Abstract
Breast cancer (BCa) is the most common cancer and the second cause of death among women. Phosphoinositide 3-kinase (PI3K) signaling pathway has a crucial role in the cellular processes such as cell survival, growth, division, and motility. Moreover, oncogenic mutations in the PI3K pathway generally involve the activation phosphatidylinositol-4,5-bisphosphate 3-kinase-catalytic subunit alpha (PIK3CA) mutation which has been identified in numerous BCa subtypes. In this review, correlations between PIK3CA mutations and their clinicopathological parameters on BCa will be described. It is reported that PIK3CA mutations which have been localized mostly on exon 9 and 20 hot spots are detected 25–40 % in BCa. This relatively high frequency can offer an advantage for choosing the best treatment options for BCa. PIK3CA mutations may be used as biomarkers and have been major focus of drug development in cancer with the first clinical trials of PI3K pathway inhibitors currently in progress. Screening of PIK3CA gene mutations might be useful genetic tests for targeted therapeutics or diagnosis. Increasing data about PIK3CA mutations and its clinical correlations with BCa will help to introduce new clinical applications in the near future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer (BCa) is a complex disease often necessitating multimodal care. It is a disease which is developed by the accumulation of genetic and epigenetic alterations. These alterations are activated oncogenes and silenced tumor suppressors in mammalian ductal or lobular tissues [1]. Furthermore, the altered phosphatidylinositol-3-kinase (PI3K) signaling pathway is frequently involved in the regulation of cellular processes required for breast carcinogenesis. Interestingly, phosphatidylinositol-4,5-bisphosphate 3-kinase-catalytic subunit alpha (PIK3CA) mutations in exons 9 (helical domain) and 20 (kinase domain) lead to amino acid changes and result in increased PI3K activity. Increased PI3K activity can cause cell proliferation and progression in cancer cells [2]. PIK3CA mutation can be an emerging tumor marker and it might affect or alter BCa treatment modalities. Further studies about this gene would be helpful to design new treatment strategies.
Breast cancer
Among cancers affecting women, BCa is the most deadliest [3]. Recently, the mortality rate of BCa has declined in the USA and Europe, by means of improved detection and treatment [4]. Statistical data predict more than 231,840 women in the USA will be diagnosed with invasive BCa in 2015. Also, 1/12 women in the West has developed BCa at some point in their life [5]. Therefore, better tools are needed for diagnosis and monitoring. Recent strategies for early diagnosis of BCa are not sufficient despite the increased prognosis rate of the disease.
It has been known that the most important indicators for identifying patients with BCa are mammography, histopathology, and blood tests [6]. Mammography detects about 80–90 % of the BCa in women without symptoms. Another important diagnostic method is magnetic resonance imaging (MRI) which is not recommended as a routine screening tool for BCa due to false positive results. Positron emission tomography (PET) scans are more powerful in identifying aggressive tumors [7]. PET scans utilize radioactive tracers to detect BCa. These tracers better define cancer regions than an MRI or CT scan [www.nlm.nih.gov]. Clinical breast examination (changes or differences in breast shape, rashes, visible lumps or swelling, nipple discharge, etc.) can also help early diagnosis. Series of tumor markers as CA15-3, CA 27–29, and CEA are suggested by some clinicians. CA 15–3 and CA 27–29 are used routinely for monitoring. And they are elevated more than 70 % of metastatic BCa patients, whereas blood CEA level is only increased 55 % [8]. Detecting BRCA1 and BRCA2 gene mutations and family history are also significant for diagnosis. There are other candidate genes such as TP53, PIK3CA, or PTEN which can be used for screening.
The prognosis of BCa and response to the treatment are affected by series of factors such as histological grade, type and size of tumor, lymph node metastasis, estrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor 2 (Her-2/neu) status, and hormone levels. These factors allow researchers to classified BCa types as “clinical” (age, tumor, and node), “histopathological” (grade, ER, and HER2, ductal, lobular, invasive) and “molecular” (normal like, luminal, basal, HER2). This classification is vital for diagnosis due to the outcomes are various. Treatment approaches may be developed based on this classification. Thus, specific and predictive biomarkers can be chosen for suitable treatment for BCa [9].
Most women with BCa suffer from the surgery which is generally combined with the other treatments (radiation therapy, chemotherapy, hormone therapy, and targeted therapy) [7]. Particularly, breast conserving surgery or lumpectomy are performed to eliminate the cancer cells but not breast itself. Lumpectomy is always followed by about 5 to 7 weeks of radiation therapy. Radiation destroys cancer cells remaining in the breast, chest wall, or underarm area. Thus, the size of tumor can be reduced. Additionally, chemotherapeutic drugs such as cyclophosphamide, 5-fluorouracil, methotrexate doxorubicin, and paclitaxel postpone the further growth and spread of the tumor. Tamoxifen is used for to ER+ patients as hormone therapy [7]. Alternatively, targeted therapy is an option for BCa treatment. It targets specific genes, proteins, or the tissues which are involved in cancer growth. This type of treatment blocks the growth and spread of cancer cells while limits damages on healthy cells [10].
Typically, any mutation in DNA and induction of the cell proliferation are basic triggers to initiate molecular abnormalities in cancer [11, 12]. Especially, a mutation which occurs on the critical genes plays an important role for regulating cell growth, death, differentiation, and replication. Depending on the location of the gene, this mutation can be beneficial, harmful, or nonsense and it can effect the prognosis of cancer. However, development of a tumor is a multistep process, only a single mutation does not give a rise to cancer; most of the time, several mutations are required (e.g., colorectal cancer). Thus, transformed cells should be proliferating before they become fully malignant [13]. Proliferation of cancer cells exerts its tumor-forming effects by promoting the expansion of a cell population [14]. The genetic profile of BCa, 5–10 % of all cases in women, is linked to hereditary mutations in autosomal dominant genes.
There are many pathways responsible for BCa including hormone signaling pathway (ESR1, PGR, and AR), PI3K/AKT/MTOR pathway (AKT1, PIK3CA, and PTEN), receptor tyrosine kinase/growth factor signaling pathway (ERBB2/HER2), and cell cycle control/DNA damage pathway (CCND1, CDK4, CDK6, RB1, and TP53). Specifically, estrogen signaling and the estrogen receptor (ER) are important in BCa. A great number of the BCa depends on the increased estrogen secretion. Most BCa are constructed on the estrogen signaling for growth promotion. Some studies reported that ER signaling affords metastasis. This finding is important for therapeutic targets to block ER-driven metastasis [15]. The other important factors are EGFRs and their signals through the RAS → PI3K → PKB pathway [16]. When RAS is hyperactive, it may enhance BCa. RAS genes, which play an important role in human cancers, have been detected in BCa. Incidence of K-RAS oncogene activation is 0–10 %. As we implied previously, the PI3K pathway has critical roles for the regulation of cell survival and proliferation in BCa [16].
PI3K signaling pathway and PIK3CA gene mutations in different types of cancer
Phosphoinositide-3-kinase (PI3K) was studied and declared by Lewis Cantley and his colleagues [17] and later, its tumorigenic role was identified [18]. PI3K activation is critical for cell survival, proliferation, differentiation, and migration. PI3Ks are the family of lipid kinases that have been compromised in signal transduction through the tyrosine kinases and G-protein coupled receptors (EGF, IGF, FGF, or PDGF). These receptors are most frequently implicated in cancers [19, 20]. Also, signaling proteins that are phosphorylated signal through class IA PI3K, which includes p85 (α and β) regulatory subunits (Fig. 1). These isoforms are encoded by genes respectively PIK3R1, PIK3R2, and PIK3R3. p110 (α, β, and δ) is catalytic subunits. These isoforms are encoded by genes respectively PIK3CA, PIK3CB, and PIK3CD. PI3K is the most common mutation pathway and PI3K p110α (PIK3CA) and p110ß (PIKCB) catalytic subunit encode genes in BCa. The PIK3CA gene is situated on the long (q) arm of chromosome 3. IK3CA subunits are composed of several modular domains: a RAS binding domain, an NH2-terminal domain, the catalytic lipid kinase domain, and the helical domain interacting with the regulatory subunit. The PIK3CA gene provides instructions for making the p110 alpha (p110α) protein, which is one of the subunit of an enzyme called phosphatidylinositol 3-kinase (PI3K). The p110α protein is called as the catalytic subunit, it activates PI3K, while the helical subunit (produced by a different gene) regulates the enzyme activity. PIK3CA is mutated or amplified in a wide spectrum of tumors [21–23].
In the PI3K pathway, p85 interacts directly or via adaptor proteins with RTKs. This interaction activates PI3K. RTKs activate RAS which activates subsequently ERK, MAPK, AKT, and mTOR pathways. Thus, inhibitory effect of p85 onto p110 is cancelled on the cell membrane. Immediately, PI3K acts as a kinase. PI3K catalyzes the conversion of PIP2 to PIP3. PIP3 activates PDK and S6 (Fig. 1). AKT signaling results in increased cell growth, proliferation, and motility. Also, it has an anti-apoptotic role. PI3K is antagonized by PTEN. Because, PTEN catalyzes the alteration of PIP3 to PIP2 [24, 25] (Fig. 1).
Another way through the activation of PI3K: G-protein-coupled receptors (GPCRs) signal through class 1B PI3K, which includes p101 regulatory and p110γ catalytic subunits [26] (Fig. 1). Hyperactive p85 removes inhibitory effects on p110. When p110 is activated, it catalyzes the phosphorylation of PIP2 to PIP3. Then, it induces Akt sub-pathway. Subsequently, phosphorylation of Akt allows to reactivation of several target proteins in the cell. Moreover, class 1B PI3K includes seven transmembrane domain receptors (serpentine receptor), chemokine receptors, and signal through heterotrimeric G proteins, Gα and Gβγ. They are important to induce cell migration. Also, GPCR ligation dissociates the Gβγ dimer, allows its binding to p101 regulatory subunits and subsequent activation of associated p110γ catalytic subunits [27].
Until now, a lot of intracellular pathways were identified and PI3K pathway is the most important one BCa in which this pathway is activated [23, 28]. Samuels et al. [23] and Philp et al. [29] reported that in breast, ovarian, and colon cancers, phosphorylated AKT (pAKT) levels are increased whereas PI3K signals are reduced. Moreover, it is suggested that PIK3CA contains p11α mutations in approximately 15 % among all tumor types [21]. Interestingly, over the 80 % of the mutations have been detected in exon 9 (E545) of the phosphatidylinositol kinase homology domain, and in exon 20 (H1047) adjacent site of the edge of the catalytic domain (Fig. 2). Other important mutation regions are exon 1, 4, and 5. On the contrary of general mechanism which is known as through the growth factor activation, PIK3CA mutations in exon 9 and 20 promote constitute PI3K signaling through distinct mechanisms.
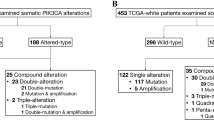
A lot of point mutations of PIK3CA gene are seen in hepatocellular, colon, brain, and BCa [17, 30–33]. As reported by the COSMIC database, distribution of PIK3CA somatic mutation in breast, endometrium, urinary tract, colon, and upper digestive tract are respectively 27, 23, 15, 17, and 7 % [34]. But, PIK3CA mutations have been observed less frequent in some cases such as head (<10 %), melanoma, prostate, pancreas, and lung cancer [35]. In a study belong to Garcia-Dios et al. [36], 172 PIK3CA mutations are detected among all tumors (16.2 %) in patients with primary endometrial carcinoma. Sun et al. [37] announced that 13 % PIK3CA amplification and 3 % PIK3CA mutation have been detected in 40 % of prostatic carcinomas. Liu et al. [38] proclaimed that PIK3CA mutations were appointed in 25 % (2 of 8) of ARMS exemplars. Tong et al. [39] have detected 22/120 mutations relevant to PIK3CA in BCa thereby using specific appliance known as high-throughput mass-spectrometry based cancer gene mutation profiling platform (Table 1).
Importance of PIK3CA gene mutations in breast cancer
A mutation occurred in PI3K pathway can be considered as an important target in BCa therapy. PI3K pathway plays a critical role in myriad of cellular actions that are necessary in both normal and cancer cell, for instance cell division, motility, growth, and survival [40]. In many cancer types, somatic mutations in PI3K pathway are recurrent. Oncogenic mutations in the PI3K pathway generally are evaluated in accordance to two different functions: activate mutation of the gene encoding PI3K (PIK3CA) or AKT (AKT1), and extinguish or reduce expression of PTEN. In BCa management, PI3K is a new way for clinicians. Samuels et al. [23] and Levine et al. [41] reported that the frequency of PIK3CA mutations in BCa are in range from 8 to 40 %. Other studies reported that activated mutations in PIK3CA were proximately 30 % of BCa and more frequent in ER (+) BCa [24, 42]. Especially, within exons 9 and 20, there would be found 80 % of PIK3CA mutations. These two exons are known as ‘hot spots’ and encode the helical and kinase domains. Helical domain is encoded sooner than kinase domain. As a result of E542K and E545K (exon 9) mutations reported by Miled et al. [43]. The inhibitory interaction between p110α and p85 may be blocked and this affect provides a gain-of-function. According to Gonzalez-Angulo et al. [44] and Stemke-Hale et al. [45], PIK3CA and AKT mutations are more frequent in HER2+ BCa. PI3K has been interacted with ER directly or indirectly. In either case ER phosphorylation is induced [46, 47]. According to Generali et al. [48], estrogen deprivation downregulates PI3K activity. PIK3CA is generally mutated in ER-positive BCa. Campbell et al. [49] at least 41.3 % of BCa is ER-positive. The researchers concluded that the presence of any PI3KCA mutation is an independent factor for survival, and effects are negatory. According to Maruyama et al. [50] PI3KCA mutation status was a significant and independent prognostic factor. These differences from the conventional prognostic factors are more predictive for a better prognosis [50].
PIK3CA mutation is detected in the range of 20–25 %, depending on the BCa subtype (HER2 tumors and triple-negative BCa (>30 %). But, it may be less frequent [42] in other subtypes. As well Kalinsky et al. [51] pointed in their study, in case of PIK3CA mutations have been detected, clinical outcomes and clinicopathologic founds are better, including survival benefits. In case of three subtypes of BCa were compared each other, gene-level mutation frequencies of PIK3CA were found significantly common in ER (+) (40 %) BCa [45–52]. But, its prognostic significance has not been explained yet. According to the Cancer Genome Atlas Network report [53], in TN BCa, even though PIK3CA mutation is seen at a low rate, the PI3K pathway activity inferred from gene expression or protein array signatures is actually the highest. Wright et al. [54] stated that Ras combined with PIK3CA (H1047R), an oncogenic mutant related to ERα (+)/luminal BCa in humans, induced metastatic luminal B-like tumors. Stachler et al. [55] presented that BCa had the highest rate of PIK3CA mutations (34 %), which correlated with estrogen receptor + status (P = 0.0002). Pogue-Geile et al. [56] reported that 741 (47.0 %) of 1578 tumors were classified as HER2-enriched (HER2E) subtype. Also, 166 (24.7 %) of 671 tumors had PIK3CA mutations. Lehmann et al. [57] displayed that activating PIK3CA mutations were enriched in AR + TNBC. Sakr et al. [58] indicated that molecular aberrations affecting the PI3K pathway may play a role in the progression from high-grade DCIS to IBC in a subset of cases (e.g., a subgroup of ER-positive/HER2-negative lesions). Arsenic et al. [59] show that the rate of PIK3CA mutations was increased in HR (+)/HER2 (−) tumors (18.6 %). In their study, the lowest rate of mutations was observed in HR (+)/HER2 (+) tumors (5.3 %). Christgen et al. [60] refer that PIK3CA mutations were positively selected for during ILBC progression to local recurrence but not distant metastasis, which might have clinical implications for PI3K inhibitor-based therapy.
In 2014, we determined an association between PIK3CA gene mutations and clinicopathologic differences in 101 Turkish BCa patients [61]. We studied PIK3CA gene mutation in exon 9 and exon 20 regions using high resolution melting (HRM) (Table 1). According to our results, PIK3CA exon 9 mutations, Q546R, E542Q, E545K, E542K, and 545D, were detected in 10 tumor samples. On the other hand, 21 mutations on exon 20 hotspot region were identified. Interestingly, one of the tumor samples had two different mutations on exon 20. The following mutations were defined using by Sanger sequencing: H1047L, H1047R, T1025T, and G1049R. Furthermore, we detected the mutation in one sample with both exon 9 (E542Q) and exon 20 (H1047R) mutations. Additionally, we should have emphasized that any correlations between PIK3CA, clinicopathological parameters (ER, HER2, stage and age, etc.) and survival analysis were detected in BCa.
Therapeutic and prognostic potential of pik3ca gene mutations in breast cancer
The biological markers are predictive and prognostic role in cancer. These biomarkers have to be confirmed by clinical and therapeutic applications. Therefore, in many molecular studies, a lot of inhibitors and antibodies have been developed for BCa treatment. These target molecules may be a gene or protein. Moreover, in some studies, these molecules have been detected considerably effective with in clinical settings (e.g., ALK or EGFR inhibitors for lung cancer).
In literature, there are lots of studies about PIK3CA-PI3K relation with cancer treatment. These studies indicate that alterations of PI3K signal pathway are important to treat cancer. There are different approaches for PI3K treatment such as trastuzumab, lapatinib, pertuzumab, anti-PI3K drugs, etc. There are lots of inhibitors for PI3K in biomedical industry. The most important kinase inhibitors for cancer therapies are perifosine (for colorectal cancer), idelalisib (for CML), PX-866 (for solid tumor), BAY 80–6946 (for PI3Kα and δ), INK1117 (for PI3Kα), and IPI-145 (for hematologic cancers) (Fig. 3 and Table 2). Other important kinase therapies use imanitib (Gleevec), trastzumab (Herceptin), and gefitinib (Iressa) [62–65], pertuzumab, lapatinib and endocrine therapies. On the other hand, there are important anti-PI3K pathway drugs. Imatinib was the first member of tyrosine kinase inhibitors [66]. Although targeted kinases like imatinib can destroy cancer cells but a resistance is developed frequently. Another study reported that p110α isoform-selective inhibitors look promising in heavily pretreated PIK3CA mutant BCa [67].
Combined therapy approaches which use PI3K-targeting pathway are also important treatment strategies. According to the findings of Roulin et al. [68], treatment of 786–0 and Caki-1 cells with NVP-BEZ235 or sorafenib resulted in diminished tumor cell proliferation and improved tumor cell apoptosis in vitro. The combination of NVP-BEZ235 and sorafenib was more effective than each compound alone. Hu et al. [69] indicate that when doxorubicin (DOX) was combined with BKM120, strong synergistic anti-proliferative effect was observed. BKM120 activity induced the blockage of PI3K/AKT signaling and NF-κB expression, which in turn led to activate caspase-3/7 and caspase-9 and changed the expression of several apoptosis-related gene expression. According to the Blackwell et al. [70] pilaralisib or voxtalisib, in combination with letrozole, was associated with an acceptable safety profile and limited efficacy in endocrine therapy-resistant HR+, HER2-negative metastatic BCa. Ayub et al. [71] used small-molecule kinase inhibitors, namely, NVP-AEW541, NVP-BKM120, KU0063794, and PD0325901 to target IGF-1R, PI3K, mTORC, and MEK, respectively. Mainly, combination treatments of PD0325901 with NVP-AEW541, NVP-BKM120 or KU0063794 and NVP-AEW541 with KU0063794 revealed a significant synergistic growth inhibition. Park et al. [72] showed that the combination of GSK2126458 and AZD6244 blocks both the RAS/RAF/MEK/ERK and PI3K/AKT/mTOR pathways simultaneously and is an effective strategy for the treatment of CRPCs. Using the mTOR inhibitors rapamycin, everolimus and PF-04691502 (a dual PI3K/mTOR inhibitor) and in combination with tamoxifen, significant reduction in mammosphere formation was observed [73]. Sweetlove et al. [74] expressed that ZSTK474 and BEZ235 also inhibited cell proliferation in all cell lines and enhanced the antitumor activity of selumetinib and vemurafenib in the majority of lines by either interacting synergistically or additively to increase potency or by inducing cytotoxicity by significantly increasing the magnitude of cell growth inhibition. Gedaly et al. [75] established that the combination of PKI-587 and sorafenib has the advantage over mono drug therapy on inhibition of HCC cell proliferation by blocking both PI3K/AKT/mTOR and Ras/Raf/MAPK signaling pathways.
PIK3CA driver mutations might be resistant to chemotherapeutic agents. Therefore, this review suggests the presence of different molecular features for breast tumors that should be considered when defining the therapeutic decisions. In the absence of PIK3CA mutations, we can estimate that clinical/preclinical efficacy for the treatment. In the present review, predictive or prognostic importance of PIK3CA for BCa will be explained and also briefly discussed.
In the researches exist so far, there is a resistance to hormonal therapy which caused by PI3K mutations. In a specific study, hyperactivation of some receptors (GFR or IGFR) induced the PI3K pathway. This hyperactivation also led to a discernible resistance to anti-estrogen therapy [76]. The mechanism of this resistance has been defined as a straight effect of estrogen receptor (ER) transcription. In some studies, PI3K mutations were shown as a downstream mediator which causes a resistance development to mTOR inhibitors. Deng et al. [77] revealed that the treatment with combination of PI3K/AKT/mTOR inhibitors and endocrine therapy has resulted with poorer outcome if there is a PIK3CA mutation in ER positive breast carcinomas. Correlatively, De La Rochefordiere et al. [78] proved that among the cervical cancer patients with mutation in PIK3CA pathway show poor response following standard radio chemotherapy +/− Cetuximab. In a trial of lapatinib monotherapy in HER2+ metastatic BCa, three PIK3CA-mutant patients were detected, and one durable PR and two stable disease responses were observed [79]. However, we have not found any specific study about resistance to antiestrogen therapy in Turkish women with BCa population.
Although it is known that PIK3CA driver mutations might be resistant to chemotherapeutic agents, the mechanism of this resistance is not clear yet. Black et al. [80] reported that oncogenic PIK3CA mutations are common in HER2/neu-amplified USC and may constitute a major mechanism of resistance to trastuzumab treatment. Boch et al. [81] suggested that the effects on the transcription were enhanced by the addition of estradiol and suppressed by the anti-ER therapies fulvestrant and tamoxifen. Moreover, fulvestrant markedly sensitized ER-positive tumors to PI3Kα inhibition, resulting in major tumor regressions in vivo. There was a correlation of the EGFR expression with sensitivity to EGFR and resistance to active agents of PI3K [82].
A much more important issue for the patients with BCa is whether a relationship exists between PIK3CA mutations and benefit with a specific type of therapy. Yet, PIK3CA mutations have been declared to confer resistance to endocrine therapy [83]. But, the data by Sabine et al. [84] showed opposite findings. Chemotherapy may reduce mutation frequency in patients with BCa. Recent studies explain that loss of PIK3CA mutations after neoadjuvant chemotherapy provides better prognosis for patients [85]. Yuan et al. [86] exhibited that phospho-PRAS40Thr246 expression was associated with activation of the PI3K pathway. Thus, these researchers have implied that there is an increased risk of tumor progression in patient with HER2+ metastatic BCa who have had trastuzumab therapy. Patients with PIK3CA/HER2+ BCa were treated with neoadjuvant therapy [87]. As a result of these neoadjuvant therapy trials, PIK3CA cannot be used as predictive biomarkers for adjuvant trastuzumab [88]. According to the Costa et al. [89] in HER2-amplified and PIK3CA mutant cancer, PIP3 rebound are prevented by the addition of the p110β inhibitor to BYL719. In this manner, a greater antitumor efficacy is induced. Pogue-Geile et al. [56] considered the benefit of adjuvant trastuzumab therapy in the NSABP (National Surgical Adjuvant Breast and Bowel Project) B-31 trial, but the results showed that there were no evidence for differences between PIK3CA wild type and mutant type. In another study, PIK3CA-metastatic HER2-positive BCa, Pilaralisib in combination with trastuzumab with or without paclitaxel did not present any differences [90]. In 55 BCa patient, a resistance to Herceptin was found associated with PIK3CA mutations or low PTEN expression. Similarly, in cell culture models, PIK3CA mutations would debate resistance to Herceptin [91]. Junttila et al. [92] recorded that the resistance to trastuzumab is mediated by PIK3CA mutation, through the E545K and H1047R-HER2 overexpressing BCa cell lines were sensitive to GDC-0941, a pan-PI3K inhibitor. According to Liu et al. [93], PIK3CA mutant tumors lead to a resistance to PI3K inhibitors via overexpression of c-MET and MYC. Sabine et al. [94] reported in the TEAM adjuvant endocrine study, in 39.8 % of PIK3CA mutant and ER-positive post-menopausal BCa patient’s response to treatment was better.
There are also lots of studies about PIK3CA treatment for other cancer types. In a study, regular aspirin use after diagnosis influences survival in PIK3CA mutated colorectal cancer in contrast to patients with wild-type PIK3CA [95]. Inhibition of PI3K pathway by using aspirin can reduce cell proliferation then promote cell death. Any alteration of this pathway, straightforward for cancer treatment. Accordingly, plenty of PI3K inhibitors have been developed. For example, rapamycin analogs: temsirolimus and everolimus that inhibit mTORC1 are suggested that these inhibitors also can be used for the treatment of advanced renal cell carcinoma [96, 97]. Use of pertuzumab and trastuzumab resulted in an improvement in progression-free survival in both the PIK3CA-mutant and wild-type groups [87].
Briefly, several studies indicate that PIK3CA may be predictive value. But, there have been some studies which not affirm PIK3CA mutations might be significant for prediction of BCa [98, 99].
Conclusion
Profiling the patients with cancer molecularly, determination of mutations in arbiter to design accurate drug combination and to settle drug sensitivity. According to the type of cancer, pathogenesis of PIK3CA mutations may be variable. It is known that these mutations lead to resistance to HER-2 targeting agents and/or hormonal agents in BCa. PIK3CA mutations are detected nearly 25–40 % in patient with BCa which is mostly caused by PIK3CA mutation. We also have detected 31 % PIK3CA mutation in Turkish BCa patients (n = 101) [61]. These results show us PIK3CA mutations could have a major role in the diagnosis and treatment of BCa.
In this point of view, certain gene alterations or modifications may be screened routinely for BCa diagnosis. In this way, detection of PIK3CA mutations might lead to a better decision of sequential therapy for many cancer types. Moreover, survey and life quality of the patients may be improved. If cancer related genes and their molecular mechanisms could be clarified, these would be innovative for new treatment strategies. In this perspective, clinically significant alterations in the PIK3CA gene which were identified previously could have an important impact to provide a “targeted treatment decision” in patient with BCa.
References
Wolfson B, Eades G, Zhou Q. Adipocyte activation of cancer stem cell signaling in breast cancer. World J Biol Chem. 2015;6(2):39–47.
Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7:606–19.
Jemal A, Siegel R, Xu J, Ward E. Cancer statistics. CA Cancer J Clin. 2010;60:277–300.
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90.
American Cancer Society. Cancer facts & figures 2015. Atlanta: American Cancer Society; 2015. Available online: http://www.cancer.org/acs/groups/content/@editorial/documents/document/acspc-044552.pdf. Accessed 23 Feb 2015.
Shi L. Racial differences in breast cancer patterns. Science. 2010;329:5987.
Sarkar S, Mahitosh Mandal. Breast cancer: classification based on molecular etiology influencing prognosis and prediction. Medicine » oncology » breast cancer - focusing tumor microenvironment, stem cells and metastasis (book). 978-953-307-766-6.
Kufe DW, Frei E, Hollander JF, Weichselbaum RR, Pollock RE, Bast RC, et al. Holland-Frei cancer medicine. 6th ed. Hamilton: B.C. Decker; 2003.
Bedard PL, Hansen AR, Ratain MJ, Siu LL. Tumour heterogeneity in the clinic. Nature. 2013;501:355–64.
Mayer EL. Understanding targeted therapy. Cancer.Net editorial board. 2015. http://www.cancer.net/navigating-cancer-care/how-cancer-treated/personalized-and-targeted-therapies/understandingtargeted-therapy. Accessed 21 Jan 2015.
Weinberg RA. Oncogenes and antioncogenes, and the molecular basis of multistep carcinogenesis. Cancer Res. 1989;49:3713–21.
Weinstein IB. The origins of human cancer: molecular mechanisms of carcinogenesis and their implications for cancer prevention and treatment twenty-seventh G.H.A. Clowes Memorial Award Lecture. Cancer Res. 1988;48:4135–43.
Bozzone D. Causes of cancer (Biology of cancer). 1st ed. Broomall: Chelsea House Publishers; 2006.
Cohen SM, Ellwein LB. Cell proliferation in carcinogenesis. Science. 1990;249(4972):1007–11.
Saha Roy S, Vadlamudi RK. Role of estrogen receptor signaling in breast cancer metastasis. Int J Breast Cancer. 2012;654–98.
Weber GF. Molecular mechanisms of cancer. Copyright: 2007.
Whitman M, Kaplan DR, Schaffhausen B, Cantley L, Roberts TM. Association of phosphatidylinositol kinase activity with polyoma middle-T competent for transformation. Nature. 1985;315(6016):239–42.
Sugimoto Y, Whitman M, Cantley LC, Erikson RL. Evidence that the Rous sarcoma virus transforming gene product phosphorylates phosphatidylinositol and diacylglycerol. Proc Natl Acad Sci U S A. 1984;81:2117–21.
Gadina M, Sudarshan C, Visconti R, Zhou YJ, Gu H, Neel BG, et al. The docking molecule gab2 is induced by lymphocyte activation and is involved in signaling by interleukin-2 and interleukin-15 but not other common gamma chainusing cytokines. J Biol Chem. 2000;275:26959–66.
Okkenhaug K. Signaling by the phosphoinositide 3-kinase family in immune cells. Annu Rev Immunol. 2013;31:675–704.
Karakas B, Bachman K, Park B. Mutation of the PIK3CA oncogene in human cancers. Br J Cancer. 2006;94:455–9.
Ligresti G, Militello L, Steelman L, Cavallaro A, Basile F, Nicoletti F, et al. PIK3CA mutations in human solid tumors. Cell Cycle. 2009;8:1352–8.
Samuels Y, Wang Z, Bardelli A, Siliman N, Ptak J, Szabo S, et al. High frequency of mutations of the PIK3CA gene in human cancers. Science. 2004;304:554.
Liu P, Cheng H, Roberts TM, Zhao JJ. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat Rev Drug Discov. 2009;8:627–44.
Engelman JA. Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nat Rev Cancer. 2009;9(8):550–62.
Vanhaesebroeck B, Guillermet-Guibert J, Graupera M, Bilanges B. The emerging mechanisms of isoform-specific PI3K signalling. Nat Rev Mol Cell Biol. 2010;11:329–41.
Andrews S, Stephens LR, Hawkins PT. PI3K class IB pathway in neutrophils. Sci STKE. 2007;cm3. doi:10.1126/stke.4072007cm3.
Bader AG, Kang S, Zhao L, Vogt PK. Oncogenic PI3K deregulates transcription and translation. Nat Rev Cancer. 2005;5:921–9.
Philp AJ, Campbell IG, Leet C, Vincan E, Rockman SP, Whitehead RH, et al. The phosphatidylinositol 3'‐kinase p85alpha gene is an oncogene in human ovarian and colon tumours. Cancer Res. 2001;61:7426–9.
Gallia GL, Rand V, Siu IM, Eberhart CG, James CD, Marie SK, et al. PIK3CA gene mutations in pediatric and adult glioblastoma multiforme. Mol Cancer Res. 2006;4:709–14.
Carson JD, Van Aller G, Lehr R, Sinnamon RH, Kirkpatrick RB, Auger KR, et al. Effects of oncogenic p110alpha subunit mutations on the lipid kinase activity of phosphoinositide 3-kinase. Biochem J. 2008;409:519–24.
Murugan AK, Hong NT, Fukui Y, Munirajan AK, Tsuchida N. Oncogenic mutations of the PIK3CA gene in head and neck squamous cell carcinomas. Int J Oncol. 2008;32:101–11.
Riener MO, Bawohl M, Clavien PA, Jochum W. Rare PIK3CA hotspot mutations in carcinomas of the biliary tract. Genes Chromosom Cancer. 2008;47:363–7.
COSMIC Database. 2015. http://www.sanger.ac.uk/cosmic. Accessed 28 July 2015.
Bowles DW, Jimeno A. New phosphatidylinositol 3-kinase inhibitors for cancer. Expert Opin Investig Drugs. 2011;20(4):507–18.
Garcia-Dios DA, Lambrechts D, Coenegrachts L, Vandenput I, Capoen A, Webb PM, et al. High-throughput interrogation of PIK3CA, PTEN, KRAS, FBXW7 and TP53 mutations in primary endometrial carcinoma. Gynecol Oncol. 2013;128(2):327–34.
Sun X, Huang J, Homma T, Kita D, Klocker H, Schafer G, et al. Genetic alterations in the PI3K pathway in prostate cancer. Anticancer Res. 2009;29(5):1739–43.
Liu CX, Li XY, Li CF, Chen YZ, Cui XB, Hu JM, et al. Compound HRAS/PIK3CA mutations in Chinese patients with alveolar rhabdomyosarcomas. Asian Pac J Cancer Prev. 2014;15(4):1771–4.
Tong L, Yang XX, Liu MF, Yao GY, Dong JY, Ye CS, et al. Mutational analysis of key EGFR pathway genes in Chinese breast cancer patients. Asian Pac J Cancer Prev. 2012;13(11):5599–603.
Klarenbeek S, van Miltenburg MH, Jonkers J. Genetically engineered mouse models of PI3K signaling in breast cancer. Mol Oncol. 2013;7(2):146–64.
Levine DA, Bogomolniy F, Yee CJ, Lash A, Barakat RR, Borgen PI, et al. Frequent mutation of the PIK3CA gene in ovarian and breast cancer. Clin Cancer Res. 2005;11:2875–8.
Saal LH, Holm K, Maurer M, Memeo L, Su T, Wang X, et al. PIK3CA mutations correlate with hormone receptors, node metastasis, and ERBB2, and are mutually exclusive with PTEN loss in human breast carcinoma. Cancer Res. 2005;65:2554–9.
Miled N, Yan Y, Hon WC, Perisic O, Zvelebil M, Inbar Y, et al. Mechanism of two classes of cancer mutations in the phosphoinositide 3-kinase catalytic subunit. Science. 2007;317:239–42.
Gonzalez-Angulo AM, Ferrer-Lozano J, Stemke-Hale K, Sahin A, Liu S, Barrera JA, et al. PI3K pathway mutations and PTEN levels in primary and metastatic breast cancer. Mol Cancer Ther. 2011;10(6):1093–101.
Stemke-Hale K, Gonzalez-Angulo AM, Lluch A, Neve RM, Kuo WL, Davies M, et al. An integrative genomic and proteomic analysis of PIK3CA, PTEN, and AKT mutations in breast cancer. Cancer Res. 2008;68(15):6084–91.
Guo RX, Wei LH, Wang JL, Sun PM, Sun XL. Activation of phosphatidylinositol 3-kinase-protein kinase B (PI3K-PKB) induced by 17beta-estradiol in endometrial carcinoma cell (Ishikawa). Zhonghua Fu Chan Ke Za Zhi. 2004;39(7):469–73.
Schuur ER, Loktev AV, Sharma M, Sun Z, Roth RA, Weigel RJ. Ligand-dependent interaction of estrogen receptoralpha with members of the forkhead transcription factor family. J Biol Chem. 2001;276(36):33554–60.
Generali D, Fox SB, Brizzi MP, Allevi G, Bonardi S, Aguggini S, et al. Down-regulation of phosphatidylinositol 3'-kinase/AKT/molecular target of rapamycin metabolic pathway by primary letrozole-based therapy in human breast cancer. Clin Cancer Res. 2008;14(9):2673–80.
Campbell M, Allen WE, Sawyer C, Vanhaesebroeck B, Trimble ER. Glucose-potentiated chemotaxis in human vascular smooth muscle is dependent on cross-talk between the PI3K and MAPK signaling pathways. Circ Res. 2004;95(4):380–8.
Maruyama N, Miyoshi Y, Taguchi T, Tamaki Y, Monden M, Noguchi S. Clinicopathologic analysis of breast cancer with PIK3CA mutations in Japanese women. Clin Cancer Res. 2007;13:408–14.
Kalinsky K, Jacks LM, Heguy A, et al. PIK3CA mutation associates with improved outcome in breast cancer. Clin Cancer Res. 2009;15:5049–59.
Perez-Tenorio G, Alkhori L, Olsson B, Waltersson MA, Nordenskjöld B, Rutqvist LE, et al. PIK3CA mutations and PTEN loss correlate with similar prognostic factors and are not mutually exclusive in breast cancer. Clin Cancer Res. 2007;13(12):3577–84.
Cancer Genom Atlas Network. http://cancergenome.nih.gov. Accessed 13 Sep 2012.
Wright KL, Adams JR, Liu JC, Loch AJ, Wong RG, Jo CE, et al. Ras signaling is a key determinant for metastatic dissemination and poor survival of luminal breast cancer patients. Cancer Res. 2015;75(22):4960–72.
Stachler MD, Rinehart EM, Garcia E, Lindeman NI. PIK3CA Mutations are common in many tumor types and are often associated with other driver mutations. Appl Immunohistochem Mol Morphol. 2015 Jun 5. doi:10.1097/PAI.0000000000000195.
Pogue-Geile KL, Song N, Jeong JH, Gavin PG, Kim SR, Blackmon NL, et al. Intrinsic subtypes, PIK3CA mutation, and the degree of benefit from adjuvant trastuzumab in the NSABP B-31 trial. J Clin Oncol. 2015;33(12):1340–7.
Lehmann BD, Bauer JA, Schafer JM, Pendleton CS, Tang L, Johnson KC, et al. PIK3CA mutations in androgen receptor-positive triple negative breast cancer confer sensitivity to the combination of PI3K and androgen receptor inhibitors. Breast Cancer Res. 2014;16(4):406.
Sakr RA, Weigelt B, Chandarlapaty S, Andrade VP, Guerini-Rocco E, Giri D, et al. King TA.PI3K pathway activation in high-grade ductal carcinoma in situ—implications for progression to invasive breast carcinoma. Clin Cancer Res. 2014;20(9):2326–37.
Arsenic R, Lehmann A, Budczies J, Koch I, Prinzler J, Kleine-Tebbe A, et al. Analysis of PIK3CA mutations in breast cancer subtypes. Appl Immunohistochem Mol Morphol. 2014;22(1):50–6.
Christgen M, Noskowicz M, Schipper E, Christgen H, Heil C, Krech T, et al. Oncogenic PIK3CA mutations in lobular breast cancer progression. Genes Chromosom Cancer. 2013;52(1):69–80.
Dirican E, Kaya Z, Gullu G, Peker I, Ozmen T, Gulluoglu BM, et al. Detection of PIK3CA gene mutations with HRM analysis and association with IGFBP-5 expression levels in breast cancer. Asian Pac J Cancer Prev. 2014;15(21):9327–33.
Hanusch C, Schneeweiss A, Loibl S, Untch M, Paepke S, Kümmel S, et al. Dual blockade with AFatinib and trastuzumab as NEoadjuvant treatment for patients with locally advanced or operable breast cancer receiving taxaneanthracycline containing chemotherapy-DAFNE (GBG-70). Clin Cancer Res. 2015;21(13):2924–31.
Druker BJ. Imatinib: a viewpoint by Brian J. Druker Drugs. 2001;61(12):1775–6.
Arteaga CL, Moulder SL, Yakes FM. HER (erbB) tyrosine kinase inhibitors in the treatment of breast cancer. Semin Oncol. 2002;3(11):4–10.
Wakeling AE. Epidermal growth factor receptor tyrosine kinase inhibitors. Curr Opin Pharmacol. 2002;2(4):382–7.
Motawi TM, Sadik NA, Fahim SA, Shouman SA. Combination of imatinib and clotrimazole enhances cell growth inhibition in T47D breast cancer cells. Chem Biol Interact. 2015;233:147–56.
Zardavas D, Phillips WA, Loi S. PIK3CA mutations in breast cancer: reconciling findings from preclinical and clinical data. Breast Cancer Res. 2014;16(1):201.
Roulin D, Waselle L, Dormond-Meuwly A, Dufour M, Demartines N, Dormond O. Targeting renal cell carcinoma with NVP-BEZ235, a dual PI3K/mTOR inhibitor, in combination with sorafenib. Mol Cancer. 2011;10:90. doi:10.1186/1476-4598-10-90.
Hu Y, Guo R, Wei J, Zhou Y, Ji W, Liu J, et al. Effects of PI3K inhibitor NVP-BKM120 on overcoming drug resistance and eliminating cancer stem cells in human breast cancer cells. Cell Death Dis. 2015;6, e2020. doi:10.1038/cddis.2015.363.
Blackwell K, Burris H, Gomez P, Lynn Henry N, Isakoff S, Campana F, et al. Phase I/II dose-escalation study of PI3K inhibitors pilaralisib or voxtalisib in combination with letrozole in patients with hormone receptor-positive and HER2-negative metastatic breast cancer refractory to a non-steroidal aromatase inhibitor. Breast Cancer Res Treat. 2015 Oct 24.
Ayub A, Yip WK, Seow HF. Dual treatments targeting IGF-1R, PI3K, mTORC or MEK synergize to inhibit cell growth, induce apoptosis, and arrest cell cycle at G1 phase in MDA-MB-231 cell line. Biomed Pharmacother. 2015;75:40–50.
Park H, Kim Y, Sul JW, Jeong IG, Yi HJ, Ahn JB, et al. Synergistic anticancer efficacy of MEK inhibition and dual PI3K/mTOR inhibition in castration-resistant prostate cancer. Prostate. 2015;75(15):1747–59.
Karthik GM, Ma R, Lövrot J, Kis LL, Lindh C, Blomquist L, et al. mTOR inhibitors counteract tamoxifen-induced activation of breast cancer stem cells. Cancer Lett. 2015;367(1):76–87.
Sweetlove M, Wrightson E, Kolekar S, Rewcastle GW, Baguley BC, Shepherd PR, et al. Inhibitors of pan-PI3K signaling synergize with BRAF or MEK inhibitors to prevent BRAF-mutant melanoma cell growth. Front Oncol. 2015;5:135.
Gedaly R, Angulo P, Hundley J, Daily MF, Chen C, Evers BM. PKI-587 and sorafenib targeting PI3K/AKT/mTOR and Ras/Raf/MAPK pathways synergistically inhibit HCC cell proliferation. J Surg Res. 2012;176(2):542–8.
Di Cosimo S, Baselga J. Management of breast cancer with targeted agents: importance of heterogeneity. Nat Rev Clin Oncol. 2010;7(3):139–47.
Deng L, Chen J, Zhong XR, Luo T, Wang YP, Huang HF, et al. Correlation between activation of PI3K/AKT/mTOR pathway and prognosis of breast cancer in Chinese women. PLoS One. 2015;10(3), e0120511.
de la Rochefordiere A, Kamal M, Floquet A, Thomas L, Petrow P, Petit T, et al. PIK3CA pathway mutations predictive of poor response following standard radiochemotherapy ± cetuximab in cervical cancer patients. Clin Cancer Res. 2015;21(11):2530–7.
Toi M, Iwata H, Fujiwara Y, Ito Y, Nakamura S, Tokuda Y, et al. Lapatinib monotherapy in patients with relapsed, advanced, or metastatic breast cancer: efficacy, safety, and biomarker results from Japanese patients phase II studies. Br J Cancer. 2009;101(10):1676–82.
Black JD, Lopez S, Cocco E, Bellone S, Altwerger G, Schwab CL, et al. PIK3CA oncogenic mutations represent a major mechanism of resistance to trastuzumab in HER2/neu overexpressing uterine serous carcinomas. Br J Cancer. 2015;113(7):1020–6.
Bosch A, Li Z, Bergamaschi A, Ellis H, Toska E, Prat A, et al. PI3K inhibition results in enhanced estrogen receptor function and dependence in hormone receptor-positive breast cancer. Sci Transl Med. 2015;7(283):283ra51.
Glaysher S, Bolton LM, Johnson P, Atkey N, Dyson M, Torrance C, et al. Targeting EGFR and PI3K pathways in ovarian cancer. Br J Cancer. 2013;109(7):1786–94.
Crowder RJ, Phommaly C, Tao Y, Hoog J, Luo J, Perou CM, et al. PIK3CA and PIK3CB inhibition produce synthetic lethality when combined with estrogen deprivation in estrogen receptor-positive breast cancer. Cancer Res. 2009;69:3955e62.
Sabine VS, Crozier C, Brookes CL, Drake C, Piper T, van de Velde CJ, et al. Mutational analysis of PI3K/AKT signaling pathway in tamoxifen exemestane adjuvant multinational pathology study. J Clin Oncol. 2014a;32(27):2951–8.
Jiang YZ, Yu KD, Bao J, Peng WT, Shao ZM. Favorable prognostic impact in loss of TP53 and PIK3CA mutations after neoadjuvant chemotherapy in breast cancer. Cancer Res. 2014;74:3399e407.
Yuan K, Wu H, Wang Y, Chen H, Jiao M, Fu R. Phospho-PRAS40Thr246 predicts trastuzumab response in patients with HER2-positive metastatic breast cancer. Oncol Lett. 2015;9(2):785–9.
Majewski IJ, Nuciforo P, Mittempergher L, et al. PIK3CA mutations are associated with decreased benefit to neoadjuvant human epidermal growth factor receptor 2-targeted therapies in breast cancer. J Clin Oncol. 2015;33(12):1334–9.
Liu S, Wang H, Zhang L, Tang C, Jones L, Ye H, et al. Rapid detection of genetic mutations in individual breast cancer patients by next-generation DNA sequencing. Hum Genomics. 2015;9(1):2.
Costa C, Ebi H, Martini M, Beausoleil SA, Faber AC, Jakubik CT, et al. Measurement of PIP3 levels reveals an unexpected role for p110β in early adaptive responses to p110α-specific inhibitors in luminal breast cancer. Cancer Cell. 2015;27(1):97–108.
Tolaney S, Burris H, Gartner E, Mayer IA, Saura C, Maurer M, et al. Phase I/II study of pilaralisib (SAR245408) in combination with trastuzumab or trastuzumab plus paclitaxel in trastuzumab-refractory HER2-positive metastatic breast cancer. Breast Cancer Res Treat. 2015;149(1):151–61.
Berns K, Horlings HM, Hennessy BT, Madiredjo M, Hijmans EM, Beelen K, et al. A functional genetic approach identifies the PI3K pathway as a major determinant of trastuzumab resistance in breast cancer. Cancer Cell. 2007;12:395–402.
Junttila TT, Akita RW, Parsons K, Fields C, Lewis Phillips GD, Friedman LS, et al. Ligand-independent HER2/HER3/PI3K complex is disrupted by trastuzumab and is effectively inhibited by the PI3K inhibitor GDC-0941. Cancer Cell. 2009;15:429–40.
Liu P, Cheng H, Santiago S, Raeder M, Zhang F, Isabella A, et al. Oncogenic PIK3CA-driven mammary tumors frequently recur via PI3K pathway-dependent and PI3K pathway-independent mechanisms. Nat Med. 2011;17:1116–20.
Sabine V, Crozier C, Drake C, Piper T, van de Velde CJ, Hasenburg A, et al. PIK3CA mutations are linked to PgR expression: a tamoxifen exemestane adjuvant multinational (TEAM) pathology study. Cancer Res. 2012;72:1–5.
Liao X, Lochhead P, Nishihara R, Morikawa T, Kuchiba A, Yamauchi M, et al. Aspirin use, tumor PIK3CA mutation, and colorectal-cancer survival. N Engl J Med. 2012;367(17):1596–606.
Courtney KD, Corcoran RB, Engelman JA. The PI3K pathway as drug target in human cancer. J Clin Oncol. 2010;28(6):1075–83.
German S, Aslam HM, Saleem S, Raees A, Anum T, Alvi AA, et al. Carcinogenesis of PIK3CA. Hered Cancer Clin Pract. 2013;11(1):5.
Souglakos J, Philips J, Wang R, Marwah S, Silver M, Tzardi M, et al. Prognostic and predictive value of common mutations for treatment response and survival in patients with metastatic colorectal cancer. Br J Cancer. 2009;101:465–72.
Mao C, Yang ZY, Hu XF, Chen Q, Tang JL. PIK3CA exon 20 mutations as a potential biomarker for resistance to anti-EGFR monoclonal antibodies in KRAS wild-type metastatic colorectal cancer: a systematic review and meta-analysis. Ann Oncol. 2012;23(6):1518–25.
Oshiro C, Kagara N, Naoi Y, Shimoda M, Shimomura A, Maruyama N, et al. PIK3CA mutations in serum DNA are predictive of recurrence in primary breast cancer patients. Breast Cancer Res Treat. 2015;150(2):299–307.
Sudhakar N, George Priya Doss C, Thirumal Kumar D, Chakraborty C, Anand K, Suresh M. Deciphering the impact of somatic mutations in exon 20 and exon 9 of PIK3CA gene in breast tumors among Indian women through molecular dynamics approach. J Biomol Struct Dyn. 2015;34(1):1–13.
Yamaguchi T, Mukai H, Yamashita S, Fujii S, Ushijima T. Comprehensive DNA methylation and extensive mutation analyses of HER2-positive breast cancer. Oncology. 2015;88(6):377–84.
Papaxoinis G, Kotoula V, Alexopoulou Z, Kalogeras KT, Zagouri F, Timotheadou E, et al. Significance of PIK3CA mutations in patients with early breast cancer treated with adjuvant chemotherapy: a hellenic cooperative oncology group (HeCOG) study. PLoS One. 2015;10(10), e0140293.
Lips EH, Michaut M, Hoogstraat M, Mulder L, Besselink NJ, Koudijs MJ, et al. Next generation sequencing of triple negative breast cancer to find predictors for chemotherapy response. Breast Cancer Res. 2015;17(1):134.
Adamczyk A, Niemiec J, Janecka A, Harazin-Lechowska A, Ambicka A, Grela-Wojewoda A, et al. Prognostic value of PIK3CA mutation status, PTEN and androgen receptor expression for metastasis-free survival in HER2-positive breast cancer patients treated with trastuzumab in adjuvant setting. Pol J Pathol. 2015;66(2):133–41.
Guarneri V, Dieci MV, Frassoldati A, Maiorana A, Ficarra G, Bettelli S, et al. Prospective biomarker analysis of the randomized CHER-LOB study evaluating the dual anti-HER2 treatment with trastuzumab and lapatinib plus chemotherapy as neoadjuvant therapy for HER2-positive breast cancer. Oncologist. 2015;20(9):1001–10.
Toro PV, Erlanger B, Beaver JA, Cochran RL, VanDenBerg DA, Yakim E, et al. Comparison of cell stabilizing blood collection tubes for circulating plasma tumor DNA. Clin Biochem. 2015;48(15):993–8.
Millis SZ, Gatalica Z, Winkler J, Vranic S, Kimbrough J, Reddy S, et al. Predictive biomarker profiling of >6000 breast cancer patients shows heterogeneity in TNBC, with treatment implications. Clin Breast Cancer. 2015;15(6):473–81.
Sueta A, Yamamoto Y, Yamamoto-Ibusuki M, Hayashi M, Takeshita T, Yamamoto S, et al. An integrative analysis of PIK3CA mutation, PTEN, and INPP4B expression in terms of trastuzumab efficacy in HER2-positive breast cancer. PLoS One. 2014;9(12), e116054.
Pestrin M, Salvianti F, Galardi F, De Luca F, Turner N, Malorni L, et al. Heterogeneity of PIK3CA mutational status at the single cell level in circulating tumor cells from metastatic breast cancer patients. Mol Oncol. 2015;9(4):749–57.
Deb S, Wong SQ, Li J, Do H, Weiss J, Byrne D, et al. Mutational profiling of familial male breast cancer reveals similarities with luminal A female breast cancer with rare TP53 mutations. Br J Cancer. 2014;111(12):2351–60.
Mendelová A, Jezková E, Zubor P, Holubeková V, Lasabová Z, Plank L, et al. Correlation between the incidence of PIK3CA mutations in breast cancer and histopathological characteristics of the tumor. Ceska Gynekol. 2014 Summer;79(4):283–8.
Loibl S, von Minckwitz G, Schneeweiss A, Paepke S, Lehmann A, Rezai M, et al. PIK3CA mutations are associated with lower rates of pathologic complete response to anti-human epidermal growth factor receptor 2 (her2) therapy in primary HER2-overexpressing breast cancer. J Clin Oncol. 2014;32(29):3212–20.
Arthur LM, Turnbull AK, Renshaw L, Keys J, Thomas JS, Wilson TR, et al. Changes in PIK3CA mutation status are not associated with recurrence, metastatic disease or progression in endocrine-treated breast cancer. Breast Cancer Res Treat. 2014;147(1):211–9.
Sabine VS, Crozier C, Brookes CL, Drake C, Piper T, van de Velde CJ, et al. Mutational analysis of PI3K/AKT signaling pathway in tamoxifen exemestane adjuvant multinational pathology study. J Clin Oncol. 2014b;32(27):2951–8.
Zhang Y, Liu M, Yang H, Wang J, Liu H, Li X, et al. PIK3CA mutations are a predictor of docetaxel plus epirubicin neoadjuvant chemotherapy clinical efficacy in breast cancer. Neoplasma. 2014;61(4):461–7.
López-Knowles E, Segal CV, Gao Q, Garcia-Murillas I, Turner NC, Smith I, et al. Relationship of PIK3CA mutation and pathway activity with antiproliferative response to aromatase inhibition. Breast Cancer Res. 2014;16(3):R68.
Bai X, Zhang E, Ye H, Nandakumar V, Wang Z, Chen L, et al. PIK3CA and TP53 gene mutations in human breast cancer tumors frequently detected by ion torrent DNA sequencing. PLoS One. 2014;9(6), e99306.
Castaneda CA, Lopez-Ilasaca M, Pinto JA, Chirinos-Arias M, Doimi F, Neciosup SP, et al. PIK3CA mutations in Peruvian patients with HER2-amplified and triple negative non-metastaticbreast cancer. Hematol Oncol Stem Cell Ther. 2014;7(4):142–8.
Hashimoto K, Tsuda H, Koizumi F, Shimizu C, Yonemori K, Ando M, et al. Activated PI3K/AKT and MAPK pathways are potential good prognostic markers in node-positive, triple-negative breast cancer. Ann Oncol. 2014;25(10):1973–9.
Wolters KL, Ang D, Warrick A, Beadling C, Corless CL, Troxell ML. Frequent PIK3CA mutations in radial scars. Diagn Mol Pathol. 2013;22(4):210–4.
Song J, Zhang J, Lv F, Cheng Y, Wang B, Feng L, et al. Multiplex detection of DNA mutations by the fluorescence fingerprint spectrum technique. Angew Chem Int Ed Engl. 2013;52(49):13020–3.
Welt A, Tewes M, Aktas B, Hoffmann O, Wiesweg M, Ting S, et al. Preemptive tumor profiling for biomarker-stratified early clinical drug development in metastatic breast cancer patients. Breast Cancer Res Treat. 2013;142(1):81–8.
Palimaru I, Brügmann A, Wium-Andersen MK, Nexo E, Sorensen BS. Expression of PIK3CA, PTEN mRNA and PIK3CA mutations in primary breast cancer: association with lymph node metastases. Springerplus. 2013;2:464.
Hohensee I, Lamszus K, Riethdorf S, Meyer-Staeckling S, Glatzel M, Matschke J, et al. Frequent genetic alterations in EGFR- and HER2-driven pathways in breast cancer brain metastases. Am J Pathol. 2013;183(1):83–95.
Kandula M, Chennaboina KK, Ys AR, Raju S. Phosphatidylinositol 3-kinase (PI3KCA) oncogene mutation analysis and gene expression profiling in primary breast cancer patients. Asian Pac J Cancer Prev. 2013;14(9):5067–72.
Karakas B, Colak D, Kaya N, Ghebeh H, Qasem A, Hendrayani F, et al. Prevalence of PIK3CA mutations and the SNP rs17849079 in Arab breast cancer patients. Cancer Biol Ther. 2013;14(10):888–96.
Deb S, Do H, Byrne D, Jene N, kConFab Investigators, Dobrovic A, et al. PIK3CA mutations are frequently observed in BRCAX but not BRCA2-associated male breast cancer. Breast Cancer Res. 2013;15(4):R69.
Schneck H, Blassl C, Meier-Stiegen F, Neves RP, Janni W, Fehm T, et al. Analysing the mutational status of PIK3CA in circulating tumor cells from metastatic breast cancer patients. Mol Oncol. 2013;7(5):976–86.
Loi S, Michiels S, Lambrechts D, Fumagalli D, Claes B, Kellokumpu-Lehtinen PL, et al. Somatic mutation profiling and associations with prognosis and trastuzumab benefit in early breast cancer. J Natl Cancer Inst. 2013;105(13):960–7.
Cizkova M, Dujaric ME, Lehmann-Che J, Scott V, Tembo O, Asselain B, et al. Outcome impact of PIK3CA mutations in HER2-positive breast cancer patients treated with trastuzumab. Br J Cancer. 2013;108(9):1807–9.
Ramirez-Ardila DE, Helmijr JC, Look MP, Lurkin I, Ruigrok-Ritstier K, van Laere S, et al. Hotspot mutations in PIK3CA associate with first-line treatment outcome for aromatase inhibitors but not for tamoxifen. Breast Cancer Res Treat. 2013;139(1):39–49.
Ang D, O'Gara R, Schilling A, Beadling C, Warrick A, Troxell ML, et al. Novel method for PIK3CA mutation analysis: locked nucleic acid--PCR sequencing. J Mol Diagn. 2013;15(3):312–8.
Flatley E, Ang D, Warrick A, Beadling C, Corless CL, Troxell ML. PIK3CA-AKT pathway mutations in micropapillary breast carcinoma. Hum Pathol. 2013;44(7):1320–7.
Harlé A, Lion M, Lozano N, Husson M, Harter V, Genin P, et al. Analysis of PIK3CA exon 9 and 20 mutations in breast cancer using PCR-HRM and PCRARMS: correlation with clinicopathological criteria. Oncol Rep. 2013;29(3):1043–52.
Nishimura R, Arima N, Toyoshima S, Ohi Y, Anan K, Sagara Y, et al. Evaluation of PTEN loss and PIK3CA mutations and their correlation with efficacy of trastuzumab treatment in HER2-positive metastatic breast cancer: a retrospective study (KBCSG 1001). Mol Clin Oncol. 2013;1(1):47–52.
Beltran H, Yelensky R, Frampton GM, Park K, Downing SR, MacDonald TY, et al. Targeted next-generation sequencing of advanced prostate cancer identifies potential therapeutic targets and disease heterogeneity. Eur Urol. 2013;63(5):920–6.
Mangone FR, Bobrovnitchaia IG, Salaorni S, Manuli E, Nagai MA. PIK3CA exon 20 mutations are associated with poor prognosis in breast cancer patients. Clinics (Sao Paulo). 2012;67(11):1285–90.
Chandarlapaty S, Sakr RA, Giri D, Patil S, Heguy A, Morrow M, et al. Frequent mutational activation of the PI3K-AKT pathway in trastuzumab-resistant breast cancer. Clin Cancer Res. 2012;18(24):6784–91.
Santarpia L, Qi Y, Stemke-Hale K, Wang B, Young EJ, Booser DJ, et al. Mutation profiling identifies numerous rare drug targets and distinct mutation patterns in different clinical subtypes of breast cancer. Breast Cancer Res Treat. 2012;134(1):333–43.
Higgins MJ, Jelovac D, Barnathan E, Blair B, Slater S, Powers P, et al. Detection of tumor PIK3CA status in metastatic breast cancer using peripheral blood. Clin Cancer Res. 2012;18(12):3462–9.
Cizkova M, Susini A, Vacher S, Cizeron-Clairac G, Andrieu C, Driouch K, et al. PIK3CA mutation impact on survival in breast cancer patients and in ERα, PR and ERBB2-based subgroups. Breast Cancer Res. 2012;14(1):R28.
Janku F, Wheler JJ, Westin SN, Moulder SL, Naing A, Tsimberidou AM, et al. PI3K/AKT/mTOR inhibitors in patients with breast and gynecologic malignancies harboring PIK3CA mutations. J Clin Oncol. 2012;30(8):777–82.
Jensen JD, Knoop A, Laenkholm AV, Grauslund M, Jensen MB, Santoni-Rugiu E, et al. PIK3CA mutations, PTEN, and pHER2 expression and impact on outcome in HER2-positive early-stage breast cancer patients treated with adjuvant chemotherapy and trastuzumab. Ann Oncol. 2012;23(8):2034–42.
Acknowledgments
The authors appreciate Dr. Pınar Uysal Onganer and Nihan Verimli for their critical revision of the manuscript. This work was supported by a grant (SBAG-111S161 to MA) from the Scientific and Technological Research Council of Turkey (TUBITAK).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None
Rights and permissions
About this article
Cite this article
Dirican, E., Akkiprik, M. & Özer, A. Mutation distributions and clinical correlations of PIK3CA gene mutations in breast cancer. Tumor Biol. 37, 7033–7045 (2016). https://doi.org/10.1007/s13277-016-4924-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-016-4924-2