Abstract
The aim of this study was to investigate the association between keratin 17 (K17) expression and the clinicopathological features of patients with epithelial ovarian cancer (EOC). K17 expression was detected by real-time quantitative RT-PCR in EOC and adjacent noncancerous tissues. In addition, K17 expression was analyzed by immunohistochemistry in 104 clinicopathologically characterized EOC cases. The expression levels of K17 mRNA and protein in EOC tissues were both significantly higher than those in noncancerous tissues. In addition, positive expression of K17 correlated with the clinical stage (p = 0.001). Furthermore, Kaplan–Meier survival analysis showed that a high expression level of K17 resulted in a significantly poor prognosis of EOC patients. Multivariate analysis revealed that EOC expression level was an independent prognostic parameter for the overall survival rate of EOC patients. Our data are the first to suggest that increased K17 expression in EOC is significantly associated with aggressive progression and poor prognosis. K17 may be an important molecular marker for predicting the carcinogenesis, progression, and prognosis of EOC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Epithelial ovarian cancer (EOC) represents one of the most common gynecologic malignancies worldwide. It has the highest mortality rate among malignant tumors in female reproductive system [1, 2]. Because of the lack of specific early symptoms or effective tumor biomarkers, most patients with EOC are diagnosed at the advanced stages, and the prognosis of these patients is still poor, even though there has been great improvement on traditional treatments, such as surgery, supplemented with radiotherapy and chemotherapy. The 5-year survival rate for EOC patients is only 30–40 % [3]. Similar with other human malignancies, tumorigenesis and tumor progression of EOC are caused by numerous reproductive, environmental, and genetic risk factors. Therefore, it is of great importance to discover and analyze the genetic changes and molecular events involving the initiation, progression, and metastasis of EOC.
Keratins are the typical intermediate filament proteins in epithelial cells. These proteins constitute an important component of the cytoskeleton and are involved in the fixation of the nucleus and the maintenance of individual epithelial cells, as well as epithelial tissue morphology, via cell-to-cell contacts [4]. Keratins display a characteristic expression pattern in epithelial cells in a manner dependent on cell type, stage of differentiation, and function. Epithelial neoplasms frequently retain specific expression of keratins associated with the organ cell type [5]. Thus, keratin profiling provides particularly useful information for detecting the primary site of carcinomas that have spread across several organs and in cases of metastases.
Keratin 17 (K17) was originally identified as a major keratin of basal cell skin carcinoma [6]. In normal human epithelia, K17 expression is distributed in various glands, respiratory epithelium, and urothelium, and K17 is regarded as a basal/myoepithelial cell keratin [7, 8]. In the last decade, studies have demonstrated that K17 is expressed in adenocarcinomas of the bile duct, and it is also regarded as a useful marker for distinguishing between pancreaticobiliary adenocarcinomas and the carcinomas arising from the digestive tract, lung, prostate, and gynecologic organs [9–11]. However, the expression patterns and involvement of K17 in EOC are still unclear. Therefore, the aim of this study was to investigate the clinical significance of K17 expression in EOC.
Materials and methods
Sample collecting
The study was approved by the Research Ethics Committee of Fourth Military Medical University, China. Informed consent was obtained from all of the patients. All specimens were handled and made anonymous according to the ethical and legal standards.
For immunohistochemistry analysis, EOC tissues were collected from 104 patients with EOC from the Department of Oncology, Fourth Military Medical University, from January 2000 to January 2006. Forty noncancerous tissues were obtained during surgery from women with EOC. All patients with only gynecology tumor were treated without preoperative radiotherapy, chemotherapy, or hormonal therapy. Surgical staging was established according to the International Federation of Gynecology and Obstetrics system. Debulking status was defined according to the size of the nodules left in the peritoneal cavity after surgery. The clinical features of 104 EOC patients were summarized in Table 1. In addition, 20 self-pairs of EOC specimens and adjacent noncancerous tissues were snap frozen in liquid nitrogen and stored at −80 °C following surgery for real-time quantitative RT-PCR.
RNA extraction and real-time quantitative RT-PCR
K17 gene expression in 20 paired tumor tissue samples and adjacent noncancerous tissues were confirmed by real-time quantitative RT-PCR. Total RNA was extracted according to the manufacturer’s instructions (TRIzol, Invitrogen, USA). RNA (2 μg) was reverse transcribed into cDNA (Promega, Madison, WI). Quantitative K17 mRNA levels were assessed using Mastercycler® ep realplex (Eppendorf, Hamburg, Germany) with an IQTM SYBR Green Supermix Kit (BIORAD, Berkeley, CA) according to the manufacturer’s protocol. GAPDH was used as an internal control. The qPCR primers were as follows: K17, sense 5′-TTGGAACCTTCCTTGGACTGCATGC-3′ and antisense 5′-GCCATGGCGGCCTTTGGAACTC-3′; GAPDH, sense 5′-TGA AGGTCGGAGTCAACGG-3′ and antisense 5′-CTGGAAGATGGTGATGGGATT-3′. The cycling conditions were as follows: 95 °C for 2 min, then 40 cycles of 95 °C for 15 s, 59 °C for 30 s, and 72 °C for 45 s, with a final extension at 72 °C for 5 min. Each reaction was performed in triplicate, and the mean K17 mRNA level for each tumor was compared with its matched noncancerous tissue. The expression level of K17 was expressed as 2−ΔΔCt, where ΔCt = Ct (K17) − Ct (GAPDH).
Immunohistochemistry analysis
K17 protein expression in 104 tumor tissue samples and 40 noncancerous tissues was confirmed by immunohistochemistry, which was performed on formalin-fixed, paraffin-embedded, 3-μm-thick tissue sections using the avidin–biotin–peroxidase complex method. The sections were deparaffinized and dehydrated using a graded series of ethanol solutions. Endogenous peroxidase activity was halted through incubation with 0.3 % hydrogen peroxidase and methanol for 20 min. Following a rinse in phosphate-buffered saline, the tissue sections were processed in a 0.01-M citrate buffer (pH 6.0) inside a heat-resistant plastic container. Sections were then irradiated in a domestic microwave oven for 20 min. After microwave irradiation, the slides were allowed to cool at room temperature. The primary antibody was a rabbit antibody specific to K17 (1:15, Atlas Antibodies, Sigma-Aldrich, USA). The sections were incubated with the primary antibody overnight at 4 °C followed by the secondary antibody. The results were visualized with diaminobenzidine. In each immunohistochemistry assay, the negative controls were stained without primary antibody.
Two independent observers experienced in immunohistochemistry evaluated the slides. Both readers were blinded to clinicopathologic data and patient outcomes. K17 expression was quantified using a visual grading system based on the extent of staining (percentage of positive tumor cells graded on a scale from 0 to 3: 0 = negative, 1 = 1–30 %, 2 = 31–60 %, and 3 >60 %) and the intensity of staining (graded on a scale of 0–3: 0 = none, 1 = weak staining, 2 = moderate staining, and 3 = strong staining). The combination of the extent (E) and intensity (I) of staining was obtained by the product of E × I called EI, and varied from 0 to 9 for each spot. The mean EI score was calculated for each EOC specimen. EI scores of 0–3 were considered low expression and EI scores >3 were considered high expression. In 95 % of the samples, the evaluations of the two observers were identical; the remaining slides were reevaluated, and consensus decisions were made.
Statistical analysis
All statistical analyses were performed using the SPSS 13.0 statistical software package. The χ 2 test and Fisher’s exact test were used to analyze the relationship between K17 expression and the clinicopathologic characteristics. Survival curves were plotted using the Kaplan–Meier method and compared between the cases with high and low K17 expression using the logrank test. Survival data were evaluated using the Cox proportional hazards model. Independent prognostic factors were determined by a multivariate analysis. p < 0.05 was considered statistically significant.
Results
K17 gene expression in EOC tissue and noncancerous tissue
K17 gene expression in EOC tissues and corresponding noncancerous tissues was analyzed using qRT-PCR. In EOC tissues, the gene expression levels of K17 were significantly higher (1.56 ± 0.47) than in noncancerous tissues (0.52 ± 0.23; p < 0.001; Fig. 1).
K17 protein expression in EOC tissue and noncancerous tissue
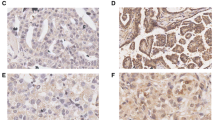
The expression of K17 protein in archived EOC tissue samples and noncancerous tissues was analyzed by immunohistochemistry. We observed that 53.8 % (56/104) of the EOC samples showed high K17 expression (Fig. 2). In comparison, the rate of high K17 protein expression was 20 % (8/40) in noncancerous epithelial cells. The protein expression level of K17 was markedly higher in EOC tissues than the level in noncancerous tissues (p < 0.001).
Association of K17 protein expression with the clinicopathological features of EOC
The association between K17 expression and the clinicopathological features of pancreatic cancer was further analyzed, as shown in Table 1. We did not find a significant association between K17 expression and age, pathological grade, histological type, and residual tumor after surgery in patients with EOC (p > 0.05). Interestingly, we observed that K17 expression was closely correlated with the clinical stage (p = 0.001) in patients with EOC.
Survival analysis
To investigate the prognostic value of K17 for EOC, we assessed the association between K17 expression and survival duration using a Kaplan–Meier analysis with a logrank test. The logrank test showed that the survival time of patients with EOC was significantly different between the groups with high K17 expression and low K17 expression (p < 0.001). In patients with EOC, the high K17 expression group had a shorter survival duration compared to the low K17 expression group (Fig. 3). To determine whether K17 expression is an independent prognostic factor for EOC, we performed a multivariate survival analysis of K17 protein expression and factors including age, clinical stage, pathological grade, histological type, and residual tumor after surgery in patients with EOC. The results showed that K17 protein expression was an independent prognostic factor for EOC (Table 2).
Discussion
As the first leading cause of cancer death in female reproductive system malignant tumors, EOC has no characteristic early symptoms or tumor markers, leading to disappointing clinical outcome. Overall survival rates remain poor despite improvements in response rates. The clinical course of remission and relapse is commonly seen in patients undergoing therapy for EOC. Discovery and analysis of the genetic changes and molecular events have contributed largely to a better understanding of the molecular mechanisms of cancer ontogenesis. Accordingly, it is of great significance to identify novel specific diagnostic or prognostic markers that contribute to progression and metastasis of EOC.
K17 is a 46–47-kDa type I keratin that plays important roles in fetal epidermal development, skin wound healing, and dermal allergic reactions [12, 13]. Overexpression of K17 is associated with specific pathological conditions including psoriasis and various neoplasms [13]. Several studies have reported K17 expression in squamous cell carcinoma arising from the oral cavity, esophagus, lung, and uterine cervix [14–17]. In a gene expression profile study of breast carcinomas, expression of K5, K14, and K17 was associated with a basal epithelial-like subgroup of breast cancer [18]. Several reports have also shown that basal cell keratin expression is associated with poor prognosis in invasive ductal breast carcinoma [19, 20].
In the present study, the overexpression of K17 gene and protein in EOC was verified by real-time quantitative RT-PCR and immunohistochemistry analysis, respectively. The expression levels of K17 mRNA and protein in EOC tissues were both significantly higher than those in noncancerous tissues. In addition, K17 expression was analyzed by immunohistochemistry in 104 clinicopathologically characterized EOC cases. High K17 expression correlated with the clinical stage (p = 0.001). Furthermore, Kaplan–Meier survival analysis showed that a high expression level of EOC resulted in a significantly poor prognosis of EOC patients. Multivariate analysis revealed that K17 expression level was an independent prognostic parameter for the overall survival rate of EOC patients.
In this study, although our results demonstrate the aberrant expression and important clinical significance of K17 in EOC patients, the exact mechanism of K17 upregulation in EOC is still not clearly understood. In this context, further studies are needed to determine the molecular mechanism of K17 dysfunction in human ovarian carcinogenesis.
In conclusion, our data suggest for the first time that K17 overexpression is associated with advanced tumor progression and poor clinical outcome of EOC patients. K17might be a novel prognostic marker of EOC.
References
Jordan SJ, Cushing-Haugen KL, Wicklund KG, Doherty JA, Rossing MA. Breast-feeding and risk of epithelial ovarian cancer. Cancer Causes Control. 2012;23:919–27. doi:10.1007/s10552-012-9963-4.
Rota M, Pasquali E, Scotti L, Pelucchi C, Tramacere I, Islami F, et al. Alcohol drinking and epithelial ovarian cancer risk. A systematic review and meta-analysis. Gynecol Oncol. 2012;125:758–63. doi:10.1016/j.ygyno.2012.03.031.
Wang M, He Y, Shi L, Shi C. Multivariate analysis by Cox proportional hazard model on prognosis of patient with epithelial ovarian cancer. Eur J Gynaecol Oncol. 2011;32:171–7.
Moll R, Divo M, Langbein L. The human keratins: biology and pathology. Histochem Cell Biol. 2008;129:705–33. doi:10.1007/s00418-008-0435-6.
Karantza V. Keratins in health and cancer: more than mere epithelial cell markers. Oncogene. 2011;30:127–38. doi:10.1038/onc.2010.456.
Moll R, Franke WW, Volc-Platzer B, Krepler R. Different keratin polypeptides in epidermis and other epithelia of human skin: a specific cytokeratin of molecular weight 46,000 in epithelia of the pilosebaceous tract and basal cell epitheliomas. J Cell Biol. 1982;95:285–95.
Troyanovsky SM, Guelstein VI, Tchipysheva TA, Krutovskikh VA, Bannikov GA. Patterns of expression of keratin 17 in human epithelia: dependency on cell position. J Cell Sci. 1989;93:419–26.
Troyanovsky SM, Leube RE, Franke WW. Characterization of the human gene encoding cytokeratin 17 and its expression pattern. Eur J Cell Biol. 1992;59:127–37.
Chu PG, Schwarz RE, Lau SK, Yen Y, Weiss LM. Immunohistochemical staining in the diagnosis of pancreatobiliary and ampulla of Vater adenocarcinoma: application of CDX2, CK17, MUC1, and MUC2. Am J Surg Pathol. 2005;29:359–67.
Goldstein NS, Bassi D. Cytokeratins 7, 17, and 20 reactivity in pancreatic and ampulla of Vater adenocarcinomas. Percentage of positivity and distribution is affected by the cut-point threshold. Am J Clin Pathol. 2001;115:695–702.
Ide M, Kato T, Ogata K, Mochiki E, Kuwano H, Oyama T. Keratin 17 expression correlates with tumor progression and poor prognosis in gastric adenocarcinoma. Ann Surg Oncol. 2012;19:3506–14. doi:10.1245/s10434-012-2437-9.
Moll R, Moll I, Wiest W. Changes in the pattern of cytokeratin polypeptides in epidermis and hair follicles during skin development in human fetuses. Differentiation. 1982;23:170–8.
Jiang CK, Flanagan S, Ohtsuki M, Shuai K, Freedberg IM, Blumenberg M. Disease-activated transcription factor: allergic reactions in human skin cause nuclear translocation of STAT-91 and induce synthesis of keratin K17. Mol Cell Biol. 1994;14:4759–69.
Wei KJ, Zhang L, Yang X, Zhong LP, Zhou XJ, Pan HY, et al. Overexpression of cytokeratin 17 protein in oral squamous cell carcinoma in vitro and in vivo. Oral Dis. 2009;15:111–7. doi:10.1111/j.1601-0825.2008.01501.x.
Takahashi H, Shikata N, Senzaki H, Shintaku M, Tsubura A. Immunohistochemical staining patterns of keratins in normal oesophageal epithelium and carcinoma of the oesophagus. Histopathology. 1995;26:45–50.
Wetzels RH, Schaafsma HE, Leigh IM, Lane EB, Troyanovsky SM, Wagenaar SS, et al. Laminin and type VII collagen distribution in different types of human lung carcinoma: correlation with expression of keratins 14, 16, 17 and 18. Histopathology. 1992;20:295–303.
Smedts F, Ramaekers F, Troyanovsky S, Pruszczynski M, Link M, Lane B, et al. Keratin expression in cervical cancer. Am J Pathol. 1992;141:497–511.
Sørlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98:10869–74. doi:10.1073/pnas.191367098.
Malzahn K, Mitze M, Thoenes M, Moll R. Biological and prognostic significance of stratified epithelial cytokeratins in infiltrating ductal breast carcinomas. Virchows Arch. 1998;433(2):119–29.
Thike AA, Iqbal J, Cheok PY, Chong AP, Tse GM, Tan B, et al. Triple negative breast cancer: outcome correlation with immunohistochemical detection of basal markers. Am J Surg Pathol. 2010;34:956–64. doi:10.1097/PAS.0b013e3181e02f45.
Acknowledgments
This work was supported by International Science and Technology Cooperation Program of China & Japan (no. 2010DFA31900), Major State Basic Research Development Program (2011CB503704), Program for Changjiang Scholars and Innovative Research Team in University, and National Natural Science Foundation of China (no. 60971055).
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Additional information
Ya-Feng Wang, Hai-Yang Lang, and Jing Yuan contributed equally to this paper.
Rights and permissions
About this article
Cite this article
Wang, YF., Lang, HY., Yuan, J. et al. Overexpression of keratin 17 is associated with poor prognosis in epithelial ovarian cancer. Tumor Biol. 34, 1685–1689 (2013). https://doi.org/10.1007/s13277-013-0703-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-013-0703-5