consistent with an identification as C. macrocarpum, both from morphological features and similarity in DNA sequences. An alternative designation could be C. brassicae. However, C. brassicae has been rarely studied beyond original diseased material, in that strains are not available in culture collections or DNA sequences in GenBank. As the C. brassicae species epithet implies, the species is associated with causing disease on Brassica plants, however, it is similar in morphology to C. macrocarpum (Bensch et al. 2012). Further, strains of C. macrocarpum have been associated with Brassica for over two centuries: for example, Schubert et al. 2007 synonymised Dematium herbarum β [equivalent to var.] brassicae, found on the stems and leaves of Brassica species (Persoon, 1801), with C. macrocarpum. Cladosporium species, including C. macrocarpum, occur on species in the Brassicaceae family, including B. napus in Canada (Clear and Patrick 1995; Corlett 1988), while in Australia C. macrocarpum has been isolated from Brassicaceae species in Victoria and wheat in Western Australia (J. Edwards and V. Lanoiselet, pers. commun.).
In a 2019 field trial at Alma, Western Australia, incidence of these new disease symptoms were noticeable, whereas no other diseases were observed at the site. The symptoms on pods and upper stem at crop maturity were significantly reduced (P < 0.05) by a double application of commercial fungicide applied at the 20% and 50% bloom stages of canola development, whereas the single applications at those stages had no significant effect (Table 3). However, no significant yield or grain quality benefit was seen in the trial. This may reflect that the 2019 growing season was extremely dry throughout the WA grain belt and therefore canola yields were low. Average yield across all treatments at the site was 0.4 t/ha. Average grain moisture was 6.7% but yield and grain quality results were standardised to 6% moisture. Average grain quality results were 42% oil, and 2.8 g for 1000 grain weight. At a similar field trial conducted at Dale, Western Australia, in 2020 a single application of the same commercial fungicide applied at 20% bloom stage of canola development had no significant effect on Cladosporium disease incidence (Table 3), but was in the presence of Sclerotinia stem rot, blackleg canker and upper canopy blackleg diseases (data not presented). Average yield across the site at Dale was 1.87 t/ha.
It is unknown why Cladosporium disease emerged to prominence starting in 2019. One hypothesis is that the species has been present at a low level in canola or other plant species and that relatively recent changes in farming to control diseases caused by L. maculans or S. sclerotiorum, such as fungicide applications during flowering [reviewed by (Van de Wouw et al. 2021)], may have altered the prevalence of fungal species in canola fields. Thus, the UoM19-17 strain was tested for its ability to grow in the presence of two demethylation inhibitor (azole) fungicides, which target ergosterol biosynthesis, and are registered in Australia for use against Sclerotinia stem rot and commonly used to control upper canopy infection caused by L. maculans. The values for the inhibition of growth by 50% (effective concentration; EC50) were 9.96 µg/ml for prothioconazole and 1.32 µg/ml for tebuconazole (as illustrated in Fig. 5). These values, particularly for prothioconazole, are higher than observed for S. sclerotiorum (Dalili et al. 2015) or L. maculans (Eckert et al. 2010; Yang et al. 2020). Analysis of the sequence of the C. macrocarpum erg11/cyp51 homolog that encodes the enzyme that is targeted by the azole fungicides, as assembled from genome sequencing reads (GenBank accession MW646549), did not reveal any overt features that would account for the high level of tolerance to these antifungals, and is consistent with the field trial data where a reduction in disease incidence was only obtained from a double application of these fungicides.
The release of fungicides that provide protection to canola later in the growing season against blackleg disease and sclerotinia stem rot could have inadvertently created a plant environment relatively free of species that may previously have outcompeted C. macrocarpum. This hypothesis is further supported by co-culturing C. macrocarpum and L. maculans, wherein the blackleg pathogen inhibited C. macrocarpum growth (Fig. 6).
Future research could explore further if infection by C. macrocarpum results in a reduction in canola seed yield or oil quality. If C. macrocarpum were to be a cause of decline in productivity or quality then control measures could be established, such as reducing or avoiding inoculum, exploring genetic resistance in B. napus cultivars, or the application of fungicides in classes other than demethylation inhibitors.
References
Al-Lami HFD, You MP, Barbetti MJ (2019) Incidence, pathogenicity and diversity of Alternaria spp. associated with alternaria leaf spot of canola (Brassica napus) in Australia. Plant Pathol 68:492–503
Bensch K, Braun U, Groenewald JZ, Crous PW (2012) The genus Cladosporium. Stud Mycol 72:1–401
Bensch K, Groenewald JZ, Braun U, Dijksterhuis J, de Jesús Yáñez-Morales M, Crous PW (2015) Common but different: the expanding realm of Cladosporium. Stud Mycol 82:23–74
Clear RM, Patrick SK (1995) Frequency and distribution of seedborne fungi infecting canola seed from Ontario and western Canada - 1989 to 1993. Can Plant Dis Survey 75:9–17
Corlett M (1988) Taxonomic studies in the genus Mycosphaerella. Some species of Mycosphaerella on Brassicaceae in Canada. Mycotaxon 31:59–78
Dalili A, Bakhtiari S, Barari H, Aldaghi M (2015) Effect of some fungicides against the growth inhibition of Sclerotinia sclerotiorum mycelial compatibility groups. J Plant Prot Res 55:354–361
David JC (1997) A contribution to the systematics of Cladosporium: revision of the fungi previously referred to Heterosporium. Mycol Pap 172:1–157
Derbyshire MC, Denton-Giles M (2016) The control of sclerotinia stem rot on oilseed rape (Brassica napus): current practices and future opportunities. Plant Pathol 65:859–877
Eckert MR, Rossall S, Selley A, Fitt BDL (2010) Effects of fungicides on in vitro spore germination and mycelial growth of the phytopathogens Leptosphaeria maculans and L. biglobosa (phoma stem canker of oilseed rape). Pest Manag Sci 66:396–405
Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, Buxton S, Cooper A, Markowitz S, Duran C, Thierer T, Ashton B, Meintjes P, Drummond A (2012) Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28:1647–1649
Kumar S, Stecher G, Tamura K (2016) MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol Biol Evol 33:1870–1874
Persoon CH (1801) Synopsis Methodica Fungorum
Pitkin JW, Panaccione DG, Walton JD (1996) A putative cyclic peptide efflux pump encoded by the TOXA gene of the plant-pathogenic fungus Cochliobolus carbonum. Microbiology 142:1557–1565
Schoch CL, Seifert KA, Huhndorf S, Robert V, Spouge JL, Levesque CA, Chen W, Fungal Barcoding Consortium (2012) Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. Proc Natl Acad Sci USA 109:6241–6216
Schubert K, Groenewald JZ, Braun U, Dijksterhuis J, Starink M, Hill CF, Zalar P, de Hoog GS, Crous PW (2007) Biodiversity in the Cladosporium herbarum complex (Davidiellaceae, Capnodiales), with standardisation of methods for Cladosporium taxonomy and diagnostics. Stud Mycol 58:105–156
Sprague SJ, Marcroft SJ, Lindbeck KD, Ware AH, Khangura RK, Van de Wouw AP (2017) Detection, prevalence and severity of upper canopy infection on mature Brassica napus plants caused by Leptosphaeria maculans in Australia. Crop Pasture Sci 69:65–78
Van de Wouw AP, Idnurm A, Davidson JA, Sprague SJ, Khangura RK, Ware AH, Lindbeck KD, Marcroft SJ (2016a) Fungal diseases of canola in Australia: identification of trends, threats and potential therapies. Australas Plant Path 45:415–423
Van de Wouw AP, Marcroft SJ, Howlett BJ (2016b) Blackleg disease of canola in Australia. Crop Pasture Sci 67:273–283
Van de Wouw AP, Marcroft SJ, Sprague SJ, Scanlan JL, Vesk PA, Idnurm A (2021) Epidemiology and management of blackleg of canola in response to changing farming practices in Australia. Australas Plant Pathol 50:137–149
White TJ, Bruns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protocols: a guide to methods and applications. Academic Press, Inc. 315–322
Yang Y, Marcroft SJ, Forsyth LM, Zhao J, Li Z, Van de Wouw AP, Idnurm A (2020) Sterol demethylation inhibitor fungicide resistance in Leptosphaeria maculans is caused by modifications in the regulatory region of ERG11. Plant Dis 104:1280–1290
Acknowledgements
This research was supported by the University of Melbourne, Australian Research Council (ARC grant LP170100548), Department of Primary Industries and Regional Development (DPIRD) and the Grains Research and Development Corporation (GRDC and DPIRD co-funded projects DAW1907-002RTX and DAW1810-007RTX). We thank Jacky Edwards (Agriculture Victoria) and Vincent Lanoiselet (DPIRD) for advice on disease reporting procedures and C. macrocarpum incidence in Australia, and for accessioning strains and diseased material. We also thank the growers who hosted the field trials, Bayer for providing the fungicide used in the trials and Syngenta for providing fungicides for in vitro, David Nicholson and Debra Donovan (DPIRD) for their assistance in season at Dale, and DPIRD research support units at Geraldton and Northam who harvested the Alma and Dale trials, respectively.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest statement
Through ARC linkage project LP170100548 A.I. receives funding for research from Syngenta Australia. Any use of registered trade names is for the purpose of research transparency, and constitutes neither an endorsement nor a recommendation against products or services by the authors, their institutions, or their funding bodies.
Rights and permissions
About this article
Cite this article
Idnurm, A., Beard, C., Smith, A. et al. Emergence of Cladosporium macrocarpum disease in canola (Brassica napus). Australasian Plant Pathol. 50, 687–694 (2021). https://doi.org/10.1007/s13313-021-00819-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13313-021-00819-8