Abstract
The Rab GTPase family protein Rab14 has been implicated in cancer development. However, its clinical significance in ovarian cancers and its biological effects have not been examined. The present study aims to examine the clinical significance, biological roles, and molecular mechanism of Rab14 in ovarian cancer progression. We examined expression pattern of Rab14 in 122 cases of ovarian cancer specimens using immunohistochemistry and found Rab14 overexpression correlated with FIGO stage (p = 0.0041). We depleted Rab14 in SKOV3 cells using siRNA and overexpressed Rab14 in SW626 cells. Knockdown of Rab14 inhibited cell growth and invasion while its overexpression facilitated cell growth and invasion. In addition, Rab14 overexpression increased paclitaxel resistance in SW626 cells while its depletion reduced drug resistance. Then, we investigated the role of Rab14 in the regulation of WNT/β-catenin signaling, demonstrating Rab14 overexpression regulated GSK3β phosphorylation and nuclear β-catenin accumulation. Rab14 depletion inhibited while its overexpression enhanced TCF transcriptional activity with corresponding change of Wnt target genes including MMP7 and c-myc. Wnt inhibitor abolished the effect of Rab14 on cell proliferation and Wnt target genes. In conclusion, the present study demonstrated that Rab14 promotes aggressiveness of ovarian cancer cell through, at least partly, Wnt signaling pathway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ovarian cancer is one of the leading causes of gynecological cancer-related mortality in women [1, 2]. Despite development of conventional therapies including surgery and chemotherapy, the prognosis of advanced ovarian cancer remains poor. The molecular mechanism of ovarian carcinogenesis remains poorly defined, which limits the development of efficient treatment. Thus, it is important to define oncoprotein conferring the malignant potential to ovarian cancer, which could be used as markers in predicting risk of ovarian cancer aggressiveness [3–7].
Rab14 belongs to RAS oncogene superfamily of small G-proteins which regulate membrane vesicle trafficking, signal transduction, and recycling of various membrane receptors [8]. Rab family proteins play various roles during development and progression of human cancers [9–15]. Rab5a and Rab25 has been reported to be overexpressed in human ovarian cancers and correlated with malignancy [16–19]. In these Rab family proteins, Rab14 has been seldom investigated and its biological role remains unclear. In one report, Rab14 was found to be overexpressed in non-small cell lung cancer and its depletion inhibited AKT phosphorylation and Bcl-2 expression [20]. The expression pattern and biological function of Rab14 in human ovarian cancer has not been explored.
In the present study, we showed that Rab14 was upregulated in ovarian cancer tissues and its overexpression promoted cell proliferation, invasion, and chemoresistance. We also demonstrated that Rab14 induced GSK3β phosphorylation, promoting β-catenin nuclear translocation, and Wnt activation, which in turn induced c-myc and MMP7. Our study provided evidences supporting tumor-promoting function of Rab14 in ovarian cancers.
Materials and methods
Clinical specimens
The protocol of present study was approved by the reviewer board of China Medical University. Clinical tissues were collected from 122 patients who were diagnosed with ovarian cancer in the Shengjing Hospital of China Medical University since 2012 to 2014. The histological diagnosis and tumor grade were evaluated by pathologist according to the WHO classification guidelines. Twelve fresh ovarian cancer tissues and corresponding normal tissues were stored at −70 °C after resection for extraction of RNA.
Immunohistochemistry
Tumor specimens were fixed with 10 % neutral formalin, and 4 μm thick paraffin sections were made. Immunostaining was performed using the S-P staining kit from Maixin (Ultrasensitive™, MaiXin, Fuzhou, China). After antigen retrieval in citrate buffer (pH 6.0) for 2 min in an autoclave. 0.3 % hydrogen peroxide was used for 15 min and then the sections were incubated with goat serum. Then, sections were incubated with Rab14 rabbit polyclonal antibody at 4 °C overnight (1:200 dilution, Proteintech, USA). Primary antibody incubation was performed at room temperature for 2 h. Biotinylated goat anti-rabbit serum IgG was incubated after washing in PBS. Then HRP conjugated streptavidin–biotin was incubated with sections. DAB kit (MaiXin, Fuzhou, China) was used for staining. Counterstaining with hematoxylin was performed and the sections were dehydrated in ethanol before mounting.
Two pathologists (Y. W. and Y. L.) examined all slides randomly. Five views were examined per slide. Staining of Rab14 was scored by evaluating the intensity and percentage of cells showing positive staining. Cytoplasmic staining was considered as positive immunostaining. The intensity of Rab14 cytoplasmic staining was also scored as 0 (negative), 1 (weak), and 2 (strong). Percentage scores were assigned as 1:1–25 %, 2: 26–50 % 3: 51–75 %, and 4: 76–100 %. The scores were multiplied to give a final score of 0 to 8 and the total expression of Rab14 was determined as either low expression: score <4 or high expression: score ≥4.
Cell culture and transfection
SW626, SKOV3, OVCAR3, and SW626 cell lines were purchased from ATCC (Manassas, VA, USA), which were cultured in 1640 medium (Invitrogen, USA) with 10 % FBS (Invitrogen). Cells were passaged every 2 days with trypsin.
For siRNA knockdown, DharmaFECT1 was employed (Dharmacon, CO, USA). The ONTARGETplus siRNA for Rab14 was also obtained from Dharmacon (Dharmacon, CO, USA). Non targeting siRNAa were used as negative control.
pCMV6-Rab14 plasmid was obtained from Origene company (Origene, Rockville, USA). Attractene transfection was used for transfection of plasmid (Qiagen, Hilden, Germany). pCMV6 empty vector was used as control. Wnt inhibitor FH535 was purchased from Santa Cruz.
Western blot analysis
Total protein was extracted using cell lysis buffer (Pierce, Rockford, IL). Protein quantification was performed using the Bradford method. Forty-microgram protein was added to SDS-PAGE, which was transferred to PVDF membranes (Millipore, MA, USA). Incubation of primary antibodies was performed overnight at 4 °C. Antibody dilution was listed as follows: Rab14(1:1000 Proteintech, USA), beta-catenin, p-GSK3beta, c-myc, MMP7 (1:800; Cell signaling, Boston, MA, USA), and antibody against GAPDH (1:2000; Santa Cruz, CA, USA). After incubation with HRP-coupled anti-mouse/rabbit IgG antibody (1:1500, Santa Cruz, CA, USA) at room temperature for 2 h. Proteins on membrane were visualized using ECL and images were captured using DNR BioImaging System (DNR, Jerusalem, Israel).
Quantitative real-time PCR
Quantitative real-time PCR was performed using SYBR Green master mix kit from Applied Biosystems. PCR was performed using 7500 Real-Time PCR System (Applied Biosystems) β-actin was used as the endogenous control. The relative levels of gene expression were represented as ΔCt = Ct gene- Ct reference, and the fold change of gene expression was calculated by the 2-ΔΔCt Method. The primer sequences are as follows:
Rab14 forward, 5′-CATGGCAACTGCACCATACAAC-3′, Rab14 reverse, 5′-GCAAGATTTTCCTACTCCCATGTC-3′; cyclin E forward, 5′-AGCCAGCCTTGGGACAATAAT-3′, cyclin E reverse, 5′-GAGCCTCTGGATGGTGCAAT-3′; p21 forward, 5′-CCTCATCCCGTGTTCTCCTTT-3′, p21 reverse, 5′-GTACCACCCAGCGGACAAGT-3′; c-myc forward, 5′-ACGGTGGTGGAGGAGCTCTT-3′, c-myc reverse, 5′-CGGTTGACGCTCTCCACAC-3′; MMP7 forward, 5′-CGGAGGAGATGCTCACTTCG-3′, MMP7 reverse, 5′-GGATCAGAGGAATGTCCCATACC-3′; β-actin forward, 5′ ATAGCACAGCCTGGATAGCAACGTAC 3′, β-actin reverse, 5′ CACCTTCTACAATGAGCTGCGTGTG 3′.
CCK-8 cell viability assay
Cell proliferation and viability was analyzed using Cell Counting Kit-8 (CCK-8) (Dojindo, Japan) according to the protocol. Forty-eight hours after transfection, cells were seeded in 96-well plates and incubated with CCK-8 solution for another 4 h. Then, plates were measured in a reader at the wavelength of 450 nm.
Wnt reporter assay
Tcf/LEF TOP-Flash reporter plasmid (0.2–0.3 μg/well) and Renilla luciferase reporter plasmid were transfected into ovarian cancer cells using Attractene reagent (Qiagen, Germany). Dual luciferase reporter kit (Promega) was used to detect luciferase activity.
Matrigel invasion assay
Invasion assay was performed using 24-well Transwell chamber with 20 ul Matrigel from BD bioscience (1:5 dilution, BD Bioscience, USA). Briefly, cells were suspended in 100 μl of serum free medium and added to the upper chambers. Six hundred microliters of medium with 15 % serum was added to the lower chamber. After 16–20 h, the cells on the upper chamber were wiped out with cotton tips. Cells that invaded through the filter to the low chamber were stained with hematoxylin. The invading cell number was counted under the microscope.
Statistical analysis
SPSS software 16 was used statistical analyses. χ2 test was used to examine the clinicopathologic data. T test was used to compare densitometry data. p < 0.05 was considered to indicate statistical significance.
Results
Expression of Rab14 in normal and ovarian cancer tissues
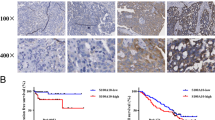
We investigated Rab14 protein expression in a panel of 122 ovarian cancer specimens by immunohistochemistry. In normal ovarian tissues, we observed negative Rab14 expression (Fig. 1a). In ovarian cancer tissues, Rab14 protein was significantly increased, which mainly localized in the cytoplasma of cancer cells (Fig. 1b–h). We stratified cancer specimens into two groups: high Rab14 expression (score 4 to 8) and low Rab14 expression (score 0 to 4). Of the 122 patients in the study, 70 cases (57.37 %) showed high Rab14 expression. We analyzed the correlation between Rab14 and the histological staging of ovarian cancer. The rates of Rab14 overexpression in serous carcinoma, mucinous carcinoma endometrioid, and clear cell carcinoma were 54.43, 58.82, 63.64, and 75 %, respectively (Table 1). The rate of Rab14 overexpression was higher in stage III–IV cancers than in stage I–II cancers (p = 0.0041). High-grade cancers (Grade 3, 64.81 %) were slightly more likely to be Rab14 overexpression than low-grade cancers (Grade 1 + 2, 51.47 %, p = 0.3264).
Expression pattern of Rab14 in ovarian cancer tissues. a Negative Rab14 staining in normal ovary epithelium specimen. b Positive Rab14 staining in clear cell carcinoma. c Positive cytoplasmic staining of Rab14 in serous carcinoma. d Negative staining of Rab14 in serous carcinoma. e Positive cytoplasmic staining of Rab14 in mucinous carcinoma. f Negative Rab14 staining in mucinous carcinoma. g Positive Rab14 expression in endometrioid carcinoma. h Negative Rab14 expression in endometrioid carcinoma. (Magnification: 400×)
We also confirmed Rab14 upregulation in 12 paired ovarian cancer tissues using real-time PCR. As shown in Fig. 2a, significant Rab14 mRNA upregulation was observed in cancer tissues compared with adjacent normal tissues, especially in case 2, 6, 9, 10, and 11.
Expression of Rab14 in ovarian cancer cell lines. a Real-timePCR showed that Rab14 mRNA expression was upregulated in ovarian cancer tissues. The average Rab14 level was high in tumor tissues than normal tissues. b Protein and mRNA expression of Rab14 in ovarian cancer cell lines. SKOV3 cell line has relatively high expression and SW626 cell line has relatively low expression. c Western blot and real-time PCR showed that siRNA treatment markedly decreases Rab14 levels in SKOV3 cells and Rab14 transfection significantly increased its expression in SW626 cells. *p < 0.05
Rab14 regulates ovarian cancer cell proliferation
We examined Rab14 protein expression in four ovarian cancer cell lines (SW626, SKOV3, OVCAR3, and SW626) by western blot and real-time PCR. High Rab14 expression was found in SKOV3 cell line and low Rab14 level was found in SW626 cell line (Fig. 2b). To investigate biological function of Rab14, specific siRNA was transfected in SKOV3 cell line and Rab14 plasmid was introduced into SW626 cell line. Knockdown and transfection efficiency was confirmed by western blot and real-time RT-PCR (Fig. 3c).
Rab14 knockdown inhibits and Rab14 overexpression promotes cell proliferation. Cell cycle progression and related proteins. a CCK-8 assay showed that Rab14 knockdown inhibited proliferation in SKOV3 cell line. Rab14 overexpression promoted proliferation in SW626 cell line. b Rab14 knockdown increased G1 phase percentage and decreased S phase percentage in SKOV3 cells. Rab14 overexpression increased S phase percentage and decreased G1 phase cells percentage in SW626 cells. c Western blot and real-time PCR revealed that knockdown of Rab14 decreased the mRNA/protein expression of cyclin E and increased mRNA/protein expression of p21. Rab14 overexpression in SW626 cells showed the opposite effects. *p < 0.05
The role of Rab14 on cancer proliferation rate was checked using CCK-8 assay and colony formation assay. As shown in Fig. 3a, Rab14 depletion decreased cell growth rate in SKOV3 cells (absorbance at day 5; Control siRNA vs Rab14 siRNA: 1.658 ± 0.043 vs 1.257 ± 0.055, p < 0.05). Rab14 overexpression promoted cell proliferation rate in SW626 cells (absorbance at day 5; pCMV6 vs Rab14 plasmid: 1.016 ± 0.0182 vs 1.326 ± 0.0159, p < 0.05). In addition, we checked cell cycle related proteins after treatment with siRNA or plasmid. As shown in Fig. 3c, we found that Rab14 overexpression in SW626 cells upregulated cyclin E and downregulated p21 expression at both mRNA and protein levels. While in Rab14 depleted SKOV3 cells, the expression of cyclin E was decreased and p21 was increased. These results indicated that Rab14 could induce cell proliferation by regulating cell cycle proteins in ovarian cancer cells.
Rab14 induces paclitaxel resistance
To characterize the impact of Rab14 on paclitaxel resistance of ovarian cancer cells, CCK-8 and Annexin V/PI staining were used in SKOV3 and SW626 cells with paclitaxel treatment (50 nM). As shown in Fig. 4a, Rab14 depletion significantly downregulated cell viability in SKOV3 cells at 24 and 48 h of paclitaxel treatment. While Rab14 overexpression in SW626 cells increased paclitaxel resistance and upregulated cell viability. These results demonstrated that Rab14 could confer paclitaxel resistance in ovarian cancer cells.
Rab14 increases paclitaxel resistance and invasion. a Depletion of Rab14 expression decreases sensitivity to chemotherapeutic drug paclitaxel after 3 days treatment. Rab14 overexpression increased SW626 cell survival rate. The absorbance rate of cancer cells treated with paclitaxel was measured by CCK-8 assay. Cell survival rate was calculated by comparing absorbance of treated groups to that of untreated group. b Rab14 depletion downregulated SKOV3 invading cell number. Rab14 overexpression upregulated invading ability of SW626 cell line. *p < 0.05
Rab14 facilitates ovarian cancer invasion
We checked change of invading ability in Rab14 overexpressed and depleted ovarian cancer cells. As shown in Fig. 4b, Rab14 siRNA decreased invading ability of SKOV3 cells (control vs Rab14 siRNA: 322 ± 18 vs 167 ± 19, p < 0.05) while Rab14 overexpression increased SW626 invasion (pCMV vs Rab14 plasmid: 198 ± 15 vs 285 ± 18, p < 0.05).
Rab14 regulates malignant behavior through Wnt signaling pathway
To check the potential mechanisms of Rab14 induced cell proliferation, invasion, and chemoresistance, we examined several signaling pathways and related proteins. Western blot and PCR analysis showed that Rab14 depletion downregulated protein and mRNA expression of MMP7 and c-myc, while Rab14 overexpression upregulated their protein and mRNA levels (Fig. 5a). Since MMP7 and c-myc are Wnt target genes, we checked if Rab14 could regulate Wnt activity using luciferase reporter assay. As shown in Fig. 5b, Rab14 siRNA downregulated TOP-Flash activity while Rab14 plasmid upregulated TOP-Flash activity. In addition, western blot showed that protein expression of nuclear β-catenin was upregulated after Rab14 overexpression and downregulated after Rab14 depletion (Fig. 5c), indicating Rab14 might regulate ovarian cancer aggressiveness through Wnt signaling.
Rab14 regulates Wnt signaling and target genes. a. Western blot and real-time PCR showed that knockdown of Rab14 decreased the mRNA and protein levels of c-myc and MMP7. Rab14 overexpression showed the opposite effects b TOP-FLASH luciferase reporter assay showed that Rab14 depletion downregulated TCF/LEF activity in SKOV3 cell line. Rab14 overexpression increased TCF/LEF luciferase activity in SW626 cell line. c Western blot showed that knockdown of Rab14 decreased protein levels of nuclear β-catenin and p-GSK3β expression. Rab14 overexpression upregulated nuclear β-catenin and p-GSK3β. *p < 0.05
To validate the involvement of Wnt signaling in Rab14 regulated cell proliferation, we employed Wnt inhibitor FH535 in Rab14 transfected SW626 cells and Rab14 depleted SKOV3 cells. As shown in Fig. 6a, Wnt inhibitor blocked the Rab14 induced MMP7 and c-myc in SW626 cell line. Wnt inhibitor treatment also abolished the effect of Rab14 siRNA. In addition, FH535 treatment also blocked the effect of Rab14 overexpression/depletion on cell proliferation rate (Fig. 6b), suggesting Rab14 exerts its biological effects through Wnt signaling.
Rab14 regulates proliferation through Wnt signaling and GSK3β interaction. a Western blot analysis revealed that Wnt inhibitor FH535 blocked the role of Rab14 overexpression/depletion on MMP7 and c-myc upregulation in both SKOV3 and SW626 cells. b CCK-8 showed that Wnt inhibitor FH535 blocked the effect of Rab14 on ovarian cancer cell proliferation. *p < 0.05
We further checked the change of GSK3β activity, which functions as a regulator of β-catenin degradation. We found that Rab14 upregulated phosphorylation of GSK3β while Rab14 depletion downregulated GSK3β phosphorylation (Fig. 5c).
Discussion
Rab14 has been reported to be involved in intracellular vesicle trafficking. Rab14 also regulates signal transduction and recycling of various membrane receptors [8]. Recently, Rab14 has been indicated in human cancer progression. Rab14 was found to be overexpressed in non-small cell lung cancer and its depletion inhibited AKT phosphorylation and Bcl-2 expression [20], suggesting Rab14 as a potential cancer-related protein. However, its expression pattern, biological function and molecular mechanism in ovarian cancer were not clear. In the present study, we found Rab14 expression was upregulated in ovarian cancer tissues using real-time PCR and immunohistochemical staining. Statistical analysis showed that there was a close correlation between high Rab14 expression and FIGO stage. Expression rate of Rab14 was higher in high stage tumors than in low-stage tumors, indicating a potential role of Rab14 during ovarian cancer progression. Ovarian cancer is a heterogeneous disease. We observed no significant association between Rab14 and histological type, suggesting its presence does not reflect the morphological heterogeneity.
CCK-8 assay showed that Rab14 depletion significantly suppressed proliferation while Rab14 overexpression promoted proliferation. Cell cycle analysis showed that in SKOV3 cell line, Rab14 depletion inhibited cell cycle transition at G1/S point and Rab14 transfection in SW626 cells facilitated cell cycle. We also examined change of proliferation related proteins and found Rab14 could induce cyclin E and inhibit p21 at protein and mRNA levels. Cyclin E and p21 participate in G1-S transition. Cyclin E correlates with malignant growth of ovarian cancer cells and predicts poor survival [21–24]. p21 is a cell cycle inhibitor which inhibits ovarian cancer growth [25, 26]. These results indicate that Rab14 regulates ovarian cancer growth through cell cycle related proteins. In addition, we found that Rab14 overexpression could induce paclitaxel resistance in SW626 cells and its depletion inhibited drug resistance, suggesting Rab14 may serve as a target for chemotherapy improvement. Cancer invasion is an important step during metastasis, which is the most common cause of cancer-related mortality. We found that Rab14 promoted ovarian cancer invasion with upregulation of MMP7. MMP7 is a marker of ovarian cancer remission, migration and invasion, which could be regulated by multiple signaling pathways [27, 28]. The above results demonstrated that Rab14 promotes ovarian cancer progression by regulating proliferation, invasion, and chemoresistance. However, the molecular mechanism underlying its biological effects remains unclear.
Wnt activation has been demonstrated in human ovarian cancers and patient prognosis [29–31]. In this study, we found that Rab14 activated Wnt signaling and induced Wnt target genes c-myc and MMP7. Rab14 induced nuclear localization of β-catenin and activation of Tcf/Lef transcriptional activity. Blockage of Wnt signal using FH535 abolished the effects of Rab14 siRNA/overexpression on cell proliferation and Wnt target proteins MMP7/c-myc. Thus, the proliferation and invasion promoting effects of Rab14 may be dependent on its role on Wnt activation. Furthermore, it is reported that Wnt activation drives drug resistance of ovarian cancer [32]. Thus, the effect of Rab14 on chemoresistance may also dependent on Wnt activation.
GSK3β is a protein which plays a central role during β-catenin degradation and Wnt trans-activation. Phosphorylation of GSK3β serine-9 inhibits its degradation of β-catenin and induces its nuclear localization [33–35]. Our results showed that after Rab14 overexpression, the level of GSK3β phosphorylation was upregulated with β-catenin nuclear localization. The mechanism of Rab14 on GSK3β was not clear. Considering its role on intracellular vesicle trafficking, we assume that Rab14 may regulate recycling of certain Wnt ligand/ receptor, which in turn activate GSK3β and Wnt signaling. Taken together, these demonstrated that Rab14 facilitates ovarian cancer progression through GSK3β/Wnt signaling pathway.
In conclusion, we showed that Rab14 was overexpressed in ovarian cancer tissues and correlated with advanced FIGO stage. Rab14 promotes ovarian cancer growth, invasion, and chemoresistance by regulation of cyclin E, p21, c-myc, and MMP7. Rab14 activates GSK3β/Wnt signaling. Our study implicated that Rab14 may serve as a potential therapeutic target in ovarian cancers.
References
Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11–30.
Permuth-Wey J, Sellers TA. Epidemiology of ovarian cancer. Methods Mol Biol. 2009;472:413–37.
Wang YF, Lang HY, Yuan J, et al. Overexpression of keratin 17 is associated with poor prognosis in epithelial ovarian cancer. Tumour Biol. 2013;34(3):1685–9.
Darcy KM, Birrer MJ. Translational research in the Gynecologic Oncology Group: evaluation of ovarian cancer markers, profiles, and novel therapies. Gynecol Oncol. 2010;117(3):429–39.
Despierre E, Lambrechts D, Neven P, et al. The molecular genetic basis of ovarian cancer and its roadmap towards a better treatment. Gynecol Oncol. 2010;117(2):358–65.
Fang Y, Li Z, Wang X, et al. CIP2A is overexpressed in human ovarian cancer and regulates cell proliferation and apoptosis. Tumour Biol. 2012;33(6):2299–306.
Zhao Y, Chen S, Gou WF, et al. Aberrant Beclin 1 expression is closely linked to carcinogenesis, differentiation, progression, and prognosis of ovarian epithelial carcinoma. Tumour Biol. 2014;35(3):1955–64.
Takai Y, Sasaki T, Matozaki T. Small GTP-binding proteins. Physiol Rev. 2001;81(1):153–208.
Ming Z, Guo C, Jiang M, et al. Bioinformatics analysis of Rab GDP dissociation inhibitor beta and its expression in non-small cell lung cancer. Diagn Pathol. 2014;9:201.
Jin L, Huo Y, Zheng Z, et al. Down-regulation of Ras-related protein Rab 5C-dependent endocytosis and glycolysis in cisplatin-resistant ovarian cancer cell lines. Mol Cell Proteomics. 2014;13(11):3138–51.
Ye F, Tang H, Liu Q, et al. miR-200b as a prognostic factor in breast cancer targets multiple members of RAB family. J Transl Med. 2014;12:17.
Ho JR, Chapeaublanc E, Kirkwood L, et al. Deregulation of Rab and Rab effector genes in bladder cancer. PLoS One. 2012;7(6):e39469.
Subramani D, Alahari SK. Integrin-mediated function of Rab GTPases in cancer progression. Mol Cancer. 2010;9:312.
Chia WJ, Tang BL. Emerging roles for Rab family GTPases in human cancer. Biochim Biophys Acta. 2009;1795(2):110–6.
Cheng KW, Lahad JP, Gray JW, et al. Emerging role of RAB GTPases in cancer and human disease. Cancer Res. 2005;65(7):2516–9.
Zhao Z, Liu XF, Wu HC, et al. Rab5a overexpression promoting ovarian cancer cell proliferation may be associated with APPL1-related epidermal growth factor signaling pathway. Cancer Sci. 2010;101(6):1454–62.
Fan Y, Wang L, Han X, et al. Rab25 is responsible for phosphoinositide 3-kinase/AKTmediated cisplatin resistance in human epithelial ovarian cancer cells. Mol Med Rep. 2015;11(3):2173–8.
Liu Y, Tao X, Jia L, et al. Knockdown of RAB25 promotes autophagy and inhibits cell growth in ovarian cancer cells. Mol Med Rep. 2012;6(5):1006–12.
Fan Y, Xin XY, Chen BL, et al. Knockdown of RAB25 expression by RNAi inhibits growth of human epithelial ovarian cancer cells in vitro and in vivo. Pathology. 2006;38(6):561–7.
Wang R, Wang ZX, Yang JS, et al. MicroRNA-451 functions as a tumor suppressor in human non-small cell lung cancer by targeting ras-related protein 14 (RAB14. Oncogene. 2011;30(23):2644–58.
Psyrri A, Bamias A, Yu Z, et al. Subcellular localization and protein levels of cyclin-dependent kinase inhibitor p27 independently predict for survival in epithelial ovarian cancer. Clin Cancer Res. 2005;11(23):8384–90.
Bedrosian I, Lu KH, Verschraegen C, et al. Cyclin E deregulation alters the biologic properties of ovarian cancer cells. Oncogene. 2004;23(15):2648–57.
Dhar KK, Branigan K, Howells RE, et al. Prognostic significance of cyclin D1 gene (CCND1) polymorphism in epithelial ovarian cancer. Int J Gynecol Cancer. 1999;9(4):342–7.
Marone M, Scambia G, Giannitelli C, et al. Analysis of cyclin E and CDK2 in ovarian cancer: gene amplification and RNA overexpression. Int J Cancer. 1998;75(1):34–9.
Baekelandt M, Holm R, Trope CG, et al. Lack of independent prognostic significance of p21 and p27 expression in advanced ovarian cancer: an immunohistochemical study. Clin Cancer Res. 1999;5(10):2848–53.
Anttila MA, Kosma VM, Hongxiu J, et al. p21/WAF1 expression as related to p53, cell proliferation and prognosis in epithelial ovarian cancer. Br J Cancer. 1999;79(11–12):1870–8.
Al-Alem LF, McCord LA, Southard RC, et al. Activation of the PKC pathway stimulates ovarian cancer cell proliferation, migration, and expression of MMP7 and MMP10. Biol Reprod. 2013;89(3):73.
Schummer M, Drescher C, Forrest R, et al. Evaluation of ovarian cancer remission markers HE4, MMP7 and Mesothelin by comparison to the established marker CA125. Gynecol Oncol. 2012;125(1):65–9.
Bodnar L, Stanczak A, Cierniak S, et al. Wnt/beta-catenin pathway as a potential prognostic and predictive marker in patients with advanced ovarian cancer. J Ovarian Res. 2014;7:16.
Yoshioka S, King ML, Ran S, et al. WNT7A regulates tumor growth and progression in ovarian cancer through the WNT/beta-catenin pathway. Mol Cancer Res. 2012;10(3):469–82.
Badiglian Filho L, Oshima CT, De Oliveira Lima F, et al. Canonical and noncanonical Wnt pathway: a comparison among normal ovary, benign ovarian tumor and ovarian cancer. Oncol Rep. 2009;21(2):313–20.
Nagaraj AB, Joseph P, Kovalenko O, et al. Critical role of Wnt/beta-catenin signaling in driving epithelial ovarian cancer platinum resistance. Oncotarget. 2015;6(27):23720–34.
Benelli R, Monteghirfo S, Vene R, et al. The chemopreventive retinoid 4HPR impairs prostate cancer cell migration and invasion by interfering with FAK/AKT/GSK3beta pathway and beta-catenin stability. Mol Cancer. 2010;9:142.
Luo J. Glycogen synthase kinase 3beta (GSK3beta) in tumorigenesis and cancer chemotherapy. Cancer Lett. 2009;273(2):194–200.
Yook JI, Li XY, Ota I, et al. A Wnt-Axin2-GSK3beta cascade regulates Snail1 activity in breast cancer cells. Nat Cell Biol. 2006;8(12):1398–406.
Acknowledgment
This study was supported by the China NSFC project (No.81302272). We thank Dr. Y Wang and Dr. Y Liu for technical assistance of results evaluation
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Rights and permissions
About this article
Cite this article
Hou, R., Jiang, L., Yang, Z. et al. Rab14 is overexpressed in ovarian cancers and promotes ovarian cancer proliferation through Wnt pathway. Tumor Biol. 37, 16005–16013 (2016). https://doi.org/10.1007/s13277-016-5420-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-016-5420-4