Abstract
Combining antitumor agents with bioactive compounds is a potential strategy for improving the effect of chemotherapy on cancer cells. The goal of this study was to elucidate the antitumor effect of the flavonoid, fisetin, combined with the multikinase inhibitor, sorafenib, against human cervical cancer cells in vitro and in vivo. The combination of fisetin and sorafenib synergistically induced apoptosis in HeLa cells, which is accompanied by a marked increase in loss of mitochondrial membrane potential. Apoptosis induction was achieved by caspase-3 and caspase-8 activation which increased the ratio of Bax/Bcl-2 and caused the subsequent cleavage of PARP level while disrupting the mitochondrial membrane potential in HeLa cells. Decreased Bax/Bcl-2 ratio level and mitochondrial membrane potential were also observed in siDR5-treated HeLa cells. In addition, in vivo studies revealed that the combined fisetin and sorafenib treatment was clearly superior to sorafenib treatment alone using a HeLa xenograft model. Our study showed that the combination of fisetin and sorafenib exerted better synergistic effects in vitro and in vivo than either agent used alone against human cervical cancer, and this synergism was based on apoptotic potential through a mitochondrial- and DR5-dependent caspase-8/caspase-3 signaling pathway. This combined fisetin and sorafenib treatment represents a novel therapeutic strategy for further clinical developments in advanced cervical cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cervical cancer is currently one of the leading causes of mortality in women [1]. Although the incidence and mortality rates have been gradually decreasing with earlier detection and advanced medical treatment, the prognosis of patients with cervical cancer remains dismal in the presence of advanced or metastatic disease [2]. Due to the minimal success of cytotoxic therapies for cervical cancer, there has been an increased interest in targeted therapy which alters molecular events in this disease [3]. Currently, the standard treatment for advanced cervical cancer is surgical tumor resection followed by chemotherapy. Moreover, when chemotherapeutic drugs with different effects have been combined, intolerable side effects have sometimes occurred [4]. Thus, novel combination strategies for cervical cancer are critically needed.
Apoptosis plays a critical role in the balance between cellular survival and death by selective cell depletion and cell morphological changes. Apoptotic bodies, DNA fragmentation, and caspase family activation can be achieved through two major signaling pathways, the intrinsic or mitochondrial-mediated pathway and the extrinsic or death receptor (DR)-induced pathway [5, 6]. The intrinsic pathway is triggered by anticancer medicines, growth factor withdrawal, or hypoxia resulting in increased permeability of the outer mitochondrial membrane and release of apoptogenic factors such as cytochrome c from the mitochondrial intermembrane space into the cytosol. Cytochrome c subsequently activates the caspase cascade [7, 8]. The extrinsic pathway is activated by the binding of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) to its receptors, i.e., death receptor 4 (DR4), also called TRAIL-R1 and death receptor 5 (DR5), also called TRAIL-R2. This receptor binding transduces an apoptotic signal downward to recruit the Fas-associated death domain and procaspase-8 in a death-inducing complex [9]. A study conducted in cervical cancer patients showed that high cytoplasmic staining with DR4 and DR5 in TRAIL and TRAIL-negative tumors showed better pathological response to radiotherapy, highlighting the critical role of apoptosis in treating cervical cancers [10]. Recently, herbal medicine and natural foods have shown a promising role in the treatment of cervical cancer [11].
Naturally occurring flavonoids, commonly found in fruits and vegetables, have gained focus as potential antitumor drugs. 3′,4′,7-Tetrahydroxyflavone (fisetin) is a naturally occurring flavonoid which possesses anticancer properties through inhibition of proliferation and angiogenesis as well as the ability to induce cell cycle arrest, apoptosis, and differentiation [12]. Fisetin has also demonstrated its effectiveness against several types of cancer, including hepatoma [13], lung adenocarcinoma [14], nonsmall cell lung cancer [15], and prostate cancer [16]. Recently, fisetin has shown an ability to induce apoptosis of human cervical cancer cells through a caspase-dependent pathway and to inhibit migration and invasion of cervical cancer cells by repression of urokinase plasminogen activator via interruption of p38 MAPK-dependent NF-κB signaling pathway [17, 18].
Sorafenib, an oral multikinase inhibitor with activities against Raf serine/threonine kinases, vascular endothelial growth factor receptor, and platelet-derived growth factor receptor, also has potent antiangiogenic and proapoptotic activities [19]. Sorafenib has been reported to improve the median overall survival and the median time to radiological progression in advanced hepatocellular carcinoma patients [20, 21]. In addition, sorafenib has been effective against multiple types of cancer by inducing apoptosis in bladder cancer cells [22], thyroid cancer cells [23], and synergistically with retinoids [24] and metformin [25] in hepatocellular carcinoma cell. Sorafenib has also been shown to be effective in combination with a benzofuroxan derivate in lung carcinoma [26] and synergistically with fusariotoxin enniatin B in cervical cancer [27]. In addition, our laboratory demonstrated that fisetin has potential anticancer properties against cervical cancer [17, 18] and glioma [28]. The goal of this study was to demonstrate the efficacy of the combination therapy of fisetin and sorafenib against cervical cancer HeLa cells and to elucidate its underlying mechanism(s) of action.
Materials and methods
Reagents and chemicals
Fisetin was purchased from Sigma-Aldrich. Sorafenib was purchased from Selleck Chemicals. The antibodies included anti-DR4, anti-DR5, anti-Bcl-2, anti-Bax, anti-cytochrome c, anti-β-actin, and siDR5 reagent were purchased from Santa Cruz Biotechnology. The anti-cleaved-caspase-8, anti-cleaved-caspase-3, and anti-cleaved-PARP were purchased from Cell Signal Technology. Annexin V-FITC and propidiumiodide (PI) kit was purchased from BD Biosciences. Z-VAD-FMK was purchased from BioVision.
Cell culture
Human cervical cancer cell line HeLa was obtained from the Bioresources Collection and Research Center, Food Industry Research and Development Institute (Hsinchu, Taiwan). HeLa cells were maintained in Dulbecco’s modified Eagle’s/Ham’s F-12 medium (DMEM/F12, Gibco-Invitrogen Corporation, CA). Cell culture media were supplemented with 10 % fetal bovine serum (FBS), 1 % penicillin, and 1 % streptomycin. Cells were maintained in a humidified 5 % CO2 atmosphere at 37 °C.
Cell viability assay
Cell growth was assessed using a MTT assay. In brief, cells (4 × 104 cells/well) were seeded on a 24-well dish. After treatment with fisetin, sorafenib, or fisetin combined with sorafenib for 24 h, the medium was changed and the cells were incubated with MTT (5 mg/ml) at 37 °C for 4 h; then, formazan crystals were dissolved in 1 mL of isopropanol, and the absorbance of the formazan product was measured at a wavelength of 570 nm on an ELISA reader. Assays were performed in triplicate in three independent experiments.
Annexin V-FITC/PI double-stained assay
Apoptosis was assessed using the Annexin V-FITC Apoptosis Detection kit according to the manufacturer’s protocol. The cells were suspended with FITC-conjugated Annexin V-FITC and PI (50 μg/ml) stain in the absence of light for 10 min. Apoptotic cells were analyzed via flow cytometry. Samples were analyzed for DNA content with the FACScan flow cytometer (BD Biosciences, San Diego, CA), and relative cell cycle distribution was analyzed using the CellQuest software (Verity Software House, Topsham, ME).
Inhibition of DR5 expression using siRNAs
The nontargeting siRNA and siRNA targeting DR5 (siDR5) HeLa cells that were 70 % confluent were transfected with the siDR5 (100 nM) using Lipofectamine 2000 (Invitrogen). After transfection for 24 h, the cells were treated with fisetin, sorafenib alone, or fisetin combined with sorafenib for 48 h. Cells were evaluated with Annexin V-FITC/PI assay and MTT assays were detected to apoptotic cells.
Measurement of mitochondrial membrane potential (MMP)
A mitochondrial membrane potential detection assay was used according to the manufacturer’s instructions, as described previously [29]. JC-1 reagent measures mitochondrial membrane potential (Δψm). Cells were treated with fisetin, sorafenib alone, or fisetin combined with sorafenib, resuspended in JC-1 solution (30 μM), and incubated at 37 °C for 15 min. Cells were then rinsed with PBS before flow cytometry. A dot plot of green fluorescence (cells lacking Δψm) versus red (living cells with intact Δψm) was generated. BD FACSCalibur and BD CellQuest Pro software was used to analyze the cells. For each treatment, a minimum of 10,000 cells within the gated region were analyzed. Data were expressed as the percentage of cells with intact Δψm.
Western blot analysis
The cells were homogenized in 200 μL of lysis buffer. Cell debris was removed by centrifugation at 13,000g for 30 min at 4 °C, and the protein determined using a Bradford assay. Samples were run on 10 % SDS-PAGE and subsequently electrotransferred to a polyvinylidene difluoride (PVDF) membrane. The membrane was blocked for 2 h with 5 % nonfat dry milk buffer. After blocking, the membrane was incubated with primary antibodies (1:1000) overnight. After washing, the membrane was incubated with HRP-conjugated anti-mouse (1:10,000), anti-goat (1:10,000), or anti-rabbit antibody (1:10,000) at room temperature for 2 h. The reaction was visualized using enhanced chemiluminescence (ECL) reagent (Millipore, Billerica, USA) and detected using a Luminescent Image Analyzer LAS-4000 mini.
Xenograft experiment
Animal experiments were conducted according to the guidelines of the Institutional Animal Care and Use Committee (IACUC) of Chung Shan Medical University (IACUC Approval No. 1071). Five-week-old BALB/c female mice were purchased from the National Laboratory Animal Center (Taipei, Taiwan). For the in vivo experiments, 1 × 106 HeLa cells (diluted in Matrigel) were established by subcutaneous injection into the animal’s right flank. After 1 week, the first group of mice received 100 μl vehicle (DMSO) orally. The second group of mice received 100 μl fisetin (4 mg/kg) orally and the third group of mice received sorafenib (10 mg/kg) orally. The fourth group of mice received 100 μl of the combination of fisetin (4 mg/kg) and sorafenib (10 mg/kg) orally two times per week (n = 5). Treatments were initiated when tumors reached a mean group size of approximately 80 mm3. Tumor volume (mm3) was measured with calipers, and calculated as 0.5236 × L (W)2, where W was the width and L was the length of each tumor.
Immunohistochemistry
The paraffin-embedded animal tumors were fixed in 10 % formalin, embedded in paraffin, and cut into 5-mm-thick sections. Immunohistochemical staining was performed on the slides which were incubated overnight at 4 °C in humidified chambers with human Ki-67 (Abcam; diluted 1:200). The slides were washed three times in a phosphate-buffered solution and further incubated with a secondary antibody for 30 min at room temperature. The immunolabeled sections were incubated with biotin-conjugated secondary antibody for 20 min at room temperature, then with peroxidase-conjugated complex (Dako) for 20 min, and finally visualized with 3,3′-diaminobenzidin and counterstained with hematoxylin, and analyzed for staining, as described previously [30].
Statistical analysis
Statistically significant differences were calculated using GraphPad Prism4 (San Diego, CA). Student’s t test or one-way analysis of variance (ANOVA) with a post hoc analysis using Tukey’s multiple-comparison test was used for obtaining parametric data. A P value <0.05 or 0.01 was considered statistically significant. All experiments were repeated three times (n = 3), and values were expressed as means ± standard deviation.
Results
Effects of combination treatment on cell viability in human cervical cancer HeLa cells
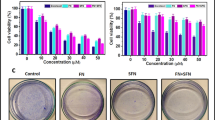
The chemical structures of fisetin and sorafenib are shown on Fig. 1a, b, respectively. To evaluate the potential of the fisetin and sorafenib combined approach, HeLa cells were treated with different concentrations of fisetin (0∼80 μM) and sorafenib (0∼10 μM). Cell viability was detected by MTT assay. Fisetin and sorafenib were significantly toxic to HeLa cells at concentrations up to 40 and 7.5 μM, respectively (Fig. 1c, d). Based on these results and those in several published reports [18, 25], the combination of fisetin (40 μM) and sorafenib (2.5 or 5 μM) were used in the following experiments.
Effect of fisetin and sorafenib on the cell viability of HeLa cells. Molecular structure of a fisetin and b sorafenib. c, d HeLa cells are incubated with fisetin (0–80 μM) or sorafenib (0–10 μM) for 24 h. Cell viability is determined by using the MTT assay. The results are expressed as the percentages of cell viability. Data are expressed as the mean ± SE of at least three independent experiments. **P < 0.01, compared with that of the untreated controls (0 μM)
Effects of combination treatment on cell apoptosis in human cervical cancer HeLa cells
Compared with either fisetin (40 μM) or sorafenib (2.5 or 5 μM) alone, the combination of fisetin (40 μM) and sorafenib (2.5 or 5 μM) induced a significant decrease in cell viability by MTT assay (Fig. 2a) and an increase in HeLa cells apoptosis as measured by Annexin V-FITC/PI double-stained assay (Fig. 2b). These results indicated that fisetin can enhance the antitumor efficacy of sorafenib in human cervical cancer HeLa cells.
Effect of fisetin and sorafenib combination on apoptosis of HeLa cells. HeLa cells are treated with fisetin (40 μM) and sorafenib (2.5 or 5 μM) alone or in combination for 24 h. Cell viability is determined by using a MTT assay and b Annexin V and PI double staining by flow cytometry. c Cell lysates are subjected to western blotting assay with anti-cleaved caspase-3, anti-cleaved caspase-8, anti-cleaved PARP, and anti-β-actin antibodies. β-Actin is used as loading control. d HeLa cells are treated with fisetin and sorafenib alone, or in combination with Z-VAD-FMK (20 μM), for 24 h, and cell viability is determined using the MTT assay and e Annexin/V and PI double staining by flow cytometry. Data are expressed as the mean (± SE) of at least three independent experiments. **P < 0.01, control versus fisetin or fisetin plus sorafenib; #P < 0.01, fisetin versus Z-VAD-FMK or plus sorafenib
Effects of combination treatment on cell apoptosis via extrinsic pathways
To understand the apoptosis mechanism underlying the synergistic effects of fisetin and sorafenib in HeLa cells, western blot assay was performed. It revealed that the combined fisetin and sorafenib treatment significantly increased the expression of cleaved-caspase-3, cleaved-caspase-8, and cleaved-PARP compared with fisetin alone (Fig. 2c). In addition, when HeLa cells were pretreated with pan-caspase inhibitor (Z-VAD-FMK, 20 μM) for 2 h and then incubated with fisetin and sorafenib either alone, or in combination, for 24 h, the MTT assay revealed significantly decreased cytotoxic effects on cell viability when treating with fisetin alone or combination with sorafenib plus Z-VAD-FMK (Fig. 2d). By performing Annexin V-FITC/PI double-stained assay and by measuring Annexin V positive cells by flow cytometry, fisetin alone, or in combination with sorafenib, was found to induce apoptosis of HeLa cells compared with pretreatment with Z-VAD-FMK (Fig. 2e). These results suggested that the fisetin and sorafenib combination induced apoptosis by activation of the caspase-3 and caspase-8 pathway.
Effect of combination treatment induces HeLa cell apoptosis through induction of death receptor 5 activation
Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) has been reported to have a remarkable ability to induce apoptosis in a variety of cancer cells and has been shown to interact with DR4 and DR5 to transduce an apoptotic signal and increase the apoptotic potential through the activation of the caspase-8/caspase-3 pathway [31]. We sought to determine whether the combination of fisetin and sorafenib could induce apoptosis through DR4 and DR5 in HeLa cells. Western blots showed that combination of fisetin and sorafenib increased the protein expression of DR5 but did not affect DR4 expression (Fig. 3a).
Effect of combination of fisetin and sorafenib on apoptosis of HeLa cells through DR5 activation. HeLa cells are treated with fisetin (40 μM) and sorafenib (2.5 or 5 μM) alone, or in combination, for 24 h. a Cell lysates are subjected to western blotting assay with anti-DR4, anti-DR5, and anti-β-actin antibodies. b HeLa cells are treated with fisetin (40 μM) and sorafenib (2.5 or 5 μM) alone, or in combination with siDR5 (100 nM) for 48 h by western blot. c Cell viability is determined using MTT assay and d Annexin V and PI double staining by flow cytometry. β-Actin is used as loading control. Data are expressed as the mean (± SE) of at least three independent experiments. **P < 0.01, control versus fisetin or fisetin versus sorafenib; #P < 0.01, fisetin versus siDR5 or plus sorafenib
In order to assess whether DR4 and DR5 expression were involved in the combination of fisetin and sorafenib-induced apoptosis, the cells were pretreated for 6 h with siDR5. The cells were then exposed to fisetin (40 μM), sorafenib (5 μM), or a combination of fisetin and sorafenib for 24 h and then analyzed by western blot assay. The results showed that the combined treatment induced DR5 cleavage of caspase-3, caspase-8, and caspase-PARP expression was attenuated by inhibiting DR5 expression with siDR5 compared with the other treatments (Fig. 3b). Furthermore, MTT assay showed that siDR5 pretreatment also completely prevented combined fisetin and sorafenib treatment-induced apoptosis of HeLa cells (Fig. 3c), consistent with Annexin V-FITC/PI double-stained assay results (Fig. 3d), suggesting that the effect of the combination treatment on HeLa cells was specifically via a DR5-mediated activation of the caspase-8 and caspase-3 pathway.
Effects of combination treatment on the mitochondrial membrane potential in HeLa cells
Mitochondria play a key role in propagating apoptotic signaling [32, 33]. We examined the effect of synergistic effects of fisetin and sorafenib on mitochondrial membrane potential (Δψm) by using a JC-1 dye and flow cytometry analysis. Quantitative analysis using flow cytometry suggested loss of ΔΨm in the combined fisetin/sorafenib-treated HeLa cells, compared with treatment with either fisetin or sorafenib alone (Fig. 4a). Additionally, we further examined whether the Bax/Bcl-2 ratio increased upon combined fisetin and sorafenib treatment of HeLa cells. We found that combined fisetin and sorafenib treatment resulted in an increase in Bax/Bcl-2 ratio compared with treatment with either fisetin or sorafenib alone (Fig. 4b).
Effect of the combination of fisetin and sorafenib on mitochondrial membrane potential of HeLa cells through activation of the DR5 pathway. HeLa cells are treated with fisetin (40 μM) and sorafenib (2.5 or 5 μM) alone, or in combination, for 24 h and mitochondrial membrane potential is determined by JC-1 staining and flow cytometry. b Cell lysates are subjected to western blot assay with anti-Bax, anti-Bcl-2, anti-cytochrome c, and anti-β-actin antibodies. c HeLa cells are treated with fisetin (40 μM) and sorafenib (2.5 or 5 μM) alone, or in combination with siDR5 (100 nM) for 48 h and mitochondrial membrane potential is determined by JC-1 staining and flow cytometry. d Bax and Bcl-2 protein levels are assessed by western blot. Data are expressed as mean (± SE) of at least three independent experiments. **P < 0.01, control versus fisetin or fisetin versus sorafenib; #P < 0.01, fisetin versus siDR5 or plus sorafenib
We transiently transfected HeLa cells with siDR5 and examined it effects on combined fisetin and sorafenib treatment-induced mitochondrial dysfunction. HeLa cells expressing siDR5 significantly blocked combined fisetin and sorafenib-induced mitochondrial dysfunction compared with other treatments (Fig. 4c). Moreover, HeLa cells expressing siDR5 also blocked combined fisetin and sorafenib-induced Bax/Bcl-2 levels and cytochrome c release (Fig. 4d). These data suggested that the apoptotic effect of combined fisetin and sorafenib treatment on Hela cells was mediated by a mitochondria-dependent apoptotic pathway.
Fisetin and sorafenib synergistically inhibit tumor growth using an in vivo xenograft cervical cancer model
To investigate the synergistic antitumour effects of the combined fisetin and sorafenib treatment, a HeLa cervical carcinoma xenograft model was used. Based on our previous studies of fisetin in a cervical cancer xenograft model [18], an in vivo scheme involving 4 mg/kg of fisetin administered orally to each mouse was utilized. Five nude mice in each group were inoculated with HeLa cervical cancer cells. They were then gavaged with DMSO, fisetin (4 mg/kg), sorafenib (10 mg/kg), or the combination treatment (fisetin 4 mg/kg and sorafenib 10 mg/kg) two times per week. The results showed that the subcutaneous tumor volume was decreased in the combination-treated group compared with the other groups (Fig. 5a, b). Consistent with the tumor volume results, the tumor weight was strongly inhibited in the combination-treated mice compared with the other groups (Fig. 5c). Moreover, we found no loss in body weight in the mice (Fig. 5d). Accordingly, the expression of Ki-67-positive proliferating cells was strongly reduced in the combination-treated mice (Fig. 5e). Immunohistochemistry assay further showed that protein levels of DR5, cleaved-caspase-3, cleaved-caspase-8, and cleaved-PARP were markedly increased in the combination-treated mice compared with the other groups (Fig. 5f). Collectively, these findings suggested that fisetin combined with sorafenib was effective as a novel therapeutic agent against cervical cancer.
Antitumor effects of the combination of fisetin and sorafenib on tumor growth in an in vivo cervical xenograft model. a Nude mice (five mice per group), previously inoculated with HeLa cervical cancer cells, are fed with fisetin (4 mg/kg) and sorafenib (10 mg/kg) alone, or in combination, and observed for 5 weeks. b Mean tumor volumes at given time points. c The weights of the tumor and (d) body weight are determined. e The tissue samples of excised xenograft tumor are examined by H&E stain and immunohistochemical stain with anti-Ki-67 antibody. f Cell lysates from the xenograft tumors are subjected to western blot assay with anti-DR5, anti-cleaved caspase-3, anti-cleaved caspase-8, anti-cleaved PARP, and anti-K-actin antibodies. **P < 0.01, compared with that of the control group
Discussion
The combination of flavonoids with anticancer drugs has been suggested as a potential strategy for tumor therapy [34]. However, such combinations have been primarily associated with low cytotoxicity, narrow therapeutic indices, and undesirable side effects. In an attempt to find a better anticancer regimen for cervical cancer cells, we investigated the activity of fisetin, a flavonoid with antitumor properties, in combination with sorafenib, a well-established multikinase inhibitor approved for the treatment of renal cell carcinoma [35] and hepatocellular carcinoma [21]. Our results showed that the less-toxic flavonoid, fisetin, in combination with sorafenib, worked synergistically to inhibit cell growth and induce apoptosis of human cervical HeLa cancer cells by increasing DR5-mediated mitochondrial dysfunction and induction of caspase-8/3 signaling both in vitro and in vivo (Fig. 6). In other words, fisetin combined with sorafenib showed enhanced therapeutic efficacy against human cervical cancer cells.
Fisetin, a flavonoid found in fruits and vegetables, has been shown to have anticancer activity against various cancer cells and to also possess chemopreventive properties [12, 36]. Studies have demonstrated fisetin-induced apoptosis in various cancer cells through activation of caspase-3 cascade and increased p53 expression in hepatocellular carcinoma SK-HEP-1 cells [13]. Fisetin treatment of A431 cells resulted in G2M arrest and induction of apoptosis, reduced expression of Bcl-2, increased expression of Bax, and activation of caspase-3/caspase-9 in NCl-H460 nonsmall lung cancer cells [19]. Fisetin also induced endoplasmic reticulum stress, inhibited cell growth, and increased activation of both extrinsic and intrinsic pathways in human melanoma cells [37]. The antitumor effects of fisetin have also been exploited to improve the efficacy of conventional chemotherapy. Our study showed that fisetin-induced apoptosis of HeLa cells, both in vitro and in vivo, is consistent with the ability of embryonal carcinoma cells to respond to the combination of fisetin and cisplatin which synergistically induced apoptosis both in vitro and in vivo [38]. Similar reports have suggested that the combination therapy of N-acetyl-L-cysteine and fisetin induced apoptosis in human colonic cancer cells [39]. Therefore, studies of combination chemotherapy have focused on identifying natural compounds that could enhance the therapeutic index in various cancer cells while decreasing the side effects [40].
Activated caspase-8 can then induce activation of proapoptotic proteins, such as Bid, which in turn could induce cytochrome c release from mitochondria and subsequently activate caspase-3 [41]. Based on our studies, fisetin and sorafenib synergistically succeeded in activating the caspase-8/caspase-3 pathway. The possibility that caspase-8 activation by combating in a death receptor or alternatively represents a secondary event derived from mitochondrial activation [42]. The Bcl-2 protein mediates antiapoptotic signaling, and Bax protein mediates proapoptotic signaling. They are crucial for inducing permeabilization of the outer mitochondrial membrane and subsequent release of cytochrome c [43], and overexpression of Bcl-2 protein showed the preventive effect against apoptosis [44]. Our results showed that the combination fisetin and sorafenib significantly increased Bax/Bcl-2 ratio levels while disrupting mitochondrial membrane potential in HeLa cells. Decreased Bax/Bcl-2 ratio levels and mitochondrial membrane potentials were also observed in siDR5-treated HeLa cells. These results provided that increased Bax/Bcl-2 ratio expression with loss of mitochondrial membrane potential is involved in fisetin/sorafenib-induced apoptosis of HeLa cells.
DR4/DR5 activation recruits Fas-associated death domain (FADD) and pro-caspase-8 to form DISC, which activates the autocleavage of pro-caspase-8 to activate caspase-8, then the downstream cleavage of caspase-3, leading to the execution of extrinsic apoptosis [45]. Several reports have shown that DR5 plays an important role in sensitizing tumor cells to induce apoptosis by chemotherapeutic agents [46, 47]. Our findings demonstrated that the fisetin/sorafenib combination increased protein levels of DR5 in HeLa cells. Interestingly, the combination of fisetin and sorafenib did not affect the protein levels of DR4, emphasizing the specific effect of the fisetin/sorafenib combination on DR5 expression. Moreover, the pan-caspase inhibitor Z-VAD-FMK and siDR5, in blocking DR5 expression, suppressed fisetin/sorafenib-induced apoptosis. In addition, some antitumor agents examined in cervical cancer HeLa cells have induced apoptotic death by activating the mitochondrial- and DR5-mediated pathway in cervical cancer [48]. The effects of one member of the flavonoid family, quercetin, has been examined in cervical cancer HeLa cells. It promoted apoptosis by increased production of reactive oxygen species and depolarization of mitochondrial membrane potentials [49]. Therefore, we suggested that fisetin and sorafenib combination could induce DR5 expression, leading to stimulation of the death receptor and mitochondria dysfunction pathway and activation of caspase-3/caspase-8, and PARP pathways.
The antitumor activities of sorafenib have been shown in a number of studies [42], and it has been approved for the treatment of advanced renal cell carcinoma [50] and advanced hepatocellular carcinoma [21]. Sorafenib was also reported to decrease viability of thyroid cancer cells by causing apoptotic cell death via cell membrane disruption and LDH release, and caspase-3/caspase-7 activation through intracellular signaling pathways including MAP kinase- and AKT-dependent pathways [23]. Based on its low toxicity and multiple functions in cancer therapy, sorafenib may be used in combination with other antitumor agents to improve therapeutic efficiency. It has been reported that sorafenib enhanced the apoptosis activity mediated by fisetin in BRAF-mutated melanoma cell through activation of mitochondrial-dependent caspase-3 apoptotic signaling via suppressing MAPK and PI3K and VEGF expression in vitro and in vivo [51]. In addition, the antimetastatic effects of a low dose of fisetin and sorafenib combination exhibited greater decrease of N-cadherin, vimentin, and fibronectin expression, and increase of E-cadherin expression in BRAF mutated melanoma cells in both in vitro and in vivo xenograft tumors [52]. ABT-737 treatment combined with sorafenib effectively suppressed levels of phosphorylated STAT3 and MCL1 in glioma cells [53]. Combined treatment of benzofuroxan derivative and sorafenib showed stronger cytotoxicity and induced apoptosis through the DR4-triggered extrinsic pathway toward lung cancer cells [26]. Taken together, sorafenib alone, or in combination with other agents, could enhance cytotoxicity of cancer cells by a variety of signaling pathways as well as apoptosis.
In conclusion, our study showed that the combination of fisetin and sorafenib exerted better synergistic effects in vitro and in vivo than either agent used alone against human cervical cancer and this synergism was based on apoptotic potential through a mitochondrial- and DR5-dependent caspase signaling pathway. Combined fisetin and sorafenib treatment represents a novel therapeutic strategy for further clinical developments in advanced cervical cancer.
References
Saslow D, Solomon D, Lawson HW, Killackey M, Kulasingam SL, et al. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. J Low Genit Tract Dis. 2012;16:175–204.
Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006;24:2137–50.
del Campo JM, Prat A, Gil-Moreno A, Perez J, Parera M. Update on novel therapeutic agents for cervical cancer. Gynecol Oncol. 2008;110:S72–6.
Chen HX, Cleck JN. Adverse effects of anticancer agents that target the VEGF pathway. Nat Rev Clin Oncol. 2009;6:465–77.
Thorburn A. Death receptor-induced cell killing. Cell Signal. 2004;16:139–44.
Riedl SJ, Salvesen GS. The apoptosome: signalling platform of cell death. Nat Rev Mol Cell Biol. 2007;8:405–13.
Babbitt SE, Sutherland MC, Francisco BS, Mendez DL, Kranz RG. Mitochondrial cytochrome c biogenesis: no longer an enigma. Trends Biochem Sci. 2015;40:446–55.
Rong Y, Distelhorst CW. Bcl-2 protein family members: versatile regulators of calcium signaling in cell survival and apoptosis. Annu Rev Physiol. 2008;70:73–91.
Murakami Y, Miller JW, Vavvas DG. RIP kinase-mediated necrosis as an alternative mechanisms of photoreceptor death. Oncotarget. 2011;2:497–509.
Maduro JH, Noordhuis MG, ten Hoor KA, Pras E, Arts HJ, et al. The prognostic value of TRAIL and its death receptors in cervical cancer. Int J Radiat Oncol Biol Phys. 2009;75:203–11.
Duenas-Gonzalez A, Cetina L, Mariscal I, de la Garza J. Modern management of locally advanced cervical carcinoma. Cancer Treat Rev. 2003;29:389–99.
Moon YJ, Wang X, Morris ME. Dietary flavonoids: effects on xenobiotic and carcinogen metabolism. Toxicol In Vitro. 2006;20:187–210.
Chen YC, Shen SC, Lee WR, Lin HY, Ko CH, et al. Wogonin and fisetin induction of apoptosis through activation of caspase 3 cascade and alternative expression of p21 protein in hepatocellular carcinoma cells SK-HEP-1. Arch Toxicol. 2002;76:351–9.
Liao YC, Shih YW, Chao CH, Lee XY, Chiang TA. Involvement of the ERK signaling pathway in fisetin reduces invasion and migration in the human lung cancer cell line A549. J Agric Food Chem. 2009;57:8933–41.
Kang KA, Piao MJ, Hyun JW. Fisetin induces apoptosis in human nonsmall lung cancer cells via a mitochondria-mediated pathway. In Vitro Cell Dev Biol Anim. 2015;51:300–9.
Khan N, Adhami VM, Mukhtar H. Apoptosis by dietary agents for prevention and treatment of prostate cancer. Endocr Relat Cancer. 2010;17:R39–52.
Chou RH, Hsieh SC, Yu YL, Huang MH, Huang YC, et al. Fisetin inhibits migration and invasion of human cervical cancer cells by down-regulating urokinase plasminogen activator expression through suppressing the p38 MAPK-dependent NF-kappaB signaling pathway. PLoS One. 2013;8, e71983.
Ying TH, Yang SF, Tsai SJ, Hsieh SC, Huang YC, et al. Fisetin induces apoptosis in human cervical cancer HeLa cells through ERK1/2-mediated activation of caspase-8-/caspase-3-dependent pathway. Arch Toxicol. 2012;86:263–73.
Bagi CM, Gebhard DF, Andresen CJ. Antitumor effect of vascular endothelial growth factor inhibitor sunitinib in preclinical models of hepatocellular carcinoma. Eur J Gastroenterol Hepatol. 2012;24:563–74.
Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34.
Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–90.
Amantini C, Morelli MB, Santoni M, Soriani A, Cardinali C, et al. Sorafenib induces cathepsin B-mediated apoptosis of bladder cancer cells by regulating the Akt/PTEN pathway. The Akt inhibitor, perifosine, enhances the sorafenib-induced cytotoxicity against bladder cancer cells. Oncoscience. 2015;2:395–409.
Broecker-Preuss M, Muller S, Britten M, Worm K, Schmid KW, et al. Sorafenib inhibits intracellular signaling pathways and induces cell cycle arrest and cell death in thyroid carcinoma cells irrespective of histological origin or BRAF mutational status. BMC Cancer. 2015;15:184.
Ishijima N, Kanki K, Shimizu H, Shiota G. Activation of AMP-activated protein kinase by retinoic acid sensitizes hepatocellular carcinoma cells to apoptosis induced by sorafenib. Cancer Sci. 2015;106:567–75.
Hsieh SC, Tsai JP, Yang SF, Tang MJ, Hsieh YH. Metformin inhibits the invasion of human hepatocellular carcinoma cells and enhances the chemosensitivity to sorafenib through a downregulation of the ERK/JNK-mediated NF-kappaB-dependent pathway that reduces uPA and MMP-9 expression. Amino Acids. 2014;46:2809–22.
Teixeira SF, Alexandre de Azevedo R, Salomon MA, Jorge SD, Levy D, et al. Synergistic anti-tumor effects of the combination of a benzofuroxan derivate and sorafenib on NCI-H460 human large cell lung carcinoma cells. Biomed Pharmacother. 2014;68:1015–22.
Dornetshuber-Fleiss R, Heilos D, Mohr T, Richter L, Sussmuth RD, et al. The naturally born fusariotoxin enniatin B and sorafenib exert synergistic activity against cervical cancer in vitro and in vivo. Biochem Pharmacol. 2015;93:318–31.
Chen CM, Hsieh YH, Hwang JM, Jan HJ, Hsieh SC, et al. Fisetin suppresses ADAM9 expression and inhibits invasion of glioma cancer cells through increased phosphorylation of ERK1/2. Tumour Biol. 2015;36:3407–15.
Kotipatruni RP, Ren X, Thotala D, Jaboin JJ. NDRG4 is a novel oncogenic protein and p53 associated regulator of apoptosis in malignant meningioma cells. Oncotarget. 2015;6:17594–604.
Tsai JP, Lee CH, Ying TH, Lin CL, Hsueh JT, et al. Licochalcone A induces autophagy through PI3K/Akt/mTOR inactivation and autophagy suppression enhances Licochalcone A-induced apoptosis of human cervical cancer cells. Oncotarget. 2015;6:28851–66.
Aggarwal BB. Signalling pathways of the TNF superfamily: a double-edged sword. Nat Rev Immunol. 2003;3:745–56.
Chipuk JE, Bouchier-Hayes L, Green DR. Mitochondrial outer membrane permeabilization during apoptosis: the innocent bystander scenario. Cell Death Differ. 2006;13:1396–402.
Williams MM, Cook RS. Bcl-2 family proteins in breast development and cancer: could Mcl-1 targeting overcome therapeutic resistance? Oncotarget. 2015;6:3519–30.
Gross A, McDonnell JM, Korsmeyer SJ. BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 1999;13:1899–911.
Lopez J, Tait SW. Mitochondrial apoptosis: killing cancer using the enemy within. Br J Cancer. 2015;112:957–62.
Ren W, Qiao Z, Wang H, Zhu L, Zhang L. Flavonoids: promising anticancer agents. Med Res Rev. 2003;23:519–34.
Syed DN, Lall RK, Chamcheu JC, Haidar O, Mukhtar H. Involvement of ER stress and activation of apoptotic pathways in fisetin induced cytotoxicity in human melanoma. Arch Biochem Biophys. 2014;563:108–17.
Tripathi R, Samadder T, Gupta S, Surolia A, Shaha C. Anticancer activity of a combination of cisplatin and fisetin in embryonal carcinoma cells and xenograft tumors. Mol Cancer Ther. 2011;10:255–68.
Wu MS, Lien GS, Shen SC, Yang LY, Chen YC. N-acetyl-L-cysteine enhances fisetin-induced cytotoxicity via induction of ROS-independent apoptosis in human colonic cancer cells. Mol Carcinog. 2014;53 Suppl 1:E119–29.
Kuo HC, Lee HJ, Hu CC, Shun HI, Tseng TH. Enhancement of esculetin on Taxol-induced apoptosis in human hepatoma HepG2 cells. Toxicol Appl Pharmacol. 2006;210:55–62.
Oikonomou E, Pintzas A. The TRAIL of oncogenes to apoptosis. Biofactors. 2013;39:343–54.
Kroemer G. The proto-oncogene Bcl-2 and its role in regulating apoptosis. Nat Med. 1997;3:614–20.
Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9:47–59.
Dole M, Nunez G, Merchant AK, Maybaum J, Rode CK, et al. Bcl-2 inhibits chemotherapy-induced apoptosis in neuroblastoma. Cancer Res. 1994;54:3253–9.
Chen JJ, Mikelis CM, Zhang Y, Gutkind JS, Zhang B. TRAIL induces apoptosis in oral squamous carcinoma cells—a crosstalk with oncogenic Ras regulated cell surface expression of death receptor 5. Oncotarget. 2013;4:206–17.
Ko H, Jeong MH, Jeon H, Sung GJ, So Y, et al. Delphinidin sensitizes prostate cancer cells to TRAIL-induced apoptosis, by inducing DR5 and causing caspase-mediated HDAC3 cleavage. Oncotarget. 2015;6:9970–84.
Tanaka R, Tomosugi M, Horinaka M, Sowa Y, Sakai T. Metformin causes G1-phase arrest via down-regulation of MiR-221 and enhances TRAIL sensitivity through DR5 Up-regulation in pancreatic cancer cells. PLoS One. 2015;10, e0125779.
Zhang Y, Ge Y, Chen Y, Li Q, Chen J, et al. Cellular and molecular mechanisms of silibinin induces cell-cycle arrest and apoptosis on HeLa cells. Cell Biochem Funct. 2012;30:243–8.
Bishayee K, Ghosh S, Mukherjee A, Sadhukhan R, Mondal J, et al. Quercetin induces cytochrome-c release and ROS accumulation to promote apoptosis and arrest the cell cycle in G2/M, in cervical carcinoma: signal cascade and drug-DNA interaction. Cell Prolif. 2013;46:153–63.
Haas NB, Manola J, Ky B, Flaherty KT, Uzzo RG, et al. Effects of adjuvant Sorafenib and Sunitinib on cardiac function in renal cell carcinoma patients without overt metastases: results from ASSURE, ECOG 2805. Clin Cancer Res. 2015;21:4048–54.
Pal HC, Baxter RD, Hunt KM, Agarwal J, Elmets CA, et al. Fisetin, a phytochemical, potentiates sorafenib-induced apoptosis and abrogates tumor growth in athymic nude mice implanted with BRAF-mutated melanoma cells. Oncotarget. 2015;6:28296–311.
Pal HC, Diamond AC, Strickland LR, Kappes JC, Katiyar SK, et al.. Fisetin, a dietary flavonoid, augments the anti-invasive and anti-metastatic potential of sorafenib in melanoma. Oncotarget. 2015. doi:10.18632/oncotarget.6237
Kiprianova I, Remy J, Milosch N, Mohrenz IV, Seifert V, et al. Sorafenib sensitizes glioma cells to the BH3 mimetic ABT-737 by targeting MCL1 in a STAT3-dependent manner. Neoplasia. 2015;17:564–73.
Acknowledgments
This work was supported by grants from Chang Bing Show Chwan Memorial Hospital (RD104025).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of interest
None
Additional information
Ming-Te Lin and Chia-Liang Lin contributed equally to this work.
Rights and permissions
About this article
Cite this article
Lin, MT., Lin, CL., Lin, TY. et al. Synergistic effect of fisetin combined with sorafenib in human cervical cancer HeLa cells through activation of death receptor-5 mediated caspase-8/caspase-3 and the mitochondria-dependent apoptotic pathway. Tumor Biol. 37, 6987–6996 (2016). https://doi.org/10.1007/s13277-015-4526-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-015-4526-4