Abstract
We attempted to develop a highly sensitive and specific method for the detection of circulating tumor DNA (ctDNA) using a digital PCR (dPCR) assay for PIK3CA mutations (i.e., H1047R, E545K, and E542K) in primary breast cancer patients. The sensitivity of the dPCR assay for the mutant alleles was examined using cell lines with PIK3CA mutations and proved to be 0.01 %. Serum samples were collected pre-operatively from 313 stage I–III breast cancer patients, of whom 110 were found to have PIK3CA mutant tumors. The serum samples from these patients with PIK3CA mutant tumors were subjected to the dPCR assay, and 25 (22.7 %) were found to be positive. No PIK3CA mutant ctDNA was detected in the serum samples of 50 healthy women and 30 breast cancer patients with PIK3CA non-mutant tumors. The patients with PIK3CA mutant ctDNA were dichotomized into mutant ctDNA-high (ctDNAhigh) and ctDNA-low (ctDNAlow) groups based on the median. The ctDNAhigh patients exhibited significantly shorter recurrence-free survival (RFS; P = 0.0002) and overall survival rates (OS; P = 0.0048) compared to those exhibited by the combined ctDNAlow patient and ctDNA-free patient group. Multivariate analysis revealed that ctDNAhigh status significantly predicted poor RFS and OS and did so independently of conventional histological parameters. These results suggest that dPCR is a highly sensitive and specific method for the detection of PIK3CA mutant ctDNA and that ctDNAhigh but not ctDNAlow status is a significant and independent prognostic factor for primary breast cancer patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cell-free DNA (cfDNA) is composed of the fragments of cellular nucleic acids that exist in the circulating blood. One type of cfDNA is composed of tumor-derived cfDNA that harbors tumor-specific alterations, including genetic or epigenetic changes, and are known as circulating tumor DNA (ctDNA) [1, 2]. ctDNA is thought to be released from cancer cells by necrosis, apoptosis, or secretion [3, 4]. In general, patients with cancer have higher levels of cfDNA than do healthy individuals, and the fractions of ctDNA within the cfDNA vary widely between 0.01 % and more than 90 % according to the tumor burden [5]. Due to fragmentation and a short half-life (1–2 h) [6], it has been difficult to detect ctDNA in early cancer patients, and most studies of ctDNA have been limited to metastatic or advanced cancer patients with greater amounts of ctDNA, and fewer studies have been performed in early cancer patients. However, the recent advances in the molecular-based technologies, including high sensitivity (<0.01 %) digital PCR (dPCR), have enabled the detection of rare ctDNA and thus its application to early cancer patients [1, 2, 7].
PIK3CA mutations are some of the most common genetic alterations in breast cancers and are detected in 20–40 % of these cancers [8, 9]. PIK3CA mutations occurs predominantly in exons 9 and 20, which are called “hot spots” and include H1047R, E545K, and E542K; these three mutations account for 70–80 % of PIK3CA mutations in breast cancers [8]. These hot spot mutations are known to modulate the activity of the PI3K signaling pathway, which regulates cell growth, motility, and other important cellular functions [10, 11]. Given that ctDNA carries tumor-derived DNA alterations, the detection of PIK3CA mutations in cfDNA with a highly sensitive assay is expected to be useful in the early detection of recurrence following surgery and in the monitoring of tumor response to systemic therapy in patients with metastatic disease and to replace conventional tumor markers such as CEA and CA15-3 [12, 13].
It has been reported that PIK3CA mutations are detectable in the cfDNA in approximately 80 % of metastatic breast cancer patients whose tumors harbor PIK3CA mutations and that PIK3CA mutations reflect responses to systemic therapies more accurately than CEA or CA15-3 [1, 5, 12]. Very recently, the successful detection of PIK3CA mutations in the cfDNA of early breast cancer patients has been reported [14], but the clinicopathological characteristics and prognostic significance of breast tumors with PIK3CA mutations in the cfDNA have not been documented [14, 15]. Therefore, in the present report, we investigated whether PIK3CA mutations could be detected in the cfDNA of early breast cancer patients using dPCR and the prognostic relevance of the presence of such mutations.
Materials and methods
Patients and sample collection
Three hundred and thirteen primary breast cancer patients (stage I–III) who had received no neoadjuvant systemic therapy and had undergone mastectomy or breast-conserving surgery followed by radiation therapy to the breast at Osaka University Hospital between June 2000 and November 2009 were retrospectively included in this study. Blood samples were collected from each patient prior to surgery and centrifuged at 3000 rpm for 10 min at 4 °C within 2 h after venipuncture, and then serum samples were stored at −80 °C until use. Samples were centrifuged again before DNA extraction to remove debris. The median follow-up period after surgery was 79 months (range 1–133). Twelve patients received no adjuvant systemic therapy, 128 received adjuvant hormonal therapy, 63 received adjuvant chemotherapy, and 110 received adjuvant chemo-hormonal therapy essentially according to the recommendations of the St. Gallen consensus conference [16–19]. The clinicopathological characteristics of these patients are shown in Table 1. The serum tumor markers CEA and CA15-3 were also measured before surgery and defined as positive when they exceeded 5.0 ng/ml and 30.0 U/ml, respectively. A tumor sample was obtained from each patient at surgery, snap frozen in liquid nitrogen and stored at −80 °C until use. Serum samples from 50 healthy women (14 premenopausal and 36 postmenopausal) were used as controls. Informed consent for participation in the study was obtained from each patient and healthy woman.
DNA extraction from tumor samples and real-time PCR
DNA was extracted from frozen tumor tissues with the DNeasy Blood & Tissue Kit® (QIAGEN, Germantown, MD, USA) according to the manufacturer’s instructions. TaqMan®-based real-time PCR analysis was conducted using a LightCycler® 480 Real-Time PCR System (Roche Applied Science, Mannheim, Germany) to detect the three “hot spot” PIK3CA mutations (H1047R, E545K and E542K). Custom TaqMan® primers and probes were designed for the three PIK3CA mutations as shown in Supplementary Table S2.
DNA extraction from the serum samples and digital PCR (dPCR)
DNA was extracted from 500 μl of serum using the QIAamp Circulating Nucleic Acid Kit® (QIAGEN, Hilden, Germany) according to the manufacturer’s instructions. The DNA was eluted into 50 μl of AVE buffer and stored at −20 °C until further processing. dPCR was performed using a QuantStudio™3D digital PCR system (Life Technologies, Carlsbad, CA) to detect the three PIK3CA mutations. The primers and probes shown in Supplementary Table S2 were used. For the dPCR, 9 μl of template DNA was mixed with 1 μl of 20× TaqMan® Assay primer/probe mix and 10 μl of 2× QuantStudio™ 3D Digital PCR Master Mix (Life Technologies) according to the manufacturer’s instructions. Fifteen-microliter aliquots of the PCR solutions were then loaded into QuantStudio™ 3D Digital PCR 20 K chips, and the PCR reaction was performed. The thermal cycler protocol was as follows: 10 min at 96 °C, 39 cycles at 60 °C for 2 min, 98 °C for 30 s, and 60 °C for 1 min. All samples were analyzed in a single assay for each mutation. The data were analyzed with the QuantStudio™3D AnalysisSuite™ v1.1.3 (Life Technologies, Carlsbad, CA) for mutation search and quantification of the DNA copies in the serum. The mutant allele fraction (MAF, %) was defined as the proportion of mutant DNA copies relative to the sum of mutant and wild-type DNA copies obtained by dPCR. The samples were defined as positive for mutations (ctDNApositive) when one or more mutant alleles were detected per assay and defined as negative (ctDNAnegative) when no mutant alleles were detected.
Immunohistochemical (IHC) examination
ER and PR were defined as positive when 10 % or more of the tumor cells were stained by immunohistochemistry (ER: clone 6F11; PR: clone 16; Ventana Japan K.K. and SRL Inc. Tokyo, Japan). Human epidermal growth factor receptor 2 (HER2) was identified immunohistochemically (anti-human c-erbB-2 polyclonal antibody; Nichirei Biosciences, Tokyo, Japan) or by means of fluorescent in situ hybridization (FISH) using the PathVysion HER2 DNA probe kits (SRL Inc. Tokyo, Japan). For the FISH scoring, the ratio of the HER2 gene signals to the chromosome 17 centromere signals was calculated for each of the specimens. When a tumor exhibited +3 immunohistostaining or a FISH ratio ≧2.0, it was considered HER2-positive. The histological grade was determined according to the Scarff-Bloom-Richardson grading system [20].
Statistical analyses
The associations between the various clinicopathological parameters and the PIK3CA mutations were evaluated with Chi square or Fisher’s exact tests. Recurrence-free survival (RFS) rates and overall survival (OS) rates were calculated with the Kaplan–Meier method and evaluated with the log-rank test. Univariate and multivariate analyses of the various parameters for the predictions of recurrence and death were conducted with the Cox proportional hazards model. For all statistical tests, differences were considered significant at P < 0.05.
Results
Detection sensitivity of PIK3CA mutation by dPCR
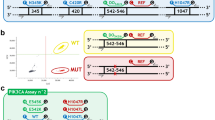
First, we evaluated the detection sensitivity of PIK3CA mutant alleles by dPCR using the genomic DNA extracted from T47D breast cancer cell lines that were known to harbor the H1047R mutation. The H1047R-mutant DNA from the T47D cells was mixed at different mutant allele concentrations (i.e., 100, 10, 1, 0.1, 0.01, 0.001, and 0 %) with wild-type DNA that was extracted from the peripheral blood mononuclear cells of the healthy women. Our dPCR assay was highly sensitive to detect at least two copies of mutant alleles within 20,000 copies of wild-type alleles (sensitivity = 0.01 %) (Fig. 1a), and the mutant allele frequencies measured by dPCR were well correlated with the expected mutant allele frequencies (Pearson’s r = 1.000). Representative dPCR plots for the H1047R mutation are shown in Fig. 1b. Next, the sensitivities for the other two PIK3CA mutations were also evaluated using genomic DNA from MCF-7 cells with the E545K mutation and BT-483 cells with the E542K mutation. The detection sensitivities for the E545K and E542K mutations were also found to be 0.01 % (Supplementary Fig. S1).
a Detection sensitivity of digital PCR is shown. Genomic DNA from T47D cell line carrying PIK3CA H1047R mutation was mixed at different mutant allele concentrations ranging from 0.001 to 100 % with wild-type genomic DNA from peripheral blood mononuclear cells. Mutant alleles were detectable at concentrations of 0.01 %, and the mutant allele fraction (MAF) (%) measured by dPCR were well correlated with expected mutant allele fraction (MAF) (Pearson’s r = 1.000). b Representative views of digital PCR plots (mutant samples). Data from sample chips are displayed in a scatter plot based on color of FAM and VIC events. Red, blue, and yellow plots mean wild-type alleles, mutant alleles, and no amplifications, respectively
PIK3CA mutations in breast tumors
The three PIK3CA hot spot mutations were screened in 313 primary breast tumors with real-time PCR, and PIK3CA mutations were identified in 110 (35.1 %) of the tumors; specifically, 79 H1047R mutations, 19 E545K mutations, and 12 E542K mutations were found. None of the patients carried multiple PIK3CA mutations. The clinicopathological characteristics of the PIK3CA mutant tumors are shown in Table 1. The PIK3CA mutant tumors were significantly more likely to be ER-positive (P = 0.024), PR-positive (P = 0.001), and HER2-negative (P = 0.033).
PIK3CA mutations in the cfDNA
We next performed the dPCR assay for the PIK3CA mutations in the cfDNA from the 110 patients with PIK3CA mutant tumors. Twenty-five patients (22.7 %) were ctDNApositive, specifically, 21 carried H1047R, 3 carried E545K, and 1 carried E542K. The median copy number and MAF of the PIK3CA in the ctDNApositive cases were 29 copies/ml (range 13–2500; Fig. 2a) and 0.37 % (range 0.04–22.00; Supplementary Table S1), respectively. To investigate the specificity of the dPCR assay, serum samples from 50 healthy women and 30 breast cancer patients with PIK3CA non-mutant tumors were assayed for three PIK3CA mutations by dPCR. No mutant ctDNA was detected in any of these samples (Fig. 2a).
a Copy numbers of PIK3CA mutant alleles (copies/ml) in serum samples from the 110 patients with PIK3CA mutant tumors are shown. No mutant ctDNA was detected in 50 healthy women and 30 breast cancer patients. ND not detected. b Mutant allele copy numbers of 25 ctDNApositive cases are shown according to the recurrent status. Black and gray bars represent recurrent and non-recurrent cases, respectively
PIK3CA mutant DNA and patient prognosis
The clinicopathological characteristics of the ctDNApositive and ctDNAnegative patients are shown in Table 1. The ctDNApositive patients were significantly (P = 0.033) more likely to have PR-negative tumors. No other parameters were significantly different between the two groups.
The ctDNApositive patients exhibited significantly (RFS; P = 0.0029, OS; P = 0.0283) lower RFS (92 % vs. 68 %) and OS rates (96 % vs. 84 %) than did the ctDNAnegative patients (Fig. 3a, b). Because there was a trend for recurrences to be more frequently observed in the patients with greater copy numbers of mutant alleles (Fig. 2b; Supplementary Table S1), the ctDNApositive patients were dichotomized into those with high (ctDNAhigh) and low (ctDNAlow) PIK3CA mutant ctDNA counts based on the median copy number (29 copies) of mutant alleles. The ctDNAhigh patients exhibited significantly worse RFS and OS rates than did the ctDNAnegative patients (RFS; P = 0.0001, OS; P = 0.0032), while the RFS and OS rates were not significantly different between the ctDNAlow and ctDNAnegative patients (RFS; P = 0.3950, OS; P = 0.4886) (Fig. 3c, d). Next, the ctDNAlow and ctDNAnegative patients were combined for the following analysis (ctDNAlow+negative). The ctDNAhigh patients exhibited significantly worse RFS and OS rates than did the combined ctDNAlow+negative patients (PFS; P = 0.0002, OS; P = 0.0048; Fig. 3e, f). The multivariate analysis revealed that ctDNAhigh was a significant prognostic factor of RFS (P = 0.005, HR = 4.783; Table 2) and OS (P = 0.128, HR = 3.917; Table 3) independently of the other parameters. Forest plot analyses revealed that the prognostic significance of the ctDNAhigh status was independent of the type of adjuvant therapy (Supplementary Fig. S2). Only two (1.8 %) and three (2.7 %) patients were CEA- and CA15-3-positive, respectively, and neither of these statuses exhibited a significant correlation with patient prognosis (Supplementary Fig. S3).
Discussion
In the present study, we first investigated whether the dPCR assay was sufficiently sensitive to detect a rare PIK3CA mutation in cfDNA. Our experiments using DNA from the several cell lines that harbored different PIK3CA mutations revealed that our dPCR assay was sensitive enough to detect as few as two copies of mutant alleles within 20,000 copies of wild-type alleles, representing the sensitivity of 0.01 % for each of the three PIK3CA hotspot mutations. Additionally, a high specificity (100 %) was confirmed by the findings that no PIK3CA mutations were observed in any of the serum samples from the 50 healthy women and the 30 breast cancer patients with PIK3CA non-mutated tumors. These results indicate that the dPCR assay was highly sensitive and specific for the detection of PIK3CA mutation.
We detected the PIK3CA mutant ctDNA in 25 patients (22.7 %) who had PIK3CA mutant tumors. We found that PIK3CA mutant ctDNA was more likely to be detected in the patients with ER-negative (P = 0.051) or PR-negative (P = 0.033) tumors, and these findings are consistent with our previous report found that GSTP1, RASSF1A, and RARb-methylated ctDNA are more likely to be detected in ER-negative tumors [21]. Additionally, the PIK3CA mutant ctDNA tended to be associated with high histological grade tumors (P = 0.066) but not tumor size or lymph node status. These results indicate that the presence of PIK3CA mutant ctDNA reflects the biologically more aggressive phenotype of breast tumors rather than the tumor size itself. Accordingly, Becker S et al. reported that ER-negative tumors are more likely to release CTCs into the blood, and CTCs are known to correlate with ctDNA [22].
We have shown that the patients with PIK3CA mutant ctDNA exhibited significantly worse RFS and OS rates than did those without this ctDNA; interestingly, the prognoses of the ctDNAhigh patients but not the ctDNAlow patients were significantly worse than those without the ctDNA. Multivariate analysis showed that high copy number of mutant alleles was a significant prognostic factor that was independent of the conventional parameters. These results suggest the possibility that the level of ctDNA might be important for the prediction of patient prognosis, but the optimal cut-off value remains to be established. We also analyzed the data based on relative mutant allele fraction (MAF) (%), but MAF (%) was not significantly associated with recurrence risk in a MAF-dependent manner unlike mutant copy number in serum (copy/ml) (Supplementary Fig. S4). Although both copy number per ml and MAF (%) are used in recent reports [12, 13], absolute amount of ctDNA in serum seems to reflect the tumor burden in patients more precisely than MAF which is dependent on wild-type alleles susceptible to various biological conditions. Our data suggest that the detection of PIK3CA mutations in the cfDNA with dPCR would be clinically useful for the prediction of the prognoses of primary breast cancer patients. In contrast, only 1.8 and 2.7 % of the patients were positive for the conventional tumor markers CEA and CA15-3, respectively, and these markers did not show any association with either DFS or OS, which indicates the superiority of PIK3CA mutant ctDNA to CEA and CA15-3 as a tumor marker and prognostic factor.
Recently, Beaver et al. [14] and Turner et al. [23] have reported the higher detection rates (93 and 75 %, respectively) of PIK3CA mutant ctDNA than our study (22.7 %) in primary breast cancer patients. This seems to be mostly explained by the difference of dPCR platform. They used droplet-dPCR which can analyze a significantly greater number of DNA molecules than our dPCR platform. In addition, Beaver et al. [14] performed “pre-amplification” in order to increase the detection sensitivity. The difference in the assay samples, i.e., serum or plasma, might be another reason for a lower detection rate in our study. Plasma is currently known to be more suitable for ctDNA study because plasma contains fewer wild-type alleles than serum. Unfortunately, we had only serum samples available for this retrospective study on prognosis since there was no consensus for samples to be used for ctDNA studies when we started collection of the samples. Besides, more advanced stage patients (i.e., node-positive or T3/T4) were included in Turner et al’ study [23] than our study, i.e., 91 % (100/110) were stage I or II patients. All these differences mentioned above seem to explain the lower detection rate of PIK3CA mutant ctDNA in our study. Nonetheless, we could show that ctDNAhigh patients, but not ctDNAlow patients, had a significantly worse prognosis than ctDNAnegative patients, suggesting that very high sensitivity might not be needed, at least, in the prediction of prognosis in early-stage breast cancer patients.
The molecular aberrations of the PI3K pathway, including PIK3CA somatic mutations, have important implications as therapeutic targets in breast cancer. Patients harboring such aberrations are thought to be more sensitive to PI3K/AKT/mTOR pathway inhibitors [24] and resistant to anti-HER2 and endocrine therapy [25]. A prospective study is currently investigating targeted regimens that are more tailored to individual metastatic breast cancer patients due to the identification of genomic alterations (SAFIR-01 study) [26]. However, in this trial, serious adverse events related to tumor biopsy have been reported in 4 patients (1 %), suggesting the need for safer and less invasive methods to identify genomic alterations in cancer patients. Mutational analysis of PIK3CA in cfDNA from metastatic breast cancer patients, i.e., the so-called liquid biopsy, is one promising option for avoiding such risks, and molecular screening of patients with ctDNA might increase the precision of personalized therapies.
In conclusion, we have shown that dPCR is a highly sensitive and specific method for detecting PIK3CA mutant ctDNA, 22.7 % of primary breast cancer patients are positive for this ctDNA, and ctDNAhigh but not ctDNAlow status is a significant prognostic factor that is independent of the other conventional prognostic factors. We also suggest that PIK3CA mutant ctDNA is a better tumor marker than CEA and CA15-3. Our preliminary results require validation in future studies.
References
Crowley E, Di Nicolantonio F, Loupakis F, Bardelli A (2013) Liquid biopsy: monitoring cancer-genetics in the blood. Nat Rev Clin Oncol 10(8):472–484. doi:10.1038/nrclinonc.2013.110
Diaz LA, Bardelli A (2014) Liquid biopsies: genotyping circulating tumor DNA. J Clin Oncol 32(6):579–586. doi:10.1200/jco.2012.45.2011
Stroun M, Lyautey J, Lederrey C, Olson-Sand A, Anker P (2001) About the possible origin and mechanism of circulating DNA apoptosis and active DNA release. Clin Chim Acta 313(1–2):139–142
van der Vaart M, Pretorius PJ (2008) Circulating DNA. Its origin and fluctuation. Ann NY Acad Sci 1137:18–26. doi:10.1196/annals.1448.022
Diehl F, Schmidt K, Choti MA, Romans K, Goodman S, Li M, Thornton K, Agrawal N, Sokoll L, Szabo SA, Kinzler KW, Vogelstein B, Diaz LA Jr (2008) Circulating mutant DNA to assess tumor dynamics. Nat Med 14(9):985–990. doi:10.1038/nm.1789
Lo YM, Zhang J, Leung TN, Lau TK, Chang AM, Hjelm NM (1999) Rapid clearance of fetal DNA from maternal plasma. Am J Hum Genet 64(1):218–224. doi:10.1086/302205
Schwarzenbach H (2013) Circulating nucleic acids as biomarkers in breast cancer. Breast Cancer Res 15(5):211. doi:10.1186/bcr3446
Comprehensive molecular portraits of human breast tumours (2012). Nature 490 (7418):61–70. doi:10.1038/nature11412
Stephens PJ, Tarpey PS, Davies H, Van Loo P, Greenman C, Wedge DC, Nik-Zainal S, Martin S, Varela I, Bignell GR, Yates LR, Papaemmanuil E, Beare D, Butler A, Cheverton A, Gamble J, Hinton J, Jia M, Jayakumar A, Jones D, Latimer C, Lau KW, McLaren S, McBride DJ, Menzies A, Mudie L, Raine K, Rad R, Chapman MS, Teague J, Easton D, Langerod A, Lee MT, Shen CY, Tee BT, Huimin BW, Broeks A, Vargas AC, Turashvili G, Martens J, Fatima A, Miron P, Chin SF, Thomas G, Boyault S, Mariani O, Lakhani SR, van de Vijver M, van’t Veer L, Foekens J, Desmedt C, Sotiriou C, Tutt A, Caldas C, Reis-Filho JS, Aparicio SA, Salomon AV, Borresen-Dale AL, Richardson AL, Campbell PJ, Futreal PA, Stratton MR (2012) The landscape of cancer genes and mutational processes in breast cancer. Nature 486(7403):400–404. doi:10.1038/nature11017
Samuels Y, Diaz LA Jr, Schmidt-Kittler O, Cummins JM, Delong L, Cheong I, Rago C, Huso DL, Lengauer C, Kinzler KW, Vogelstein B, Velculescu VE (2005) Mutant PIK3CA promotes cell growth and invasion of human cancer cells. Cancer Cell 7(6):561–573. doi:10.1016/j.ccr.2005.05.014
Kang S, Bader AG, Vogt PK (2005) Phosphatidylinositol 3-kinase mutations identified in human cancer are oncogenic. Proc Natl Acad Sci USA 102(3):802–807. doi:10.1073/pnas.0408864102
Dawson SJ, Tsui DW, Murtaza M, Biggs H, Rueda OM, Chin SF, Dunning MJ, Gale D, Forshew T, Mahler-Araujo B, Rajan S, Humphray S, Becq J, Halsall D, Wallis M, Bentley D, Caldas C, Rosenfeld N (2013) Analysis of circulating tumor DNA to monitor metastatic breast cancer. N Engl J Med 368(13):1199–1209. doi:10.1056/NEJMoa1213261
Murtaza M, Dawson SJ, Tsui DW, Gale D, Forshew T, Piskorz AM, Parkinson C, Chin SF, Kingsbury Z, Wong AS, Marass F, Humphray S, Hadfield J, Bentley D, Chin TM, Brenton JD, Caldas C, Rosenfeld N (2013) Non-invasive analysis of acquired resistance to cancer therapy by sequencing of plasma DNA. Nature 497(7447):108–112. doi:10.1038/nature12065
Beaver JA, Jelovac D, Balukrishna S, Cochran RL, Croessmann S, Zabransky DJ, Wong HY, Valda Toro P, Cidado J, Blair BG, Chu D, Burns T, Higgins MJ, Stearns V, Jacobs L, Habibi M, Lange J, Hurley PJ, Lauring J, VanDenBerg DA, Kessler J, Jeter S, Samuels ML, Maar D, Cope L, Cimino-Mathews A, Argani P, Wolff AC, Park BH (2014) Detection of cancer DNA in plasma of patients with early-stage breast cancer. Clin Cancer Res 20(10):2643–2650. doi:10.1158/1078-0432.ccr-13-2933
Bettegowda C, Sausen M, Leary RJ, Kinde I, Wang Y, Agrawal N, Bartlett BR, Wang H, Luber B, Alani RM, Antonarakis ES, Azad NS, Bardelli A, Brem H, Cameron JL, Lee CC, Fecher LA, Gallia GL, Gibbs P, Le D, Giuntoli RL, Goggins M, Hogarty MD, Holdhoff M, Hong SM, Jiao Y, Juhl HH, Kim JJ, Siravegna G, Laheru DA, Lauricella C, Lim M, Lipson EJ, Marie SK, Netto GJ, Oliner KS, Olivi A, Olsson L, Riggins GJ, Sartore-Bianchi A, Schmidt K, Shih IM, Oba-Shinjo SM, Siena S, Theodorescu D, Tie J, Harkins TT, Veronese S, Wang TL, Weingart JD, Wolfgang CL, Wood LD, Xing D, Hruban RH, Wu J, Allen PJ, Schmidt CM, Choti MA, Velculescu VE, Kinzler KW, Vogelstein B, Papadopoulos N, Diaz LA Jr (2014) Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci Transl Med 6(224):224ra224. doi:10.1126/scitranslmed.3007094
Goldhirsch A, Wood WC, Gelber RD, Coates AS, Thurlimann B, Senn HJ (2003) Meeting highlights: updated international expert consensus on the primary therapy of early breast cancer. J Clin Oncol 21(17):3357–3365. doi:10.1200/jco.2003.04.576
Goldhirsch A, Glick JH, Gelber RD, Coates AS, Thurlimann B, Senn HJ (2005) Meeting highlights: international expert consensus on the primary therapy of early breast cancer 2005. Ann Oncol 16(10):1569–1583. doi:10.1093/annonc/mdi326
Goldhirsch A, Wood WC, Gelber RD, Coates AS, Thurlimann B, Senn HJ (2007) Progress and promise: highlights of the international expert consensus on the primary therapy of early breast cancer 2007. Ann Oncol 18(7):1133–1144. doi:10.1093/annonc/mdm271
Goldhirsch A, Ingle JN, Gelber RD, Coates AS, Thurlimann B, Senn HJ (2009) Thresholds for therapies: highlights of the St Gallen International Expert Consensus on the primary therapy of early breast cancer 2009. Ann Oncol 20(8):1319–1329. doi:10.1093/annonc/mdp322
Bloom HJ, Richardson WW (1957) Histological grading and prognosis in breast cancer; a study of 1409 cases of which 359 have been followed for 15 years. Br J Cancer 11(3):359–377
Fujita N, Nakayama T, Yamamoto N, Kim SJ, Shimazu K, Shimomura A, Maruyama N, Morimoto K, Tamaki Y, Noguchi S (2012) Methylated DNA and total DNA in serum detected by one-step methylation-specific PCR is predictive of poor prognosis for breast cancer patients. Oncology 83(5):273–282. doi:10.1159/000342083
Becker S, Becker-Pergola G, Banys M, Krawczyk N, Wallwiener D, Solomayer E, Schuetz C, Fehm T (2009) Evaluation of a RT-PCR based routine screening tool for the detection of disseminated epithelial cells in the bone marrow of breast cancer patients. Breast Cancer Res Treat 117(2):227–233. doi:10.1007/s10549-008-0174-3
Turner NC, Garcia-Murillas I, Schiavon G, Hrebien S, Osin P, Nerurkar A, Kozarewa I, Garrido JA, Dowsett M, Smith IE (2014) Tracking tumor-specific mutations in circulating-free DNA to predict early relapse after treatment of primary breast cancer. J Clin Oncol 32:511
Miller TW, Rexer BN, Garrett JT, Arteaga CL (2011) Mutations in the phosphatidylinositol 3-kinase pathway: role in tumor progression and therapeutic implications in breast cancer. Breast Cancer Res 13(6):224. doi:10.1186/bcr3039
Berns K, Horlings HM, Hennessy BT, Madiredjo M, Hijmans EM, Beelen K, Linn SC, Gonzalez-Angulo AM, Stemke-Hale K, Hauptmann M, Beijersbergen RL, Mills GB, van de Vijver MJ, Bernards R (2007) A functional genetic approach identifies the PI3 K pathway as a major determinant of trastuzumab resistance in breast cancer. Cancer Cell 12(4):395–402. doi:10.1016/j.ccr.2007.08.030
Andre F, Bachelot T, Commo F, Campone M, Arnedos M, Dieras V, Lacroix-Triki M, Lacroix L, Cohen P, Gentien D, Adelaide J, Dalenc F, Goncalves A, Levy C, Ferrero JM, Bonneterre J, Lefeuvre C, Jimenez M, Filleron T, Bonnefoi H (2014) Comparative genomic hybridisation array and DNA sequencing to direct treatment of metastatic breast cancer: a multicentre, prospective trial (SAFIR01/UNICANCER). Lancet Oncol 15(3):267–274. doi:10.1016/s1470-2045(13)70611-9
Acknowledgments
This work was supported in part by Grants-in-Aid from the Knowledge Cluster Initiative of the Ministry of Education, Culture, Sports, Science and Technology, Japan.
Conflict of interest
The authors of this study have no conflicts of interest and no financial disclosures to make.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Oshiro, C., Kagara, N., Naoi, Y. et al. PIK3CA mutations in serum DNA are predictive of recurrence in primary breast cancer patients. Breast Cancer Res Treat 150, 299–307 (2015). https://doi.org/10.1007/s10549-015-3322-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-015-3322-6