Abstract
Laparoscopic resection of liver tumors located in the posterosuperior segments is a challenging operation that could be facilitated by robotic assistance. Laparoscopic resection of 12 tumors located in posterosuperior segments (IVa: 1; VII: 5; VIII: 6) was carried out under robotic assistance. All patients had a single tumor nodule. Data were collected prospectively and analyzed retrospectively. Surgery required a mean of 260.4 min (115–430) and was completed laparoscopically in all but one patient, who required conversion to mini-laparotomy because of intolerance of pneumoperitoneum (8.3 %). Mean estimated blood loss was 252.7 ml (50–600), making transfusion necessary in 3 patients (25.0 %). Post-operative complications occurred in 4 patients (33.3 %), being of Clavien–Dindo grade II in 3 patients (25.0 %) and Clavien–Dindo grade IV in 1 patient (8.3 %). Reoperation was required in 1 patient, who subsequently had a long hospital stay, because of decompensated cirrhosis. Median length of hospital stay was 8.5 days (7–96). No patient was readmitted. Pathology showed hepatocellular carcinoma in 7 patients (58.3 %), liver metastasis in 2 patients (16.6 %), and hepatic adenoma, focal nodular hyperplasia, and hemangioma in one patient each (8.3 %). All patients had a margin negative resection. After a mean follow-up period of 21.4 months (±24.4), no patient with malignant histology developed recurrence. Our initial experience confirms that laparoscopic robot-assisted resection of tumors located in the posterosuperior segments is feasible. Further experience is needed before final conclusions can be drawn and meaningful comparison with other surgical techniques becomes possible.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
After a long gestation [1], laparoscopic liver resection is gaining momentum, especially in referral centers for liver surgery and amongst younger surgeons [2]. Laparoscopic liver resection was initially introduced into clinical practice based on case series, demonstrating the usual benefits of laparoscopy [3], but did not gain widespread popularity until the publication of several major series [4], a review of world experience [5], and the results of the first world consensus conference [6].
Despite gifted surgeons have shown that conventional laparoscopy allows resection of tumors located in all liver segments [7] as well as all types of liver resections, including extended hepatectomies [8], the intrinsic limitations of laparoscopy, the need for extensive training, and the challenges presented by safe liver transection, have not made laparoscopy equally used across all types of liver resections. Laparoscopy is indeed particularly suitable when tumors are located in the anteroinferior liver segments (IVb, V, and VI) and when straight resection lines are required, such as in left lateral segmentectomy [6]. Instead, when dealing with tumors located in the posterosuperior liver segments (IVa, VII, and VIII), or when curvilinear resection planes are required to pursue parenchymal sparing liver resection [9], laparoscopy is confronted by most of its technical limitations. Because of these challenges, resection of posterosuperior liver segments was confirmed to be a major resection by the second world consensus conference on laparoscopic liver resection [10], and the difficult scoring system for this type of surgery [11] allocates tumors located in segments VII and VIII to the highest difficulty score.
The enhanced operative abilities offered by the da Vinci Surgical System (dVSS) (Intuitive Surgical Inc. Sunnyvale, CA, USA) could be particularly useful when pursuing laparoscopic resection of liver tumors located in the posterosuperior segments. The dVSS, indeed, when compared to standard laparoscopy, offers significant operative advantages (i.e., stereoscopic steady vision, wristed instruments, tremor filtration, and scaled motion) that are known to enhance surgical dexterity [12].
We herein present our experience with laparoscopic robot-assisted resection of tumors located in the posterosuperior liver segments.
Materials and methods
Laparoscopic liver resection was considered in all patients admitted to our Division with a diagnosis of resectable liver tumors or metastases, between October 1, 2008 and February 28, 2015. Contraindications to laparoscopy were diagnosis of Klatskin tumor, need for extensive node dissection, need for associated major abdominal procedures, history of previous major surgery in upper abdominal quadrants, tumor size not permitting safe liver mobilization, and anticipated poor tolerance to pneumoperitoneum. Patients suitable for a laparoscopic approach having tumors located in the anteroinferior liver segments (I, IVb, V, and VI) or in the left lateral liver segments (II and III) were operated upon by standard laparoscopy. Robotic assistance was selectively employed in patients otherwise suitable for laparoscopy who required major hepatectomy or resection of posterosuperior liver segments (IVa, VII, and VIII).
Surgical technique
The surgical team comprised of 2 surgeons and a surgical trainee. The senior surgeon operated from the robotic console, while the second surgeon and the surgical trainee exchanged robotic instruments and managed classical laparoscopic tasks at the bedside (i.e., suction/irrigation, insertion/withdrawal of needles, etc.).
Pneumoperitoneum was induced using a Veress needle and maintained at 12 mmHg, during most of the procedure. If necessary, pneumoperitoneum pressure was temporarily increased, up to 20 mmHg, and airway pressure decreased, to reduce back-bleeding and facilitate achievement of permanent hemostasis. All resections were guided by laparoscopic contact ultrasound, managed by the surgeon at the table. Ultrasound images were visualized by the surgeon at the console, as a picture-in-picture image, using the Tilepro® system [12].
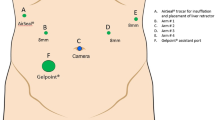
Patients with tumors located in segments VIII and IVa were placed supine with the legs parted. The table was placed in 25°–30° reverse Trendelenburg position and titled 15°–20° to the left side. The patient side cart was docked over the head of the patient. Five trocars were placed, all close to the costal margin. The optic port was placed along the midclavicular line. Robotic ports and the assistant port were placed along a curvilinear line as shown in Fig. 1. Liver mobilization was not usually required. Specific attention was paid in patients with cirrhotic livers to preserve the round ligament to maintain the collateral circulation that may develop by means of recanalized umbilical vein. For tumors located in the most lateral portion of segment VIII, close to segment VII, division of round and falciform ligaments was instead required, to improve exposure. Pulling the liver stump of the round ligament on the left side, resulted in clockwise rotation of the right hemiliver, especially after additional division of right triangular ligament and right coronary ligament. Extensive liver mobilization, however, was not required.
For tumors located in segment VIII or IVa, the patient is placed supine and the table is oriented in the reverse Trendelenburg position and tilted to the left side. The patient side cart is docked over the head of the patient (arrow), with 2 robotic arms (RA) on the patient’s left side (see set up of patient side cart as shown in the box). Five ports are used (3, 8 mm; 2, 12 mm). The optic port (OP) is placed along the midclavicular line, and the assistant port (AP) is placed between OP and RA1
Patients with tumors located in segment VII were placed on the left flank position and the patient side cart was docked at 30°–45° clockwise over the head of the patient. The table was placed in 25°–30° reverse Trendelenburg position. Five trocars were placed, all close the costal margin. No intercostal trocar was placed. The optic port was placed along the anterior axillary line. Robotic ports and assistant port were placed along a curvilinear line as shown in Fig. 2. The right liver lobe was mobilized until the target area came into clear view, so that it could be safely dominated.
For tumors located in segment VII, the patient is placed on the left flank position and the table is oriented in the reverse Trendelenburg position. The patient side cart is docked between 30° and 45° clockwise over the head of the patient (arrow), with 2 robotic arms (RA) on the patient’s left side (see set up of patient side cart as shown in the box). Five ports are used (3, 8 mm; 2, 12 mm). The optic port (OP) is placed along the anterior axillary line, and the assistant port (AP) is placed between OP and RA1
Parenchymal transection was carried out by clamp-crushing technique using bipolar precise forceps and electrified monopolar curved scissors (Fig. 3). Harmonic scalpel was not used routinely since this instrument cannot articulate, thus reducing the possibility to follow curvilinear resection lines and pursue parenchymal sparing surgery especially in posterosuperior segments. Pringle maneuver was not employed. Portal and hepatic pedicles were secured by Hem-o-lock clips, ligature, or suture-ligature as appropriate. Anatomical resection, including segmentectomy or sub-segmentectomy as appropriate, was pursued in hepatocellular carcinoma under ultrasound guidance. Portal pedicles were identified and selectively ligated [10].
Robotic liver transection using bipolar Maryland forceps, as crushing and coagulating instrument, and monopolar curved scissors, as cutting and coagulating instrument. A Cadiere forceps, holding a peanut gauze, can be used to improve exposure, or to achieve temporary bleeding control. Suction/irrigation is concurrently applied by the surgeon at the table
After completing resection, the specimen was placed into an endobag and extracted through an enlarged port site. Hemostatic products such as collagen or fibrin glue were not routinely used. One or two closed suction drains were left close to resection area.
Outcome metrics
Data from all patients were prospectively entered in a computer database and analyzed retrospectively. Operative time was estimated from induction of pneumoperitoneum to closure of skin incisions. Intraoperative blood loss was calculated by the difference between instilled and aspirated liquids. Liver anatomy and type of resection were defined according to the Brisbane classification [13]. Complications were classified according to the Clavien–Dindo classification [14]. Post-operative mortality, presented at 90 days, was defined as any death occurring at any time during the initial hospital stay or at the time of readmission if related to initial surgery or its complications.
Results
Twelve patients underwent laparoscopic robot-assisted resection of tumors located in the posterosuperior segments (Table 1). All patients had a single tumor nodule.
Laparoscopic robot-assisted liver resection was successfully carried out in all but one patient, who required conversion to mini-laparotomy, when the operation was nearly completed, because of poor tolerance to pneumoperitoneum (1/12; 8.3 %).
Main intraoperative and post-operative outcome measures are summarized in Table 2. Blood transfusions were required in 3 patients (25 %). Two patients received 2 and 3 blood units, respectively, while the third patient, with advanced liver cirrhosis, bled twice, 2 and 4 weeks after the initial surgery, and eventually required a total of 12 blood units. Hemostasis in this patient was not easily achieved even through an open approach and despite wide liver mobilization. At both reoperations he was found to have arterial bleeding from several small arteries scattered all through the resection bed. This patient had a long hospital stay (96 days), including intensive care support, mostly required to recover from decompensated liver cirrhosis.
Pathology of resected specimens showed hepatocellular carcinoma in seven patients (58.3 %), liver metastasis in two patients (16.6 %), and hepatic adenoma, focal nodular hyperplasia, and hemangioma in one patient each (8.3 %). All patients had margin negative resection. After a mean follow-up period of 21.4 months (±24.4 months), no patient with malignant histology developed tumor recurrence and none was readmitted.
Discussion
Parenchymal sparing surgery and anatomic resections in posterosuperior liver segments require to follow curvilinear resection planes. Resection of tumors located in these segments using conventional laparoscopic techniques may be troublesome, requires extensive training, and probably can be safely and reliably performed only by a minority of surgeons with innate abilities for laparoscopy [9]. As already reported by Casciola et al., the use of endowristed robotic instruments is particularly useful when dealing with this type of liver resections [9]. Despite the limited range of available instruments making the crush-clamp technique the only enforceable method for liver resection under robotic assistance, we were impressed by efficacy and precision of robotic liver transection. In particular it is worth noting that biliary and vascular pedicles are identified quite easily during parenchymal transection and are rarely cut across inadvertently. We do not have an explanation to justify the efficacy of robotic crush-clamp technique but we assume that the ability to freely decide the angulation of the crushing instrument, coupled with the need to proceed with very small bites, could play a role. Further, when major bleeding occurs, the possibility of toggling between three robotic arms offers some practical advantages. For instance, the fourth robotic arm, holding a peanut gauze, can be used to press on the bleeding vessel to achieve temporary hemostasis while the other two arms are used to throw transfixing sutures. In other instances, the fourth robotic arm can be used to hold a suture placed on one extremity of a divided vessel, thus achieving bleeding control on one side, while hemostasis is completed using the other 2 robotic arms on the other side.
Our experience confirms that laparoscopic robot-assisted resection of tumors located in the posterosuperior liver segments is feasible. The limited number of patients included in this study, however, does not allow us to draw firm conclusions. It is indeed likely that we are still along our learning process, so that the technique described herein will evolve and almost certainly will be refined. Further developments of robotic technology are also expected to occur, and could extend the indications of robotic assistance beyond current limits. When trying to foresee future development of robotic assistance in hepato-biliary surgery, one should consider that the dVSS was initially introduced for heart surgery [15], and subsequently become very popular in urology and gynecology. As a consequence it is not surprising that most of the instruments that are currently available were developed in these surgical specialities. For instance, while there are several robotic instruments that can be used only for heart valve repair [16], none was specifically conceived for liver resection. In particular, current robotic equipment for liver resection lacks of a dedicated device for parenchymal transection. Instruments developed for conventional laparoscopy can still be used by the surgeon at the table, but this approach largely reduces the potential advantages of robotic assistance. Further, the range of motion of the surgeon at the table is often limited, because the bulk of robotic arms may cause collisions with laparoscopic instruments and certainly shrinks the working space. When Intuitive Surgical, or one of its potential competitors, will decide to invest in general surgery we will see the real potential of robotic assistance also in other surgical fields, including liver resection. Until that moment, we should realize that we are operating on the liver with a set of instruments mostly developed to operate on the urinary tract and on the female genital apparatus.
Some of our operative decisions may deserve a specific comment. We elected not to use a Pringle maneuver. In a multi-institutional study comparing laparoscopic and robot-assisted major hepatectomies, we already reported that Pringle maneuver could be omitted when using robotic assistance [17]. While we agree that preparing for a Pringle maneuver could be prudent, and that inflow occlusion may be necessary in some patients, we do not feel that it should be routine when using robotic assistance. Further, especially when the patient is on the flank position, being ready for a Pringle maneuver may not be straightforward, particularly in obese patients.
We prefer to place suction drains close to the resection bed. This decision is based on anticipated difficulty to reach these posterior areas in case of symptomatic fluid collections which could require percutaneous catheter drainage. Actually, we have not come across bile leaks, so that drains could have been probably avoided in the patients presented in this study. However, the limited number of patients shown here does not allow us to make final conclusions in this regard, and change our practice.
Based on available information, it was not possible to address the issue of costs in our study. In a previous study, we have shown that operative costs of robotic pancreaticoduodenectomy exceeded those of conventional surgery by more than €6000 [18], but quite surprisingly, when a similar analysis was performed for distal pancreatectomy the Indiana group reported that reduction in hospital costs produced a positive economic balance, despite confirmed higher operative costs [19]. Reliable economic evaluation should be carried out prospectively because it requires a very sophisticated analysis, including a contemporary comparator operation and more specific consideration of outcomes, such as clinical effectiveness or survival expressed as quality-adjusted life-years.
In conclusion, we have confirmed the feasibility and the safety of laparoscopic robot-assisted resection of tumors located in the posterosuperior liver segments. Further experience is needed before final conclusions can be drawn and meaningful comparison with other surgical techniques becomes possible.
References
Gagner M, Rheault M, Dubuc J (1992) Laparoscopic partial hepatectomy for liver tumor. Surg Endosc 6:99 (abstract)
Hibi T, Cherqui D, Geller DA, Itano O, Kitagawa Y, Wakabayashi G (2014) International survey on technical aspects of laparoscopic liver resection: a web-based study on the global diffusion of laparoscopic liver surgery prior to the 2nd international consensus conference on laparoscopic liver resection in Iwate, Japan. J Hepatobiliary Pancreat Sci 21:737–744
Edwin B, Mala T, Gladhaug I, Fosse E, Mathisen Y, Bergan A, Søreide O (2001) Liver tumors and minimally invasive surgery: a feasibility study. J Laparoendosc Adv Surg Tech A 11:133–139
Koffron AJ, Auffenberg G, Kung R, Abecassis M (2007) Evaluation of 300 minimally invasive liver resections at a single institution: less is more. Ann Surg 246:385–392
Nguyen KT, Gamblin TC, Geller DA (2009) World review of laparoscopic liver resection—2804 patients. Ann Surg 250:831–841
Buell JF, Cherqui D, Geller DA, O’Rourke N, Iannitti D, Dagher I, Koffron AJ, Thomas M, Gayet B, Han HS, Wakabayashi G, Belli G, Kaneko H, Ker CG, Scatton O, Laurent A, Abdalla EK, Chaudhury P, Dutson E, Gamblin C, D’Angelica M, Nagorney D, Testa G, Labow D, Manas D, Poon RT, Nelson H, Martin R, Clary B, Pinson WC, Martinie J, Vauthey JN, Goldstein R, Roayaie S, Barlet D, Espat J, Abecassis M, Rees M, Fong Y, McMasters KM, Broelsch C, Busuttil R, Belghiti J, Strasberg S, Chari RS, World Consensus Conference on Laparoscopic Surgery (2009) The international position on laparoscopic liver surgery. The Louisville statement. Ann Surg 250:825–830
Coles SR, Besselink MG, Serin KR, Alsaati H, Di Gioia P, Samim M, Pearce NW, Abu Hilal M (2015). Total laparoscopic management of lesions involving liver segment 7. Surg Endosc. doi:10.1007/S00464-014-4052-2
Nomi T, Fuks D, Agrawal A, Kawaguchi Y, Ogiso S, Gayet B (2015) Totally laparoscopic right hepatectomy combined with resection of the inferior vena cava by anterior approach. Ann Surg Oncol 22:851
Casciola L, Patriti A, Ceccarelli G, Bartoli A, Ceribelli C, Spaziani A (2011) Robot-assisted parenchymal-sparing liver surgery including lesions located in the posterosuperior segments. Surg Endosc 25:3815–3824
Wakabayashi G, Cherqui D, Geller DA, Buell JF, Kaneko H, Han HS, Asbun H, O’Rourke N, Tanabe M, Koffron AJ, Tsung A, Soubrane O, Machado MA, Gayet B, Troisi RI, Pessaux P, Van Dam RM, Scatton O, Abu Hilal M, Belli G, Kwon CH, Edwin B, Choi GH, Aldrighetti LA, Cai X, Cleary S, Chen KH, Schön MR, Sugioka A, Tang CN, Herman P, Pekolj J, Chen XP, Dagher I, Jarnagin W, Yamamoto M, Strong R, Jagannath P, Lo CM, Clavien PA, Kokudo N, Barkun J, Strasberg SM (2015) Recommendations for laparoscopic liver resection. A report from the second international consensus conference held in Morioka. Ann Surg 261:619–629
Ban D, Tanabe M, Ito H, Otsuka Y, Nitta H, Abe Y, Hasegawa Y, Katagiri T, Takagi C, Itano O, Kaneko H, Wakabayashi G (2014) A novel difficulty scoring system for laparoscopic liver resection. J Hepatobiliary Pancreat Sci 21:745–753
Boggi U, Caniglia F, Amorese G (2014) Laparoscopic robot-assisted major hepatectomy. J Hepatobiliary Pancreat Sci 21:3–10
Terminology Committee of the IHPBA (2000) Terminology of liver anatomy and re-sections. HPB 2:333–339
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240:205–213
Autschbach R, Onnasch JF, Falk V, Walther T, Krüger M, Schilling LO, Mohr FW (2000) The Leipzig experience with robotic valve surgery. J Card Surg 15:82–87
Algarni KD, Suri RM, Daly RC (2014). Robotic-assisted mitral valve repair: surgical technique. Multimed Man Cardiothorac Surg. doi:10.1093/mmcts/mmu022
Spampinato MG, Coratti A, Bianco L, Caniglia F, Laurenzi A, Puleo F, Ettorre GM, Boggi U (2014) Perioperative outcomes of laparoscopic and robot-assisted major hepatectomies: an Italian multi-institutional comparative study. Surg Endosc 28:2973–2979
Boggi U, Signori S, De Lio N, Perrone VG, Vistoli F, Belluomini M, Cappelli C, Amorese G, Mosca F (2013) Feasibility of robotic pancreaticoduodenectomy. Br J Surg 100:917–925
Waters JA, Canal DF, Wiebke EA, Dumas RP, Beane JD, Aguilar-Saavedra JR, Ball CG, House MG, Zyromski NJ, Nakeeb A, Pitt HA, Lillemoe KD, Schmidt CM (2010) Robotic distal pancreatectomy: cost effective? Surgery 148:814–823
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Standard
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Research involving human participants or animals
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Patients were extensively counseled about their disease, the operation that was planned, and the use of robotic assistance. All patients signed an informed consent.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Boggi, U., Caniglia, F., Vistoli, F. et al. Laparoscopic robot-assisted resection of tumors located in posterosuperior liver segments. Updates Surg 67, 177–183 (2015). https://doi.org/10.1007/s13304-015-0304-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13304-015-0304-5