Abstract
Anastomotic leak (AL) is a feared complication of esophago-gastric surgery. Non-steroidal anti-inflammatory drugs (NSAIDs) are widely used to treat postoperative pain. Previous analyses conveyed heterogeneous data for colorectal surgery with a tendency toward high risk for AL after NSAIDs administration. In the setting of upper gastrointestinal (GI) surgery data are even more puzzled. The purpose of the present study was to assess whether an association exists between postoperative NSAIDs administration and AL after esophago-gastric surgery. PubMed, MEDLINE, Scopus, and Web of Science were searched up to November 2022. The included studies evaluated outcomes for NSAIDs vs. no NSAIDs administration after esophago-gastric surgery. The primary outcome was anastomotic leak (AL). Risk ratio (RR) and 95% confidence intervals (95% CI) were used to assess pooled effect size and relative inference. Six studies (43,784 patients) were included. The patient age ranged from 31 to 84 years, 82.4% were males and preoperative BMI ranged from 15 to 31 kg/m2. Esophagectomy was performed in 95% of patients. NSAIDs were administered in 18,075 (41.3%) patients. The cumulative incidence of AL was similar for NSAIDs vs. no NSAIDs (13.6% vs. 13.4%). The risk for postoperative AL was similar for NSAIDs vs. no NSAIDs administration (RR 1.49; 95% CI 0.81–2.75; p = 0.19). The cumulative incidence of postoperative gastrointestinal bleeding (0.36% vs. 0.39%), acute kidney injury (0.62% vs. 0.71%), and in-hospital mortality (2.39% vs. 2.66%) were comparable. NSAIDs administration for postoperative analgesia seems not associated with an increased risk for AL after esophago-gastric surgery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Anastomotic leak (AL) is a feared complication of esophago-gastric surgery with a wide range of reported incidence from 0 to 30% [1,2,3]. AL is associated with prolonged hospital stay, increased reoperation risk, reduced quality of life, increased hazard for anastomotic stricture, augmented costs, risk for 90-day mortality, and reduced overall survival [4,5,6,7]. Different risk factors for AL have been described while its prevention is of paramount importance [8,9,10].
Non-steroidal anti-inflammatory drugs (NSAIDs) are extensively used to treat postoperative pain. With the development of intravenous formulations, use of NSAIDs in the early postoperative period has significantly augmented [11]. Furthermore, NSAIDs play a foremost role as a key opioid-sparing element of multimodal analgesia in enhanced recovery after surgery (ERAS) protocols [12,13,14,15,16]. However, some surgeons dissent with the use of NSAIDs in the early postoperative period since few reports suggested a potential association with AL. Specifically, previous analyses conveyed heterogeneous data for colorectal surgery with a tendency toward higher risk for leak after elective resections [17]. In the setting of upper gastrointestinal (GI) surgery, data are even more sparse while a robust indication on the potential correlation between NSAIDs and AL is unclear.
Hence, the purpose of this study was to perform a systematic review and meta-analysis to determine whether an association exists between postoperative NSAIDs administration and AL in the setting of upper GI surgery for esophageal and gastric anastomoses.
Materials and methods
This study was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement and Meta-analyses Of Observational Studies in Epidemiology (MOOSE) guidelines [18, 19]. The Institutional Review Board approval was not required. PubMed, MEDLINE, Scopus, Web of Science, Cochrane Central Library, and ClinicalTrials.gov were queried [20]. We searched articles from January 1st 2000 November 30th 2022. A combination of the following MeSH (Medical Subject Headings) terms was used for the literature search (“leak” (tiab), OR “anastomotic leak” (tiab) AND (“NSAIDs” (tiab), OR “non-steroidal” (tiab), OR “ketorolac” (tiab), OR “diclofenac” (tiab), OR “nonsteroidal” (tiab)). AA and GB evaluated the title of each study, and suitable abstracts were extracted. The search was completed by consulting the references of each article. The study protocol was registered at the International prospective register of systematic reviews (PROSPERO registration number: CRD42022328749).
Eligibility criteria
The inclusion criteria were (a) cohort studies and randomized controlled trials (RCTs) comparing outcomes for postoperative NSAIDs versus no NSAIDs administration in adult patients (> 18 years) undergoing elective upper GI surgery (esophagectomy or gastrectomy), (b) reported in English, (c) when two or more studies were published using the same dataset, we included the study with the longest follow-up period or the largest sample size, (d) for duplicate studies, we only included the study with the complete dataset for quantitative analysis. The exclusion criteria were (a) non-English articles, (b) no clear outcome distinction between NSAIDs versus no NSAIDs administration, (c) studies including less than 10 patients for each treatment arm.
Data extraction
The following variables were collected: authors, year of publication, country, study design, number of patients, age, sex, body mass index (BMI), Charlson Comorbidity Index (CCI), American Society of Anesthesiologists (ASA) physical status, comorbid conditions, surgical indications, tumor characteristics, histological type, tumor location, cancer stage, use of neoadjuvant chemoradiation therapy, surgical approach (open vs. minimally invasive), NSAIDs formulation, starting time from the index procedure, daily dosage (mg/day), route of administration (oral vs. intravenous), and postoperative outcomes. All data were computed independently by three investigators (AA, MM, JG) and compared at the end of the review process. Another author (DB) reviewed the database and evaluated for discrepancies.
Outcomes and definitions
The primary outcome was AL. Secondary outcomes were gastrointestinal bleeding (GIB), acute kidney injury (AKI), and in-hospital mortality. AL was defined as radiographic evidence of contrast extravasation on postoperative swallow study and/or computed tomography, or endoscopic visualization of anastomotic dehiscence/fistula, or surgical drain output consistent with saliva. GIB was defined as any bleeding from the gastrointestinal tract requiring endoscopic hemostasis or surgical reintervention. AKI was defined in accordance with the Acute Kidney Injury Working Group of KDIGO (Kidney Disease: Improving Global Outcomes) criteria [21].
Quality assessment
Three authors (AA, MM, GG) independently assessed the quality of methodology for each study. The ROBINS-I tool was used for observational studies [22]. The following domains were considered: confounding bias, selection bias, classification bias, intervention bias, missing data bias, outcomes measurement bias, and reporting bias. Each domain was evaluated and categorized into one of the following: “yes”, “probably yes”, “probably no”, or “no”. The categories of judgement for each study were low, moderate, serious, and critical risk of bias. We used the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) tool to assess the quality of the body of evidence across studies [23].
Statistical analysis
The results of the systematic review were quantified into Frequentist random effect meta-analysis of pooled Risk Ratio (RR). An inverse-variance method and DerSimonian–Laird estimator for the variance of the true effect size (τ2) were performed [24, 25]. Heterogeneity among studies was evaluated by the I2 index and Cochran’s Q test [26]. Statistical heterogeneity was considered low, moderate, and high for I2 values of 25, 50, and 75%, respectively, and significant when p < 0.10 [27, 28]. The Wald-type 95% confidence interval (CI) was computed for pooled measurements; otherwise, the 95% CI for the I2 index was calculated according to Higgins and Thompson [29]. The prediction interval for the treatment effect of a new study was calculated according to Borestein [26, 30]. As the sample size was not the same in all studies, we performed a sensitivity analysis by excluding one study each time and rerunning the analysis to verify the robustness of the overall results. The publication bias was also investigated with the Trim and Fill funnel plot and Egger test. A two-sided p value was considered statistically significant when < 0.05. All analyses were performed using the R software program, version 3.2.2 [31].
Results
Systematic review
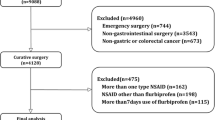
The PRISMA flow chart is reported in Fig. 1. A total of 198 publications were identified. After duplicates were excluded, 171 titles were screened, and abstracts were reviewed. After full text evaluation of 7 articles, 6 studies met the inclusion/exclusion criteria and were comprised in the quantitative synthesis. All studies were of observational design. Propensity score matching and stabilized inverse probability of treatment weighting (IPTW) were used in three studies. Four studies reported data focused on esophageal surgery, one study was focused on gastric surgery while one study reported mixed results for esophageal and gastric surgery. The quality of each study is depicted in Supplementary Table 1.
Overall, 43,784 patients were included (Table 1). The patient age ranged from 31 to 84 years and the majority (82.4%) were males. Preoperative BMI was specified in four studies and ranged from 15 to 31 kg/m2. Patients’ comorbidities were reported according to the CCI (3 studies) [32, 33, 36] and the ASA score (2 studies) [32, 33]. Tumor histology was specified in 3 studies [32, 34, 37] while the pathological stage was not informed in any of the included studies. Open, hybrid, and totally minimally Ivor-Lewis or McKeown esophagectomy were described in 95% of patients according to tumor location, operating surgeon preferences and oncologic principles. Open or minimally invasive approaches were reported for total or subtotal gastrectomy in 5% of patients. The use of neoadjuvant therapy with different protocols was reported in three studies [33, 34, 36]. The extent of lymphadenectomy was reported in four studies [32, 33, 36, 37] and varied depending on surgeon expertise, tumor clinical stage, and location. NSAIDs were administered in 18,075 (41.3%) patients in the postoperative period. Different formulations, type (selective vs. non-selective cyclooxygenase inhibitors), daily dose (mg/day) and route of administration (intravenous vs. oral) were used across included studies depending of hospital availability and clinician preference. No differences were found for the comparison NSAIDs vs. no NSAIDs in term of postoperative GIB (0.36% vs. 0.39%), AKI (0.62% vs. 0.71%), and in-hospital mortality (2.39% vs. 2.66%) cumulative incidence.
Meta-analysis—anastomotic leak
AL was reported in six studies (43,784 patients). The cumulative incidence of AL was similar for NSAIDs vs. no NSAIDs (13.6% vs. 13.4%). The quantitative analysis did not show significant differences between NSAIDs vs. no NSAIDs administration (RR 1.49; 95% CI 0.81–2.75; p = 0.19) (Fig. 2). The prediction lower and upper limits were 0.19 and 11.65, respectively. The heterogeneity was high (I2 = 88.0%, 95% CI 75–94%; p < 0.01) and τ2 = 0.449. The sensitivity analysis showed the robustness of these findings in terms of point estimation, relative confidence intervals, and heterogeneity. Using the GRADE tool, we rated the quality of evidence for AL as moderate mainly because of limitations in study design (Supplementary Table 2).
Discussion
This study indicates that postoperative NSAIDs administration in the setting of upper GI surgery seems not associated with increased risk for AL. No robust data exist for GIB and AKI while any presumed correlation with NSAIDs mandates future focused trials.
ERAS protocols have been widely introduced into surgical practice across a variety of surgical fields [13]. In the setting of upper GI surgery perioperative optimization, expedient return to baseline mobility, fluid balance, early mobilization, prompt oral feeding, and adequate postoperative pain control have been implemented [14,15,16, 38, 39]. To attain an optimal opioid-sparing pain control, multimodal analgesic approaches have been proposed based on NSAIDs administration. In this direction, several studies reported an important increase in NSAIDs utilization for pain control after upper GI surgery [32,31,32,33,34,37]. The pharmacological mechanism of NSAIDs is related to the suppression of the cyclooxygenase (COX) enzyme resulting in prostaglandins (PGs) synthesis inhibition [40,39,42]. Specifically, the analgesic effect of NSAIDs is related with PGE2 and PGI2 synthesis suppression both involved in central and peripheral nociceptive responses. The reduced PGs synthesis is even associated with the related anti-inflammatory and anti-pyretic effect. Interestingly, it has been argued in previous studies a theoretical supplementary effect of NSAIDs on tissue healing [43,42,43,46]. Specifically, there is experimental evidence that COX-produced PGs modulate fibroblast growth factor-beta (β-FBF) and vascular endothelial growth factor (EGF) [47, 48]. Fibroblast, epithelial cells migration, and neo-angiogenesis are critical component of tissue healing. Previous experimental studies performed in mice models reported a decreased recruitment and migration of fibroblast, myofibroblast and epithelial cells at the anastomotic site with consequent altered collagen deposition, delayed anastomotic healing and reduced tissue strength [43,42,43,46]. Therefore, tissue healing at the anastomotic site might be theoretically altered by NSAIDs. Several studies focused on elective colorectal resections, described a strong association with postoperative NSAIDs use and AL. Specifically, Klein et al., reported an almost seven-fold increase in AL rate (OR 7.2, 95% CI 3.8–13) for patients assuming diclofenac after colonic resection for cancer [49]. Similarly, two recent meta-analyses by Kastora et al. [50], Modasi et al. [51] and Smith and colleagues [52] stated that colo-colic anastomoses appear to be more sensitive to AL thus recommending caution with NSAIDs utilization. However, the topic is intensely debated while other recent meta-analyses reported no significant association with AL [53, 54].
In our study, focused on upper GI surgery and esophageal/gastric anastomosis, we did not find any significant differences in terms of postoperative AL for NSAIDs vs. no NSAIDs administration (RR 1.49; 95% CI 0.81–2.75; p = 0.19). Interestingly, the sensitivity analysis supported the robustness of this finding. This is similar to what recently reported by Hirano et al. that, in their dataset study from the Japanese nationwide database, reported no difference in term of AL when comparing NSAIDs vs. no NSAIDs administrations after elective esophageal resections (15% vs. 14%; p = 0.644) [36]. Similarly, Corsini et al. in a retrospective cohort study of 1016 patients (2006–2018) from a high-volume cancer center also showed that NSAID use was not associated with AL after esophagectomy (OR 0.99; p = 0.958) despite the significant increase in term of NSAIDs utilization during the study period [34]. By contrast, a previous cohort study showed a strong association between early NSAIDs use and AL with an almost fivefold increased risk after elective esophageal resection (OR 5.24, 95% CI 1.85–14.8) [32]. Similarly, Kim and colleague recently described their experience with 2150 patients undergoing laparoscopic gastrectomy. The authors reported a significantly higher AL rate in patients assuming NSAIDs in the early postoperative period (2.4% vs. 0.7%; p = 0.002) [37]. While our results are based on a relatively large patient sample, their interpretation should be cautious because the remarkable heterogeneity (I2 = 88%) and possible confounders related to lack of standardized anastomotic techniques, preoperative patient selection, age and comorbidities, smoking status, neoadjuvant treatment, surgical approach (open vs. minimally invasive), operating surgeon experience, hospital volumes, anastomotic technique (hand-sewn vs. stapled), anastomosis location, blood flow assessment with indocyanine green, NSAIDs treatment duration, daily dose (mg/day), type of administered NSAIDs (i.e. flurbiprofen, diclofenac, ketorolac, loxoprofen, etc.), selective (celecoxib) or non-selective pharmacodynamics, oral/intravenous administration, and multimodal analgesia protocols (i.e. epidural catheters).
Gastrointestinal bleeding is a potential drawback of NSAIDs treatment. Bleeding may necessitate additional endoscopic or surgical intervention with longer length of stay and increased costs. The mechanism is related to the effect on thromboxane A2 (TXA2) that is an agonist for circulating platelet activation [55]. NSAIDs decrease PGs synthesis with direct interference on arachidonic acid (AA) metabolism and consequent reduced TXA2 synthesis. By preventing TXA2 production, platelet aggregation is inhibited with a potential increase in bleeding rate. In our preliminary analysis no differences were found in term of postoperative GIB. This is similar to Hirano and colleagues that described no differences (0.3% vs. 0.4%; p = 0.242) [36] while Kim et al. stated higher rates of gastrointestinal bleeding (2.1% vs. 0.7%; p = 0.005) in patients assuming NSAIDs [37]. Again, no differences were found in terms of NSAIDs induced AKI. This is similar to what described by Hirano et al. (0.6% vs. 0.7%; p = 0.26) [36] and Kawakami et al. (1% vs. 1%; p = ns) [33] that reported comparable rates of AKI. Notably, a robust quantitative synthesis was not practicable since studies are few and data sparce. Therefore, evidence is unclear while future trials are warranted to shed the light onto these topics.
To the best of our knowledge, this is the first meta-analysis analyzing the association between NSAIDs administration and AL in the setting of esophago-gastric surgery. We acknowledge several limitations, particularly those commonly discussed in the meta-analysis including observational studies. First, the large majority of studies did not have the standardized protocols for surgical techniques and perioperative analgesic protocols. Second, because different formulations and heterogeneous treatment duration our results might not be generalizable while a possible dose–response relationship could not be excluded. Third, the definitions and classifications of AL varied between included articles. Finally, we were not able to assess the hospital volume and experience of operating surgeons.
Conclusions
NSAIDs administration for postoperative analgesia seems not associated with an increased risk for AL after esophago-gastric surgery. NSAIDs could be safely employed in the postoperative period as key component of multimodal analgesia for pain control in the setting of upper GI surgery.
Data availability
Data generated at a central, large-scale facility, available upon request.
References
Low DE, Kuppusamy MK, Alderson D et al (2019) Benchmarking complications associated with esophagectomy. Ann Surg 269(2):291–298
Aiolfi A, Sozzi A, Bonitta G et al (2022) Linear-versus circular-stapled esophagogastric anastomosis during esophagectomy: systematic review and meta-analysis. Langenbecks Arch Surg 407(8):3297–3309
Kuppusamy MK, Low DE, International Esodata Study Group (IESG) (2022) Evaluation of international contemporary operative outcomes and management trends associated with esophagectomy: a 4-year study of >6000 patients using ECCG definitions and the online esodata database. Ann Surg 275(3):515–525.
Blencowe NS, Strong S, McNair AG, Brookes ST, Crosby T, Griffin SM, Blazeby JM (2012) Reporting of short-term clinical outcomes after esophagectomy: a systematic review. Ann Surg 255(4):658–666
Low DE, Alderson D, Cecconello I et al (2015) International consensus on standardization of data collection for complications associated with esophagectomy: esophagectomy complications consensus group (ECCG). Ann Surg 262(2):286–294
Roh CK, Choi S, Seo WJ et al (2021) Incidence and treatment outcomes of leakage after gastrectomy for gastric cancer: experience of 14,075 patients from a large volume centre. Eur J Surg Oncol 47(9):2304–2312
Trapani R, Rausei S, Reddavid R, Degiuli M; Italian Research Group for Gastric Cancer (GIRCG) Clinical Investigators (2020) Risk factors for esophago-jejunal anastomosis leakage after total gastrectomy for cancer. A multicenter retrospective study of the Italian research group for gastric cancer. Eur J Surg Oncol 46(12):2243–2247
Li H, Zhuang S, Yan H, Wei W, Su Q (2021) Risk factors of anastomotic leakage after esophagectomy with intrathoracic anastomosis. Front Surg 21(8):743266
Grigor EJM, Kaaki S, Fergusson DA, Maziak DE, Seely AJE (2021) Interventions to prevent anastomotic leak after esophageal surgery: a systematic review and meta-analysis. BMC Surg 21(1):42
Wang WJ, Li R, Guo CA et al (2019) Systematic assessment of complications after robotic-assisted total versus distal gastrectomy for advanced gastric cancer: a retrospective propensity score-matched study using Clavien–Dindo classification. Int J Surg 71:140–148
Hakkarainen TW, Steele SR, Bastaworous A et al (2015) Nonsteroidal anti-inflammatory drugs and the risk for anastomotic failure: a report from Washington State’s Surgical Care and Outcomes Assessment Program (SCOAP). JAMA Surg 150(3):223–228. https://doi.org/10.1001/jamasurg.2014.2239. (Erratum in: JAMA Surg. 2015 May;150(5):492)
Wick EC, Grant MC, Wu CL (2017) Postoperative multimodal analgesia pain management with nonopioid analgesics and techniques: a review. JAMA Surg 152(7):691–697
Ljungqvist O, Scott M, Fearon KC (2017) Enhanced recovery after surgery: a review. JAMA Surg 152:292–298
Low DE, Allum W, De Manzoni G, Ferri L, Immanuel A, Kuppusamy M et al (2019) Guidelines for perioperative care in esophagectomy: enhanced recovery after surgery (ERAS(®)) society recommendations. World J Surg 43:299–330
Rosa F, Longo F, Pozzo C et al (2022) Enhanced recovery after surgery (ERAS) versus standard recovery for gastric cancer patients: the evidences and the issues. Surg Oncol 41:101727
Romario UF, Ascari F, De Pascale S, GIRCG (2022) Implementation of the ERAS program in gastric surgery: a nationwide survey in Italy. Updates Surg 28:1–8
Jamjittrong S, Matsuda A, Matsumoto S et al (2020) Postoperative non-steroidal anti-inflammatory drugs and anastomotic leakage after gastrointestinal anastomoses: systematic review and meta-analysis. Ann Gastroenterol Surg 4:64–75
Liberati A, Altman DG, Tetzlaff J et al (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 21(339):b2700
Stroup DF, Berlin JA, Morton SC, et al (2000) Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 283(15):2008–2012
Goossen K, Tenckhoff S, Probst P et al (2018) Optimal literature search for systematic reviews in surgery. Langenbecks Arch Surg 403(1):119–129
(2012) Kidney disease improving global outcomes (KDIGO) Clinical practice guideline for acute kidney injury. Kidney Int Suppl 2:1–138
Sterne JA, Hernán MA, Reeves BC, et al (2016) ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 355:i4919. https://doi.org/10.1136/bmj.i4919.
Guyatt GH, Oxman AD, Vist GE, et al. GRADE Working Group (2008) GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 336(7650):924–6.
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7(3):177–188
Tornese S, Aiolfi A, Bonitta G et al (2019) Remnant gastric cancer after Roux-en-Y gastric bypass: narrative review of the literature. Obes Surg 29(8):2609–2613
Borenstein M, Hedges LV, Higgins JP et al (2010) A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods 1(2):97–111
Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327:557–560
Davide, Bona Giancarlo, Micheletto Gianluca, Bonitta Valerio, Panizzo Marta, Cavalli Emanuele, Rausa Silvia, Cirri Alberto, Aiolfi (2019) Does C-reactive Protein Have a Predictive Role in the Early Diagnosis of Postoperative Complications After Bariatric Surgery? Systematic Review and Bayesian Meta-analysis Obesity Surgery 29(11) 3448-3456 10.1007/s11695-019-04013-0
Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21(11):1539–1558
Aiolfi A, Tornese S, Bonitta G, Rausa E, Micheletto G, Bona D. Roux-en-Y gastric bypass: systematic review and Bayesian network meta-analysis comparing open, laparoscopic, and robotic approach. Surg Obes Relat Dis. 2019 Jun;15(6):985-994. doi: 10.1016/j.soard.2019.03.006.
R Development Core Team (2015) A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna (ISBN 3–900051–07–0).
Fjederholt KT, Okholm C, Svendsen LB et al (2018) Ketorolac and other nsaids increase the risk of anastomotic leakage after surgery for GEJ cancers: a cohort study of 557 patients. J Gastrointest Surg 22(4):587–594
Kawakami J, Abe T, Higaki E, Hosoi T, Fukaya M, Komori K, Ito S, Nakatochi M, Nagino M, Shimizu Y (2020) Scheduled intravenous acetaminophen versus nonsteroidal anti-inflammatory drugs (NSAIDs) for better short-term outcomes after esophagectomy for esophageal cancer. Surg Today 50(10):1168–1175
Corsini EM, Hofstetter WL, Anderson Esophageal Cancer Working Group (2021) Ketorolac use and anastomotic leak in patients with esophageal cancer. J Thorac Cardiovasc Surg 161(2):448–454
STARSurg Collaborative (2022) Perioperative nonsteroidal anti-inflammatory drugs (NSAID) administration and acute kidney injury (AKI) in major gastrointestinal surgery: a prospective, multicenter, propensity matched cohort study. Ann Surg 275(5):904–910.
Hirano Y, Konishi T, Kaneko H, Itoh H, Matsuda S, Kawakubo H, Uda K, Matsui H, Fushimi K, Daiko H, Itano O, Yasunaga H, Kitagawa Y (2022) Early postoperative non-steroidal anti-inflammatory drugs and anastomotic leakage after oesophagectomy. Br J Surg:znac399.
Kim SJ, Jeon CH, Lee HH, Song KY, Seo HS (2022) Impact of postoperative NSAIDs (IV-PCA) use on short-term outcomes after laparoscopic gastrectomy for the patients of gastric cancer. Surg Endosc.
Puccetti F, Wijnhoven BPL, Kuppusamy M, Hubka M, Low DE (2022) Impact of standardized clinical pathways on esophagectomy: a systematic review and meta-analysis. Dis Esophagus 35(2):doab027
Jin H, Song S, Lu T, Ma S, Wang Y, Fu L, Zhang G, Han X, Zhang L, Yang K, Cai H (2022) The application of enhanced recovery after surgery in minimally invasive gastrectomy for gastric cancer: a meta-analysis of randomized controlled trials. Expert Rev Gastroenterol Hepatol 16(11–12):1089–1100
Vonkeman HE, van de Laar MA (2010) Nonsteroidal anti-inflammatory drugs: adverse effects and their prevention. Semin Arthritis Rheum 39(4):294–312
Bacchi S, Palumbo P, Sponta A, Coppolino MF (2012) Clinical pharmacology of non-steroidal anti-inflammatory drugs: a review. Antiinflamm Antiall Agents Med Chem 11(1):52–64
Bindu S, Mazumder S, Bandyopadhyay U (2020) Non-steroidal anti-inflammatory drugs (NSAIDs) and organ damage: a current perspective. Biochem Pharmacol 180:114147
Inan A, Koca C, Sen M (2006) Effects of diclofenac sodium on bursting pressures of anastomoses and hydroxyproline contents of perianastomotic tissues in a laboratory study. Int J Surg 4:222–227
Ji C, Xiong Y, Pan X, Guo X, Li Z, Qian S et al (2015) Effect of non-steroidal anti-inflammatory drugs on the increasing the incidence of colonic anastomosis in rats. Int J Clin Exp Pathol 8:6126–6134
Reisinger KW, Schellekens DH, Bosmans JW, Boonen B, Hulsewé KWE, Sastrowijoto P et al (2017) Cyclooxygenase-2 is essential for colorectal anastomotic healing. Ann Surg 265:547–554
Gulcicek OB, Solmaz A, Yigitbas H, Ercetin C, Yavuz E, Ozdogan K et al (2018) Role of diclofenac sodium and paracetamol on colonic anastomosis: an experimental rodent model. Asian J Surg 41:264–269
Kokoska ER, Smith GS, Wolff AB, Deshpande Y, Miller TA (1999) Nonsteroidal anti-inflammatory drugs attenuate epidermal growth factor-induced proliferation independent of prostaglandin synthesis inhibition. J Surg Res 84(2):186–192
Diller R, Stratmann U, Helmschmied T, Bäumer G, Bahde R, Minin E, Spiegel HU (2008) Microcirculatory dysfunction in endotoxemic bowel anastomosis: the pathogenetic contribution of microcirculatory dysfunction to endotoxemia-induced healing impairment. J Surg Res 150(1):3–10
Klein M, Gögenur I, Rosenberg J (2012) Postoperative use of non-steroidal anti-inflammatory drugs in patients with anastomotic leakage requiring reoperation after colorectal resection: cohort study based on prospective data. BMJ 26(345):e6166
Kastora SL, Osborne LL, Jardine R, Kounidas G, Carter B, Myint PK (2021) Non-steroidal anti-inflammatory agents and anastomotic leak rates across colorectal cancer operations and anastomotic sites: a systematic review and meta-analysis of anastomosis specific leak rate and confounding factors. Eur J Surg Oncol 47(11):2841–2848
Modasi A, Pace D, Godwin M, Smith C, Curtis B (2019) NSAID administration post colorectal surgery increases anastomotic leak rate: systematic review/meta-analysis. Surg Endosc 33(3):879–885
Smith SA, Roberts DJ, Lipson ME, Buie WD, MacLean AR (2016) Postoperative nonsteroidal anti-inflammatory drug use and intestinal anastomotic dehiscence: a systematic review and meta-analysis. Dis Colon Rectum 59(11):1087–1097
Burton TP, Mittal A, Soop M (2013) Nonsteroidal anti-inflammatory drugs and anastomotic dehiscence in bowel surgery: systematic review and meta-analysis of randomized, controlled trials. Dis Colon Rectum 56(1):126–134
Arron MNN, Lier EJ, de Wilt JHW, Stommel MWJ, van Goor H, Ten Broek RPG (2020) Postoperative administration of non-steroidal anti-inflammatory drugs in colorectal cancer surgery does not increase anastomotic leak rate—a systematic review and meta-analysis. Eur J Surg Oncol 46(12):2167–2173
Scharf RE (2012) Drugs that affect platelet function. Semin Thromb Hemost 38(8):865–883
Acknowledgements
OGSA Group for Esophagogastric Surgery: Francesca Lombardo, Marta Cavalli, Michele Manara, Juxhin Guraj, Guglielmo Guerrazzi
Funding
None.
Author information
Authors and Affiliations
Consortia
Contributions
AA, MM, JG and GG did the literature search. AA, GB, and DB formed the study design. Data collection done by AA, MM, FL, and MC. AA and GB analyzed the data. AA, GC, and DB interpreted the data. AA wrote the manuscript. All authors critically reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethics approval
For this type of article, ethical approval is not required because does not contain any studies with human participants or animals performed by any of the authors.
Consent to participate
For this type of study, formal consent was not necessary.
Research involving human participants and/or animals
Research involved animals and humans. There is no consent required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
OGSA Group for Esophagogastric Surgery members name listed in acknowledgement section.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Aiolfi, A., Bonitta, G., Campanelli, G. et al. Impact of postoperative NSAIDs administration on anastomotic leak after esophago-gastric surgery: systematic review and meta-analysis. Updates Surg 75, 817–824 (2023). https://doi.org/10.1007/s13304-023-01515-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13304-023-01515-6