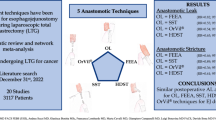
Abstract
Background
Different techniques have been described for esophagogastric anastomosis. Over the past decades, surgeons have been improving anastomotic techniques with a gradual shift from hand-sewn to stapled anastomosis. Nowadays, circular-stapled (CS) and linear-stapled (LS) anastomosis are commonly used during esophagectomy.
Methods
PubMed, MEDLINE, Scopus, and Web of Science were searched up to June 2022. The included studies evaluated short-term outcomes for LS vs. CS anastomosis in patients undergoing esophagectomy for cancer. Primary outcomes were anastomotic leak (AL) and stricture (AS). Risk ratio (RR) and standardized mean difference (SMD) were used as pooled effect size measures whereas 95% confidence intervals (95%CI) were used to assess relative inference.
Results
Eighteen studies (2861 patients) were included. Overall, 1371 (47.9%) underwent CS while 1490 (52.1%) LS. Compared to CS, LS was associated with a significantly reduced RR for AL (RR = 0.70; 95% CI 0.54–0.91; p < 0.01) and AS (RR = 0.32; 95% CI 0.20–0.51; p < 0.0001). Stratified subgroup analysis according to the level of anastomosis (cervical and thoracic) still shows a tendency toward reduced risk for LS. No differences were found for pneumonia (RR 0.78; p = 0.12), reflux esophagitis (RR 0.74; p = 0.36), operative time (SMD −0.25; p = 0.16), hospital length of stay (SMD 0.13; p = 0.51), and 30-day mortality (RR 1.26; p = 0.42).
Conclusions
LS anastomosis seems associated with a tendency toward a reduced risk for AL and AS. Although surgeon’s own training and experience might direct the choice of esophagogastric anastomosis, our meta-analysis encourages the use of LS anastomosis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Esophageal cancer is the sixth most common cancer and the eighth most common cause of cancer-related death worldwide [1]. The prognosis is poor while related 5-year overall survival ranges from 15 to 20% [2]. Esophagectomy, lymphadenectomy, and restoration of the gastrointestinal continuity via gastric conduit reconstruction represent the gold standard treatment [3]. The esophagogastric anastomosis is the most delicate and trickiest part of the operation while related complications are feared problems [4]. Anastomotic leak (AL) may occur up to 10% of patients. It has been reported to be associated with a 3-fold increase in mortality, prolonged hospital stay, delayed oral feeding, risk of reintervention, increased risk of recurrence, and decrease of overall/disease-free survival [5, 6]. Anastomotic stricture (AS) may occur up to 30% of patients and may require endoscopic dilation with a negative effect of postoperative recovery, nutritional status, and quality of life [7].
Different techniques have been described for esophagogastric anastomosis such as hand-sewn and mechanical stapled anastomosis [8, 9]. Over the past few decades, surgeons have been improving anastomotic techniques with a gradual shift from hand-sewn to stapled anastomosis [10,11,12,13]. Nowadays, circular-stapled (CS) and linear-stapled (LS) anastomosis are largely adopted into clinical practice for both cervical and thoracic anastomoses [14]. CS and LS have their own advantages and weaknesses while the decision to use one technique over another mainly depends on surgeon expertise and personal preference [15]. Currently, a definitive indication on the best stapling technique for esophageal anastomosis is still to be defined since previous studies showed contrasting results.
Hence, aim of the present systematic review and meta-analysis was to perform an updated literature analysis to compare outcomes for LS vs. CS anastomosis in the setting of esophagectomy for the treatment of esophageal cancer.
Materials and methods
We conducted this study according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement and MOOSE guidelines [16, 17]. Institutional review board approval was not required. PubMed, MEDLINE, Scopus, Web of Science, Cochrane Central Library, and ClinicalTrials.gov were used [18]. The last date of search was the June 30th, 2022. A combination of the following MeSH terms (Medical Subject Headings) was used (“esophagectomy” (tiab), OR “esophagectomies” (tiab), OR, “esophagogastric” (tiab), OR “esoph*” (tiab)) AND (“anastomosis” (tiab), OR “suture” (tiab)) AND (“linear” (tiab), OR “circular” (tiab)) AND (“outcomes” (tiab), OR “complication” (tiab)) AND (“leak” (tiab), OR “leakage” (tiab)) AND (“stricture” (tiab), OR “stenosis” (tiab)). All titles were evaluated and suitable abstracts extracted. The search was completed by consulting the references of each article. The study protocol was registered at the PROSPERO (international prospective register of systematic reviews) (Registration Number: CRD42022328741).
Eligibility criteria
Inclusion criteria: (a) cohort studies and randomized controlled trials (RCTs) comparing outcomes for LS vs. CS among adult patients (>18 year old) undergoing elective esophagectomy for cancer; (b) English-written; (c) when two or more papers were published by the same institution, study group, or used the same dataset, articles with the longest follow-up or the largest sample size; (d) in case of duplicate studies with accumulating numbers of patients only the most complete reports were included for quantitative analysis. Exclusion criteria: (a) not English-written; (b) poor methodology; (c) no clear outcome distinction between LS vs. CS; (d) articles with less than 7 patients per study arm.
Data extraction
The following data were collected: authors, year of publication, country, study design, number of patients, sex, age, body mass index (BMI), American Society of Anesthesiologists (ASA) physical status, comorbidities, surgical indication, tumor characteristics, histological type, tumor location, cancer stage, neoadjuvant chemoradiotherapy, and postoperative outcomes. All data were computed independently by three investigators (AA, AS, FL) and compared at the end of the reviewing process. A fourth author (DB) reviewed the database and determined discrepancies.
Outcomes
Primary outcomes were postoperative AL and AS. The quantitative analysis was performed for the global population and after stratification according to the level of anastomosis (cervical and thoracic). Secondary outcomes were reflux esophagitis, pulmonary complications, operative time (OT) (minutes), hospital length of stay (HLOS) (days), and 30-day mortality. AL was defined as evidence of contrast extravasation at postoperative swallow study and/or CT scan, or endoscopic visualization of anastomotic dehiscence/fistula, or visible loss of saliva through surgical drains combined with clinical signs. AS was diagnosed in case of postoperative dysphagia, evidence of anastomotic lumen stricture, and need for endoscopic dilatation up to 6 months after the operation. Reflux esophagitis was defined as higher than grade A according to the Los Angeles Classification of severity.
Quality assessment
Three authors (AA, AS, MC) independently assessed the methodological quality of included studies. The ROBINS-I tool was used for observational studies [19]. The following domains were considered: confounding bias, selection bias, classification bias, intervention bias, missing data bias, outcomes measurement bias, and reporting bias. Each domain is evaluated with one of the following: “yes,” “probably yes,” “probably no,” or “no.” The categories of judgment for each study are low, moderate, serious, and critical risk of bias. The methodological quality of randomized controlled trials (RCTs) was appraised with the Cochrane risk of bias tool [20]. This tool evaluates the following criteria: [1] method of randomization; [2] allocation concealment; [3] baseline comparability of study groups; and [4] blinding and completeness of follow-up. Trials were graded as follows: A = adequate, B = unclear, and C = inadequate on each criterion. Thus, each RCT was graded as having low, moderate, or high risk of bias. Disagreements were solved by discussion. We used the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) tool to assess the quality of the body of evidence across studies [21].
Statistical analysis
The results of the systematic review were summarized quantitatively into frequentist random effect meta-analysis of pooled risk ratio (RR) and standardized mean difference (SMD). An inverse-variance method and DerSimonian–Laird estimator for the variance of the true effect size (τ2) were performed [22, 23]. Heterogeneity among studies was evaluated by the I2 index and Cochran’s Q test [24]. Statistical heterogeneity was considered low, moderate, and high for I2 values of 25, 50, and 75%, respectively, and significant when p < 0.10 [25, 26]. The Wald-type 95% confidence interval (CI) was computed for pooled measurements; otherwise, the 95% CI for the I2 index was calculated according to Higgins and Thompson [27]. The prediction interval for the treatment effect of a new study was calculated according to Borenstein et al. [24]. As the sample size was not the same in all studies, we performed a sensitivity analysis by excluding one study each time and rerunning the analysis to verify the robustness of the overall results. The publication bias was also investigated with the trim and fill funnel plot and Egger test. A two-sided p value was considered statistically significant when p < 0.05. All analyses and figures were carried out using the R software program, version 3.2.2 [28].
Results
Systematic review
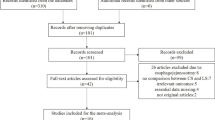
The PRISMA flow chart is reported in Fig. 1. Four-hundred fifty-eight publications were identified. After duplicates removal, 402 titles were screened. Sixteen articles were excluded after title assessment. Overall, 386 abstracts were reviewed while 21 articles were found possibly relevant for full-text assessment. After full-text evaluation, 18 studies meet the inclusion/exclusion criteria and were included in the quantitative synthesis. Fifteen studies were of retrospective design while three were RCTs. The quality of observational studies and RCTs is reported in Supplementary Table 1 and Supplementary Figure 1.
Overall, 2861 patients were included (Table 1). Of those 1371 (47.9%) underwent CS while 1490 (52.1%) underwent LS. The age of the patient population ranged from 47 to 87 years, the BMI ranged from 15.3 to 30.2 kg/m2, and the majority (74.9%) were males. The tumor was located in the upper (12.4%), middle (51.6%), and lower (36%) esophagus. Tumor histology was specified in 9 studies; squamous cell carcinoma and adenocarcinoma were diagnosed in 83% and 15.5% of patients, respectively. Pathological tumor staging according to the 7th and 8th edition of the American Joint Committee on Cancer (AJCC) and Japanese gastric cancer classification (JGCC) was detailed in 7 studies (stage 0–I: 25.1%, stage II: 37.1%, stage III: 34.5%, and stage IV: 3.3%). Open, hybrid, or totally minimally invasive esophagectomy were performed depending on operating surgeon preference and expertise. Both cervical and intrathoracic anastomoses were described according to tumor location, operating surgeon preferences, and oncological principles. The use of neoadjuvant chemoradiation therapy was heterogeneously reported in nine studies (i.e., protocols, regimens, etc.). The extent of lymphadenectomy (2-field and 3-field) was specified in 5 studies and varied depending on surgeon expertise and tumor clinical stage/location.
Meta-analysis—primary outcomes
AL was reported in eighteen studies (2861 patients). The cumulative incidence of AL was reduced for LS vs. CS (7.9% vs. 12.2%). Compared to CS, LS was associated with a significantly reduced AL risk (RR = 0.70; 95% CI 0.54–0.91; p < 0.01) (Fig. 2A). The prediction lower and upper limits were 0.47 and 1.06, respectively. The heterogeneity was zero (I2 = 0.0%, 95% CI 0.0–50%; p < 0.01) and τ2 = 0.01. The funnel plot (Fig. 2B) and the Egger test (p = 0.49) did not show evidence of publication bias. The sensitivity analysis showed the robustness of these findings in terms of point estimation, relative confidence intervals, and heterogeneity. After stratification, LS showed a significant AL risk reduction for cervical anastomosis (7 studies; 952 patients) (RR = 0.61; 95% CI 0.38–0.96; I2 = 11%; p = 0.039) while no significant differences were found for thoracic anastomosis (9 studies, 1581 patients) (RR = 0.78; 95% CI 0.57–1.05; I2 = 0.0%; p = 0.10) (Supplementary Figure 2 A-B).
AS was reported in 15 studies (1922 patients). The cumulative incidence of AS was reduced for LS vs. CS (7.7% vs. 18.9%). Compared to CS, LS was associated with a significantly reduced AS risk (RR = 0.32; 95% CI 0.20–0.51; p < 0.0001) (Fig. 3A). The prediction lower and upper limits were 0.11 and 1.05, respectively. The heterogeneity was moderate (I2 = 33.3%, 95% CI 0.0–50%; p < 0.01) and τ2 = 0.24. The funnel plot (Fig. 3B) and the Egger test (p = 0.04) showed that publication bias could not be excluded. The sensitivity analysis showed that omitting the study by Xu et al., the heterogeneity decreased to low (I2 = 12%). After stratification, LS showed a significant AL risk reduction for both cervical (7 studies; 1162 patients) (RR = 0.42; 95% CI 0.30–0.58; p < 0.001; I2 = 43%) and thoracic anastomosis (7 studies, 749 patients) (RR = 0.32; 95% CI 0.14–0.77; p < 0.01; I2 = 33%) (Supplementary Figure 3 A-B).
Subgroup analysis by including only RCTs (3 studies; 285 patients) showed a trend toward reduced AL (RR = 0.56; 95% CI 0.19–1.09; p = 0.32; I2 = 0.0%) and AS risk (RR = 0.17; 95% CI 0.02–1.88; p = 0.15; I2 = 29%) for LS vs. CS.
Meta-analysis—secondary outcomes
Reflux esophagitis (RR = 0.74; 95% CI 0.30–1.41; p = 0.36; I2 = 62%), pneumonia (RR = 0.78; 95% CI 0.57–1.06; p = 0.12; I2 = 0.0%), OT (SMD −0.25; 95% CI −0.25, 0.09; p = 0.16; I2 = 77%), HLOS (SMD 0.13; 95% CI −0.25, 0.51; p = 0.51; I2 = 91%), and 30-day mortality (RR = 1.26; 95% CI 0.72–2.21; p = 0.42; I2 = 0.0%) were similar for LS vs. CS (Fig. 4A-C). Using the GRADE tool, we rated the quality of evidence supporting each outcome as low-moderate mainly because of limitations in study design (Supplementary Table 2).
Discussion
This meta-analysis shows that compared to CS, LS seems associated with a reduced RR of AL and AS during esophagectomy. After stratification for both cervical and thoracic anastomosis, LS shows a trend toward reduced AL and AS risk. Reflux esophagitis, pneumonia, and 30-day mortality seem similar among treatments.
The esophagogastric anastomosis represents a central part of esophagectomy and may potentially contribute to the significant short- and long-term morbidity and mortality [4, 6, 47]. AL is a major complication with a reported incidence ranging from 10 and 20% [48]. It is associated with increased postoperative morbidity, high postoperative mortality rates, prolonged hospital stay, and increased costs [49,50,51,52]. In addition, it has been shown to decrease long-term quality of life and oncological survival [6, 53]. Factors that may contribute to anastomosis failure are lack of serosa layer, tension, inadequate blood supply of the gastric conduit, surgical technique, malnutrition, and patient comorbidities [54]. Furthermore, it has been suggested that gut microbiome may influence the suture line healing process thus possibly contributing to anastomosis breakdown [55]. Various techniques for esophagogastric anastomosis (hand-sewn vs. stapled) have been described [56,57,58]. Interestingly, there has been a gradual shift from hand-sewn to stapled anastomosis because technical simplicity, comparable safety, and time saving [59]. Currently, both LS and CS anastomosis are used for esophagogastric anastomosis. Their utilization is mainly dependent on surgeon preference while each technique has its pros and cons. Specifically, CS facilitates the anastomosis at the apex of the thorax (cupula pleuralis); however, the anastomotic lumen is dependent on the original esophageal diameter with problems related to possible size mismatch. Furthermore, CS creates an inverted anastomosis where esophageal and gastric mucosa margins are separated by muscular layers thus potentially resulting in a high stricture rates. Conversely, LS anastomosis makes a larger anastomotic diameter, minimizes problems related to visceral mismatch, and makes an extroverted anastomosis which leads to improved mucosa-to-mucosa apposition [29, 37]. Furthermore, it has been postulated that LS might be advantageous in terms of blood supply as the staple line results parallel to the axis of the gastric conduit thus leading to maximum preservation of the vasculature network at the anastomosis mainly perfused through intramural capillaries [44, 60, 61]. Recently Nickel et al. explored the principles of gastric conduit capillaries dynamics in live porcine models undergoing minimally invasive esophagectomy with LS anastomosis. Authors concluded that the dominant direction of flow through the conduit mainly arises through the right gastroepiploic artery and capillaries along the transverse conduit axis thus suggesting the use of short (30 mm) linear staplers to preserve optimal tissue oxygenation at the anastomotic site [62]. Finally, LS anastomosis requires a longer esophageal remnant (at least 2 cm); thus, fashioning an aligned high intra-thoracic tension-free anastomosis is challenging because of limited space.
While both techniques are currently used, robust evidence describing the best technique for stapled-esophageal anastomosis is to be defined. A previous study by Yanni et al. reported significantly reduced thoracic AL for LS vs. CS (4.1% vs. 15.3%; p = 0.019) [38]. Equally, Huang et al. in their 2017 retrospective study described a significantly reduced incidence of cervical AL for LS vs. CS (7.7% vs. 19%) [37]. Despite the lack of statistical significance, Fabbi et al. [46] and Hosoi and colleagues [44] reported a tendency toward reduced AL for LS. In contrast, Zhou et al. in a 2015 meta-analysis affirmed no differences for postoperative AL comparing LS vs. CS (RR = 0.80; p = 0.52) [63]. In our study, we found that LS seems associated with a significantly reduced RR for AL (RR = 0.67; p < 0.01). The related heterogeneity was 0.0% thus adding robustness to the result. Possible explanations include a better vascular/oxygen supply to the anastomotic site, superior anastomosis orientation with concomitantly reduced traction-related tension, and minimization of visceral twisting. The stratification analysis still evidences a significant difference for cervical anastomosis (RR = 0.61; p = 0.04) while no significant differences were found for thoracic anastomosis. Notably, the point estimation was below 1.00 (RR = 0.61) thus suggesting a clinical tendency toward reduced RR. However, this initial indication mandates future analysis and possible confirmation by large trials. Despite the low heterogeneity, our result should be interpreted with caution because the presence of possible confounders related to technical variations (Collard vs. Orringer procedure), closure of the anterior wall (i.e., single layer vs. double layer vs. stapling), suture material (absorbable vs. non-absorbable vs. antibacterial suture), blood flow assessment with indocyanine green, gastric ischemic preconditioning, esophageal diameter (dilated or not), omental wrapping, route of reconstruction (retrosternal vs. posterior mediastinal), patient selection, baseline comorbidities (i.e., diabetes), smoking status, neoadjuvant treatment, tumor characteristics (i.e., grading, size, location, etc.), tumor-free resection margins (R0), and surgical approach.
In our meta-analysis, LS seems associated with a significantly reduced RR for AS (RR = 0.31; p < 0.0001) irrespective from the level of the anastomosis. This is similar to what previously reported by Zhou et al. that acknowledged a significant tendency toward reduced stricture risk for LS (RR = 0.26; p = 0.002) [63]. Similar results are reported by several retrospective analyses [32, 37, 44]. Possible explanations include a wider anastomotic diameter and reduced risk of staple line scarring/fibrosis for LS. Again, these results should be interpreted prudently because lacking of standardized techniques, different sizes (25 mm vs. 28 mm) and techniques for CS (i.e., purse-string suture vs. EEA™ vs. Orvil™), patient selection, postoperative leak occurrence, patient age, comorbidities (i.e., diabetes), smoking status, neoadjuvant treatment, tumor-free resection margins (R0), surgical approach (open vs. minimally invasive), and surgeon proficiency. Interestingly, no significant differences were found for reflux esophagitis (RR = 0.74; p = 0.36). This may be attributable to the increased utilization of proton pump inhibitors to reduce acid production and esophageal exposure. This is different from Hosoi et al. that affirmed a tendency toward higher rates of esophagitis for LS anastomosis [44]. Finally, no significant differences were found for postoperative pneumonia, 30-day mortality, OT, and HLOS.
Notably, both AL and AS may not directly reflect the quality of a specific technique but are also influenced by surgeon proficiency, learning curves, structured training/mentorship programs, and hospital volumes [64,65,66]. Surgical volume and operating surgeon proficiency are critical to obtain optimal surgical outcomes after esophagectomy. It has been shown that case-load centralization in high-volume centers significantly reduces mortality and may improve outcomes [67]. Specifically, during the learning curve, AL has been shown to decrease from 18% in the early phase to 4.5% after 119 cases [68]. Based on these considerations, it should be pondered that AL and AS may not entirely reflect the anastomotic technique but are also influenced by learning curve effect and surgeon experience. Finally, the introduction of new technologies such as fluorescence imaging with indocyanine green to assess the gastric conduit perfusion/anastomosis and the advent of robot-assisted esophagectomy may potentially improve outcomes [69,70,71].
Our study presents the typical limitations of a meta-analysis including observational studies. The lack of inclusion criteria defined a priori, lack of homogenous surgical approach, and lack of globally defined postoperative management protocols. Second, AL and AS were not uniformly defined and classified among included studies. Third, the majority of included studies (12/18) were performed in Asian countries; therefore, results may not be generalizable. Fourth, surgeon experience and volume were not measured with a conceivable effect on outcomes. Lastly, the creation of a single type of anastomosis might have diverse technical variations among different esophageal surgeons and should therefore be considered as possible source of heterogeneity.
Conclusions
There is a variety of different techniques for esophagogastric anastomosis. Compared to CS, LS anastomosis seems associated with a tendency toward a reduced risk for AL and AS even after stratification according to the level of anastomosis (cervical and thoracic). Although surgeon’s own training, learning curve, and experience might direct the choice toward a definite technique for esophagogastric anastomosis, our meta-analysis encourages the implementation of LS anastomosis.
Data availability
Data generated at a central, large-scale facility, available upon request.
References
Murphy G, McCormack V, Abedi-Ardekani B et al (2017) International cancer seminars: a focus on esophageal squamous cell carcinoma. Ann Oncol. 28(9):2086–2093
Kutup A, Nentwich MF, Bollschweiler E et al (2014) What should be the gold standard for the surgical component in the treatment of locally advanced esophageal cancer: transthoracic versus transhiatal esophagectomy. Ann Surg. 260(6):1016–22
Bonavina L (2021) Progress in the esophagogastric anastomosis and the challenges of minimally invasive thoracoscopic surgery. Ann Transl Med. 9(10):907
Goense L, Meziani J, Ruurda JP et al (2019) Impact of postoperative complications on outcomes after oesophagectomy for cancer. Br J Surg 106:111–9
Markar S, Gronnier C, Duhamel A et al (2015) The impact of severe anastomotic leak on long-term survival and cancer recurrence after surgical resection for esophageal malignancy. Ann Surg 262:972–80
Shiraishi O, Yasuda T, Kato H et al (2022) Circular stapler method for avoiding stricture of cervical esophagogastric anastomosis. J Gastrointest Surg. 26(4):725–732
Kim RH, Takabe K (2010) Methods of esophagogastric anastomosis following esophagectomy for cancer: a systematic review. J Surg Oncol 101:527–33
Schlottmann F, Angeramo CA, Bras Harriott C et al (2022) Transthoracic esophagectomy: hand-sewn versus side-to-side linear-stapled versus circular-stapled anastomosis: a systematic review and meta-analysis. Surg Laparosc Endosc Percutan Tech. 32(3):380–392
Steichen F (2001) Mechanical sutures in esophageal replacement: fashion or resource? Dis Esophagus 14:1–12
Collard JM, Romagnoli R, Goncette L et al (1998) Terminalized semimechanical side-to-side suture technique for cervical esophagogastrostomy. Ann Thorac Surg 65:814–7
Kondra J, Ong SR, Clifton J et al (2008) Change in clinical practice: a partially stapled cervical esophagogastric anastomosis reduces morbidity and improves functional outcome after esophagectomy for cancer. Dis Esophagus. 21(5):422–9
Orringer MB, Marshall B, Iannettoni MD (2000) Eliminating the cervical esophagogastric anastomotic leak with a side-to-side stapled anastomosis. J Thorac Cardiovasc Surg. 119(2):277–88
Kamarajah SK, Bundred JR, Singh P, Pasquali S, Griffiths EA (2020) Anastomotic techniques for oesophagectomy for malignancy: systematic review and network meta-analysis. BJS Open. 4(4):563–576
Herron R, Abbas G (2021) Techniques of esophageal anastomoses for esophagectomy. Surg Clin North Am. 101(3):511–524
Liberati A, Altman DG, Tetzlaff J et al (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 339:b2700. https://doi.org/10.1136/bmj.b2700
Stroup DF, Berlin JA, Morton SC et al (2000) Meta-analysis of observational studies in epidemiology: a proposal for reporting Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 283(15):2008–12
Goossen K, Tenckhoff S, Probst P et al (2018) Optimal literature search for systematic reviews in surgery. Langenbecks Arch Surg. 403(1):119–129
Sterne JA, Hernan MA, Reeves BC, et al. 2016 ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. i4919:355.
Higgins JP, Altman DG, Gotzsche PC et al (2011) The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343:d5928
Guyatt GH, Oxman AD, Vist GE et al (2008) GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 336(7650):924–926
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7(3):177–188
Porta A, Aiolfi A, Musolino C, Antonini I, Zappa MA (2017) Prospective comparison and quality of life for single-incision and conventional laparoscopic sleeve gastrectomy in a series of morbidly obese patients. Obes Surg 27(3):681–687. https://doi.org/10.1007/s11695-016-2338-2
Borenstein M, Hedges LV, Higgins JP, Rothstein HR (2010) A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods 1(2):97–111
Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327:557–560
Aiolfi A, Tornese S, Bonitta G et al (2019) Roux-en-Y gastric bypass: systematic review and Bayesian network meta-analysis comparing open, laparoscopic, and robotic approach. Surg Obes Relat Dis. 15(6):985–994
Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21(11):1539–1558
R Development Core Team (2015) A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna (ISBN 3-900051-07-0)
Furukawa Y, Hanyu N, Hirai K et al (2005) Usefulness of automatic triangular anastomosis for esophageal cancer surgery using a linear stapler (TA-30). Ann Thorac Cardiovasc Surg. 11(2):80–6
Blackmon SH, Correa AM, Wynn B, Hofstetter WL, Martin LW, Mehran RJ, Rice DC, Swisher SG, Walsh GL, Roth JA, Vaporciyan AA (2007) Propensity-matched analysis of three techniques for intrathoracic esophagogastric anastomosis. Ann Thorac Surg. 83(5):1805–13
Xu QR, Wang KN, Wang WP, Zhang K, Chen LQ (2011) Linear stapled esophagogastrostomy is more effective than hand-sewn or circular stapler in prevention of anastomotic stricture: a comparative clinical study. J Gastrointest Surg. 15(6):915–21
Wang WP, Gao Q, Wang KN, Shi H, Chen LQ (2013) A prospective randomized controlled trial of semi-mechanical versus hand-sewn or circular stapled esophagogastrostomy for prevention of anastomotic stricture. World J Surg. 37(5):1043–50
Price TN, Nichols FC, Harmsen WS, Allen MS, Cassivi SD, Wigle DA, Shen KR, Deschamps C (2013) A comprehensive review of anastomotic technique in 432 esophagectomies. Ann Thorac Surg. 95(4):1154–60
Li J, Shen Y, Tan L, Feng M, Wang H, Xi Y, Leng Y, Wang Q. Cervical triangulating stapled anastomosis: technique and initial experience. J Thorac Dis. 2014 May;6 Suppl 3(Suppl 3):S350-4.
Mungo B, Lidor AO, Stem M, Molena D (2016) Early experience and lessons learned in a new minimally invasive esophagectomy program. Surg Endosc. 30(4):1692–8
Hayata K, Nakamori M, Nakamura M et al (2017) Circular stapling versus triangulating stapling for the cervical esophagogastric anastomosis after esophagectomy in patients with thoracic esophageal cancer: a prospective, randomized, controlled trial. Surgery. 162(1):131–138
Huang C, Xu X, Zhuang B, Chen W, Xu X, Wang C, Lin S (2017) A comparison of cervical delta-shaped anastomosis and circular stapled anastomosis after esophagectomy. World J Surg Oncol. 15(1):31
Yanni F, Singh P, Tewari N, Parsons SL, Catton JA, Duffy J, Welch NT, Vohra RS (2019) Comparison of outcomes with semi-mechanical and circular stapled intrathoracic esophagogastric anastomosis following esophagectomy. World J Surg. 43(10):2483–2489
Schröder W, Raptis DA, Schmidt HM et al (2019) Anastomotic techniques and associated morbidity in total minimally invasive transthoracic esophagectomy: results from the EsoBenchmark database. Ann Surg 270:820–6
Wang ZQ, Jiang YQ, Xu W, Cai HR, Zhang Z, Yin Z, Zhang Q (2018) A novel technique for cervical gastro-oesophageal anastomosis during minimally invasive oesophagectomy. Int J Surg. 53:221–229
Zhang H, Wang Z, Zheng Y, Geng Y, Wang F, Chen LQ, Wang Y (2019) Robotic side-to-side and end-to-side stapled esophagogastric anastomosis of Ivor Lewis esophagectomy for cancer. World J Surg. 43(12):3074–3082
Tian Y, Li L, Li S, Tian H, Lu M (2020) Comparison of circular stapling, triangulating stapling and T-shape stapling for cervical anastomosis with minimally invasive esophagectomy. Ann Transl Med. 8(24):1679
Hirano Y, Fujita T, Sato K, Kurita D, Sato T, Ishiyama K, Fujiwara H, Oguma J, Daiko H (2020) Totally mechanical collard technique for cervical esophagogastric anastomosis reduces stricture formation compared with circular stapled anastomosis. World J Surg. 44(12):4175–4183
Hosoi T, Abe T, Higaki E, Fujieda H, Nagao T, Ito S, Komori K, Iwase M, Oze I, Shimizu Y (2022) Circular stapled technique versus modified collard technique for cervical esophagogastric anastomosis after esophagectomy: a randomized controlled trial. Ann Surg. 276(1):30–37
Sugita H, Sakuramoto S, Oya S, Fujiwara N, Miyawaki Y, Satoh H, Okamoto K, Yamaguchi S, Koyama I (2021) Linear stapler anastomosis for esophagogastrostomy in laparoscopic proximal gastrectomy reduce reflux esophagitis. Langenbecks Arch Surg. 406(8):2709–2716
Fabbi M, van Berge Henegouwen MI, Fumagalli Romario U, Gandini S, Feenstra M, De Pascale S, Gisbertz SS. 2022. End-to-side circular stapled versus side-to-side linear stapled intrathoracic esophagogastric anastomosis following minimally invasive Ivor-Lewis esophagectomy: comparison of short-term outcomes. Langenbecks Arch Surg. https://doi.org/10.1007/s00423-022-02567-9
Oesophago-Gastric Anastomosis Audit study group on behalf of the West Midlands Research Collaborative. 2022 The influence of anastomotic techniques on postoperative anastomotic complications: results of the oesophago-gastric anastomosis audit. J Thorac Cardiovasc Surg. 164(3):674-684.e5.
Griffiths E A, Oesophago-Gastric Anastomosis Study Group on behalf of the West Midlands Research Collaborative. 2022 Rates of anastomotic complications and their management following esophagectomy: results of the oesophago-gastric anastomosis audit (OGAA). Ann Surg; 275 e382–91.
Derogar M, Orsini N, Sadr-Azodi O, Lagergren P (2012) Influence of major postoperative complications on health-related quality of life among long-term survivors of esophageal cancer surgery. J Clin Oncol. 30(14):1615–9
Messager M, Warlaumont M, Renaud F, Marin H, Branche J, Piessen G, Mariette C (2017) Recent improvements in the management of esophageal anastomotic leak after surgery for cancer. Eur J Surg Oncol. 43(2):258–269
Aiolfi A, Asti E, Rausa E, Bonavina G, Bonitta G, Bonavina L (2018) Use of C-reactive protein for the early prediction of anastomotic leak after esophagectomy: systematic review and Bayesian meta-analysis. PLoS One. 13(12):e0209272
Rausa E, Asti E, Aiolfi A, Bianco F, Bonitta G, Bonavina L. 2018 Comparison of endoscopic vacuum therapy versus endoscopic stenting for esophageal leaks: systematic review and meta-analysis. Dis Esophagus. 31(11).
Low DE, Alderson D, Cecconello I et al (2015) International consensus on standardization of data collection for complications associated with esophagectomy: Esophagectomy Complications Consensus Group (ECCG). Ann Surg 262:286–94
Kamarajah SK, Lin A, Tharmaraja T, Bharwada Y, Bundred JR, Nepogodiev D, Evans RPT, Singh P, Griffiths EA. 2020 Risk factors and outcomes associated with anastomotic leaks following esophagectomy: a systematic review and meta-analysis. Dis Esophagus.;33(3):doz089.
Roos D, Dijksman LM, Tijssen JG et al (2013) Systematic review of perioperative selective decontamination of the digestive tract in elective gastrointestinal surgery. Br J Surg 100:1579–88
Deng XF, Liu QX, Zhou D, Min JX, Dai JG (2015) Hand-sewn vs linearly stapled esophagogastric anastomosis for esophageal cancer: a meta-analysis. World J Gastroenterol. 21(15):4757–64
Honda M, Kuriyama A, Noma H, Nunobe S, Furukawa TA (2013) Hand-sewn versus mechanical esophagogastric anastomosis after esophagectomy: a systematic review and meta-analysis. Ann Surg. 257(2):238–48
Markar SR, Karthikesalingam A, Vyas S, Hashemi M, Winslet M (2011) Hand-sewn versus stapled oesophago-gastric anastomosis: systematic review and meta-analysis. J Gastrointest Surg. 15(5):876–84
Haverkamp L, Seesing MF, Ruurda JP, Boone J, Hillegersberg V, R. (2017) Worldwide trends in surgical techniques in the treatment of esophageal and gastroesophageal junction cancer. Dis Esophagus. 30(1):1–7
de Groot EM, Kuiper GM, van der Veen A et al (2022) Indocyanine green fluorescence in robot-assisted minimally invasive esophagectomy with intrathoracic anastomosis: a prospective study. Updates SurgAug. https://doi.org/10.1007/s13304-022-01329-y
van Rossum PSN, Haverkamp L, Verkooijen HM et al (2015) Calcification of arteries supplying the gastric tube: a new risk factor for anastomotic leakage after esophageal surgery. Radiology. 274(1):124–32. https://doi.org/10.1148/radiol.14140410
Nickel F, Studier-Fischer A, Özdemir B, et al. 2021 Optimization of anastomotic technique and gastric conduit perfusion with hyperspectral imaging in an experimental model for minimally invasive esophagectomy. bioRxiv .10.03.462901; https://doi.org/10.1101/2021.10.03.462901
Zhou D, Liu QX, Deng XF, Min JX, Dai JG (2015) Comparison of two different mechanical esophagogastric anastomosis in esophageal cancer patients: a meta-analysis. J Cardiothorac Surg. 8(10):67
van Workum F, Stenstra MHBC, Berkelmans GHK et al (2019) Learning curve and associated morbidity of minimally invasive esophagectomy: a retrospective multicenter study. Ann Surg 269:88–94
Markar SR, Mackenzie H, Lagergren P, Hanna GB, Lagergren J (2016) Surgical proficiency gain and survival after esophagectomy for cancer. J Clin Oncol. 34(13):1528–36
Halliday LJ, Doran SLF, Sgromo B, et al. 2020 Variation in esophageal anastomosis technique-the role of collaborative learning. Dis Esophagus;33:doz072.
Tapias LF, Morse CR (2014) Minimally invasive Ivor Lewis esophagectomy: description of a learning curve. J Am Coll Surg. 218(6):1130–40
van Workum F, Stenstra MHBC, Berkelmans GHK, Slaman AE, van Berge Henegouwen MI, Gisbertz SS, van den Wildenberg FJH, Polat F, Irino T, Nilsson M, Nieuwenhuijzen GAP, Luyer MD, Adang EM, Hannink G, Rovers MM, Rosman C (2019) Learning curve and associated morbidity of minimally invasive esophagectomy: a retrospective multicenter study. Ann Surg. 269(1):88–94
Bonavina L, Asti E, Sironi A, Bernardi D, Aiolfi A (2017) Hybrid and total minimally invasive esophagectomy: how I do it. J Thorac Dis. 9(Suppl 8):S761–S772
Ladak F, Dang JT, Switzer N, Mocanu V, Tian C, Birch D, Turner SR, Karmali S (2019) Indocyanine green for the prevention of anastomotic leaks following esophagectomy: a meta-analysis. Surg Endosc. 33(2):384–394
van Boxel G, van Hillegersberg R, Ruurda J (2019) Outcomes and complications after robot-assisted minimally invasive esophagectomy. J Vis Surg 5:21
Author information
Authors and Affiliations
Contributions
AA, AS, FL, and MC did the literature search. AA, GB, and DB formed the study design. Data collection done by AA, AS, FL, and MC. AA, GB, AS, and DB analyzed the data. AA, SC, PD, GC, and DB interpreted the data. AA wrote the manuscript. All authors critically reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
For this type of article, ethical approval is not required because does not contain any studies with human participants or animals performed by any of the authors.
Consent to participate
For this type of study, formal consent was not necessary.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Aiolfi, A., Sozzi, A., Bonitta, G. et al. Linear- versus circular-stapled esophagogastric anastomosis during esophagectomy: systematic review and meta-analysis. Langenbecks Arch Surg 407, 3297–3309 (2022). https://doi.org/10.1007/s00423-022-02706-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00423-022-02706-2