Abstract
Oral cancer incidence of 77,003 poses a major health concern in India, with 5–10 % tobacco habitués developing oral cancer. The current study examined the role of specific genomic variants in oral cancer. We examined five genomic variants represented as single nucleotide polymorphisms (SNPs) in genes associated with cell proliferation and cellular invasion. The SNPs rs2124437 (RASGRP3), rs1335022 (GRIK2), rs4512367 (PREX2), rs4748011 (CCDC3), and rs1435218 (LNX1) were analyzed in 500 histopathologically confirmed oral cancers and 500 healthy controls with a minimum of 10 years of tobacco usage. Allelic discrimination real-time PCR SYBR Green assay was used. The genotypic and allelic frequencies between cases and controls were analyzed using SPSS software (version 19) and odds ratio (OR) using Hutchon.net, indicating increased risk to oral cancers. A significant association of the SNPs in oral cancer was observed in RASGRP3 AA (rs2124437) (p < 0.000, OR 1.34, 95 % confidence interval (CI) 1.01–1.76), GRIK2 TT (rs1335022) (p = 0.008, OR 1.58, 95 % CI 1.23–2.03), PREX2 CC (p = 0.008, OR 1.56, 95 % CI 1.15–2.1), and TT (p < 0.000, OR 2.77, 1.68–4.57) genotypes, whereas the heterozygous genotypes showed higher frequencies in controls, i.e., GRIK2 CT (rs1335022) (p = 0.029, OR 0.68, 95 % CI 0.53–0.87) and PREX2 CT (p = 0.004, OR 0.49, 95 % CI 0.37–0.64), indicating protection. Coinheritance of the SNPs was associated with further increase in the risk. Thus, the SNP genotypes in the three genes, present singly or as a coinherited panel constituted “Predictive Biomarkers” indicating increased risk of oral cancer in tobacco habitués.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Globally, oral cancer is the 13th most common cancer with an incidence of 300,373 and a mortality rate of 145, 238 [1]. In India, oral cancer is a major health problem, being the most common cancer in males and fourth most common cancer in females, and contributes 26 % of the global oral cancer burden with an annual incidence of 77,003 new cases [1]. The high incidence is attributed to the highly prevalent chewing tobacco habit, compounded by smoking in a majority of the tobacco chewers. The additional risk factors in oral cancer are alcohol and high-risk oncogenic virus HPV types 16/18 [2]. Oral cancers include cancer of buccal mucosa, tongue, palate, floor of the mouth, and gingiva, with lip cancer being considered as a distinct cancer. Despite easy accessibility of the oral cavity and advances in treatment protocols of surgery, chemotherapy, radiotherapy, and targeted biological therapy, prognosis of cancer in Indian population is poor, partly due to late diagnosis in advanced stage. An increasing trend is observed in younger age groups of 20–45 years with a 60 % increase in oral cancer cases in <40 years of age in the past 25 years [3]. The average 5-year survival of oral cancer patients is 40 %, and the transformation of premalignant to a malignant phenotype is in 3–8.1 % cases [4].
Besides the well accepted high-risk factors of tobacco, alcohol, and HPV 16/18, individual genomic variants represented as single nucleotide polymorphisms (SNPs) and epigenetic profile of the genome contribute to the risk of oral cancer [5]. SNPs are low penetrance genes and observed in >1 % of ethnic populations [6]. SNPs may be present in the exonic regions of the genome and accessible for transcription or the intronic non-coding regions which directly or indirectly affects gene expression. The SNPs in the exonic regions are either non-synonymous resulting in a substitution of amino acid or synonymous with no amino acid alterations. The SNPs in the intronic regions may alter the conformation of the DNA molecule resulting in increase or decrease of Gibbs free energy and subsequently affect stability of the molecule and impact DNA polymerase processivity, transcription factor binding, and nucleosome assembly function [7]. SNPs are generated due to substitution mutations which are said to occur at the rate of 10−8 per base pairs. The SNPs may result in heterozygous or homozygous genotypes with the SNP present singly or in both alleles, with the original allelotype being wild type. The genotypic and allelotypic frequencies differentially increased or decreased in a population in association with various diseases make SNPs potential markers for association studies.
BRCA1 and BRCA2 are well-defined germline mutations indicating increase risk in early onset breast and ovarian cancer [8]. Pilato and colleagues reported several SNPs with the same frequency as the BRCA mutations in individuals. K118R was seen to be the most common SNP in the mutated families (p = 0.004) and various haplotypes associated with the mutations. The study showed the importance of analyzing polymorphisms to define hereditary risk [9]. The study of SNPs yields insight into exposure and cancer and lays the foundation for preventive strategies related to lifestyle [10].
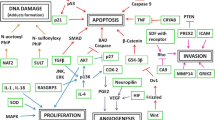
Deregulation of genes by somatic mutations or functioning genomic SNPs, in signal transduction pathways may lead to increased cell proliferation, angiogenesis, migration, and resistance to apoptosis [11]. Whole genomic association studies have demonstrated association of SNPs in several cancers including lung, breast, colorectal cancer [12], and oral cancer [5]. The microarray studies are often reported in smaller sample sizes, and validation in samples is required. Hence, the focus of the current paper was to investigate SNPs in signal transduction genes RAS guanyl releasing protein 3 (RASGRP3), phosphatidylinositol-3,4,5-trisphosphate-dependent Rac exchange factor 2 (PREX2), glutamate receptor, ionotropic, kainate 2 (GRIK2), ligand of Numb protein X 1, E3 ubiquitin protein ligase (LNX1), and coiled-coil domain containing 3 (CCDC3) in oral cancer based on a GWAS study [5]. RASGRP3 activates the Ras signaling cascade. RasGRPs are upstream activators of Ras isoforms and are regulated by diacylglycerol binding to C1 domain. These molecules have the ability to activate multiple domains together and link downstream Ras family members to growth factors and cytokines [13]. PREX2 links Rac activation and P-13 kinase pathway, GPCRs, RTKs, and Rac and Cdc42, leading to increased cell migration and reactive oxygen species [14]. The enhanced expression of PREX2 inhibits tumor suppressor PTEN and activates AKT pathway, enhancing proliferation and migration [15]. GRIK2, a metabotropic glutamate receptor, processes excitatory neurotransmission signals and is deregulated in gastric cancer [16] and esophageal cancer [17]. Wu and colleagues reported hypermethylation of GRIK2 and consequent silencing of the gene showed increased colony formation and migration [16]. LNX1 contains PDZ domains which interact with Numb protein and RhoC, regulating transcription of activator protein 1 (AP-1) which has a critical role in several cancers [18]. CCDC3 is associated with suppressing TNF-α in inflammation [19] and shows increased expression in differentiated adipocytes (Fig. 1).
Functional association of genes containing the SNPs in proliferation and migration of cancer cells. The figure highlights PREX2 activities through inhibition of PTEN and activation of mTOR pathway, GRIK2 association with migration, CCDC3 inhibition of TNF-α, RASGRP3 activation of Rac1 and PI3K, and LNX1 activation of AP-1
The aim of the current study is to examine specific SNPs in RASGRP3 (rs2124437), GRIK2 (rs1335022), PREX2 (rs4512367), CCDC3 (rs4748011), and LNX1 (rs1435218) in oral cancer patients and normal healthy controls with long-term tobacco habits and no personal or family history of cancer. The frequency distribution of the genotypes in the oral cancer patients and controls will be analyzed for association with risk to oral cancer.
Material and methods
Study subjects
The study included 500 histopathologically confirmed oral squamous cell carcinoma patients admitted to Prince Aly Khan Hospital, Mumbai, India, and 500 long-term tobacco users (LTTUs) with a minimum of 10 years of tobacco habit as controls with no history of cancer. The controls were obtained from cancer screening camps conducted by Cancer Patients Aid Association, Mumbai, India. The age, gender, tobacco habits, and clinicopathological profile of patients were recorded.
The study was approved by the Institute Ethics Committees of NMIMS (deemed-to-be) University, Mumbai; Cancer Patients Aid Association, Mumbai; and Prince Aly Khan Hospital, Mumbai. All subjects gave written informed consent for voluntary participation in the study.
DNA extraction
DNA was extracted from peripheral blood samples, using the PureLink DNA extraction kit as per the manufacturer’s instructions (Invitrogen, CA, USA). The DNA quality and quantity was checked using NanoDrop Spectrophotometer 2000 (Thermo Scientific, Walthman, USA).
SNP genotyping
SNPs were determined using Allelic Discrimination Real-Time PCR assay with SYBR green chemistry on ABI StepOne Plus Real-Time PCR (Applied Biosystems, Foster City, CA, USA). The melt curves of the SNPs were analyzed using StepOne software v2.3. Amplification and sequencing primers were designed using AlleleID software. The primers purchased from Eurofins (Luxemborg City, Luxemborg) included specific primers for the wild-type (WT) and SNP alleles and a common primer for both the alleles. A GC clamp was added to either the forward or reverse primer to ensure a melt temperature difference between wild-type and SNP PCR products. The primer sequences are listed in Supporting Information Table S1.
The final volume of the reactions was 10 μl, containing 5 μl SYBR green Master Mix, 1.5 μl DNA (30–50 ng), 1.5 μl primers (5 pmol each), and Milli-Q water. The amplification was performed with an initial denaturation 95 °C for 2 min, followed by 40 cycles at 95 °C for 15 s, annealing 55–58 °C for 30 s, and extension at 72 °C for 1 min. The PCR products varied from 89 to 168 bp and were subjected to melt curve stage at 95 °C for 15 s, 60 °C for 1 min, and 95 °C for 15 s.
Representative samples were subjected to nucleotide sequencing for confirmation of the alleles. The sequencing primers are listed in Supporting Information Table S1. AmpliTaq Gold 360 Master Mix was purchased from Applied Biosystems (Foster City, CA, USA). PCR was performed using ABI 2720 thermal cycler (Applied Biosystems, Foster City, CA, USA). The PCR products of 430 to 544 bp were sequenced on automated genetic analyzer ABI 3730xl (Foster City, CA, USA) at SciGenom Adyar, Chennai, India.
Statistical analysis
Hardy-Weinberg equilibrium was checked using SNPstats software [20]. Genotype and allelotype frequencies in oral cancer and controls and their associations were analyzed by chi-square test using SPSS software (version 19). p values less than 0.05 were considered statistically significant. The odds ratio (OR) and 95 % confidence intervals were determined using Hutchon.net software [21].
Results
Clinicopathological data of oral cancer patients
Five hundred oral cancer patients and 500 LTTU with no history of oral cancer as controls were included in the study (Table 1). The oral cancer group comprised 462 (92.4 %) males and 38 (7.6 %) females, aged 25 to 80 years, and the control group comprised 414 (82.8 %) males and 86 (17.2 %) females aged 20 to 82 years. The average duration of tobacco chewing in the oral cancer group was 14.2 years (median 10 years), and in the control group, average duration was 18.1 years (median 15 years). The major cancer sites included buccal mucosa (58.8 %), tongue (27.6 %), alveolus, and gingiva (8.6 %), and 3.4 % of cancers were retromolar trigone, labial mucosa, and palate. Majority of the oral cancers were moderately differentiated (89.2 %), with 7.2 % well differentiated and 3.6 % poorly differentiated. The patients were diagnosed in early stages I and II (50.8 %) and advanced stages III and IV (49.2 %) (Table 1).
SNP genotyping
The wild-type and SNP genotypes were determined by melt curve peaks at specific temperatures indicating homozygous WT, heterozygous WT/SNP, and homozygous SNP. The observed temperatures are as follows: PREX2 rs4512367 WT, 78.7 °C and SNP, 74.24 °C; RASGRP3 rs2124437 WT, 78.5 °C and SNP, 75.29 °C; GRIK2 rs1335022 WT, 81.25 °C and SNP, 78.4 °C; CCDC3 rs4748011 WT, 82.15 °C and SNP, 85.27 °C; and LNX1 rs1435218 WT, 78.71 °C and SNP, 74.96 °C. The melt curve data for representative PREX2 genotype is depicted in Fig. 2. The sample alleles were confirmed by nucleotide sequencing as shown for PREX2 in the electropherogram (Fig. 3).
The frequencies of the various alleles and genotypes in the oral cancer and control groups in the Indian population are tabulated (Tables 2 and 3). Evidence for departure from Hardy-Weinberg equilibrium (HWE) among the Indian group was observed for three SNPs, rs2124437, rs4512367, rs4748011, whereas rs1335022 and rs1435218 followed HWE. The minor allele frequency (MAF) in oral cancer cases and controls was significantly different for rs1335022 (p = 0.008; OR 0.73 (95 % confidence interval (CI) 0.60–0.88)) (Table 2).
Genotyping indicating homozygous wild-type, heterozygous, and homozygous SNP genotypes was performed using allele discrimination real-time PCR with SYBR Green chemistry. A significant difference between oral cancer and control groups was observed in the homozygous SNP genotypes in rs2124437 (RASGRP3) (p < 0.000; OR 1.34 (95 % CI 1.01–1.76)), rs1335022 (GRIK2) (p = 0.008; OR 1.58 (95 % CI 1.23–2.03)), and rs4512367 (PREX2) (p < 0.000; OR 2.77 (95 % CI 1.68–4.57)) (Table 3). The homozygous genotype CC (WT) in rs4512367 (PREX2) (p = 0.008; OR 1.56 (95 % CI 1.15–2.10)) showed a significant difference between oral cancer and control groups (Table 3). The heterozygous genotypes rs1335022 and rs4512367 demonstrated a significantly higher frequency in control group as compared to oral cancer cases (Table 3). The genotypes for rs4748011 (CCDC3) and rs1435218 (LNX1) did not demonstrate a significant difference (Table 3).
Coinheritance of risk genotypes in oral cancers
Genotypes for rs2124437, rs4512367, and rs1335022 showed significant differences between oral cancer and control groups. The coinheritance of homozygous SNP rs2124437 (AA) and rs4512367 (TT) genotypes showed increased risk with high OR 18.63, albeit large confidence interval (95 % CI 2.48–140.13). Coinheritance of rs1335022 TT, rs2124437 AA, and rs4512367 TT genotypes showed an increased risk (p = 0.007; OR 10.18 (95 % CI 1.29–79.86)) (Table 4).
Discussion
Oral cancer is a complex disease with genetic, epigenetic, and well-established environmental factors contributing to development and progression of cancer. However, only 5–10 % of high-risk individuals with tobacco-chewing habits develop oral cancer. Thus, inherent genomic variants may contribute to the risk or susceptibility in oral cancer. The genomic variants represented as SNPs are less deterministic and more probabilistic besides being low-penetrance variants, and hence, a single SNP may increase disease risk [22], whereas a panel of high-risk SNPs may provide a better estimate of the risk ratio with increased odds risk in oral cancer patients. Our study investigated a panel of five SNPs in genes associated with cellular signal transduction pathways leading to proliferation and migration in cancer [14, 16, 23]. Allelic discrimination real-time PCR assay with SYBR green dye was used to determine the SNP distribution in oral cancer patients (n = 500) and controls (n = 500) constituting healthy LTTUs with no history of oral cancer. The SNPs were identified in an earlier genome-wide association study [5].
Our results indicated prevalence of five SNP alleles and genotypes in genes associated with signal transduction in an Indian population group (n = 1000) comprising oral cancer patients and healthy controls (Table 3). The SNPs rs2124437, rs4512367, and rs4748011 showed HWE deviation, which may be due to the sample size, as a large sample is usually required to conform to the “infinity population” requirement for the locus to exhibit HWE [24]. The allelic and genotypic distribution was compared to various populations including Indians, Han Chinese, Japanese, African tribals, and Central Europeans in the HapMap database [25], and generally, distinct distribution of the alleles and genotypes was observed, which may be due to the different ethnicities examined and/or due to the differences in the sample sizes.
We analyzed the SNPs with respect to the allelotypes and genotypes in oral cancers and controls to indicate preferential distribution in the two groups and consequent increased susceptibility to oral cancer. The rs1335022 MAF was significantly increased in controls (p = 0.008, OR 0.73), thereby indicating a decreased risk to oral cancer. Significant allelic differences were not observed in oral cancer and control groups for the other SNPs (Table 3). The homozygous SNP genotypes in rs2124437 (AA), rs1335022 (TT), and rs4512367 (TT) and WT (CC) showed higher frequencies in oral cancer cases indicating increased risk to the cancer (Table 3). Besides PREX2, SNP rs4512367 (CC) was significantly increased in advanced stages III and IV (p = 0.046) implying a role in progression of the cancer, whereas the heterozygous genotypes rs1335022 (CT) and rs4512367 (CT) showed higher frequencies in the control group indicating decreased risk to developing oral cancer (Table 3). The coinheritance of a panel of high-risk SNPs further increased risk of oral cancer (Table 4). Our SNPs were intronic SNPs, which may be associated with alterations in the three-dimensional conformation of the DNA molecule, affecting Gibbs free energy and thus the stability of the molecule. The shift in Gibbs free energy will in turn affect DNA polymerase processivity and several functions in the cell [7].
The five associated genes RASGRP3, GRIK2, PREX2, CCDC3, and LNX1 have been implicated in various cancers. RASGRP3 is deregulated in prostate cancer [26], melanoma [13], and leukemia [27], enhancing cell proliferation and anchorage independent growth. GRIK2 functions as a tumor suppressor gene in gastric cancer and is associated with cell migration [16]. A recent study analyzing an alternative GRIK2 SNP rs6570989 reported a significant association with nicotine dependence [28], a high-risk factor in oral cancer. The PREX2 gene is deregulated in lung cancer [29] and breast cancer [15]. On the other hand, significant association of PREX2 SNPs in lung adenocarcinoma was not observed [29].
Several SNPs in various genes have been associated with increased risk in oral cancer, including proliferation-associated gene lL-4, with SNP rs2243250 showing an OR of 1.7 and rs2070874 OR of 1.53 [30, 31]. Besides, SNPs in interleukins IL-6, IL-10, IL-23R, IL-alpha, IL-beta, and IL-18 showed increased risk of oral cancer with OR ranging from 1.97 to 3.27 [32]. SNP rs1982073 in TGF-B1 showed increased OR of 2.823 and 11.1 (rs1800471) [33, 34]. The SNP rs5498 in ICAM-1 associated with invasion and migration of cancer cells showed an increased risk of oral cancer (OR 1.69) [35]. Similarly, various SNPs in different DNA repair and xenobiotic metabolism genes were associated with increased risk. The SNPs were analyzed in hMLH1 (OR 2.36) [36] and GSTM1 (OR 1.63) [37–40] and associated with increased susceptibility to oral cancer.
Our study emphasizes that genomic variants represented as SNPs which abound in the human genome and reflect the genomic constitution with an estimated ten million SNPs in our genome play an important role in oral carcinogenesis. The specific SNPs investigated in the current study contribute to increased susceptibility in oral cancer, and a panel of SNPs may reflect “Predictive Biomarkers” to screen high-risk individuals prone to oral cancer with tobacco habits, thus providing an objective unbiased test assay to assess oral cancer risk in individuals.
Abbreviations
- SNP:
-
Single nucleotide polymorphisms
- WT:
-
Wild type
- HWE:
-
Hardy-Weinberg equilibrium
- OR:
-
Odds ratio
- CI:
-
Confidence interval
- bp:
-
Base pair
References
Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer. 2013;49:1374–403.
D’Costa J, Saranath D, Dedhia P, Sanghvi V, Mehta A. Detection of HPV-16 genome in human oral cancers and potentially malignant lesions from India. Oral Oncol. 1998.
Chakrabarti S, Multani S, Dabholkar J, Saranath D. Whole genome expression profiling in chewing-tobacco-associated oral cancers: a pilot study. Med Oncol. 2015;32(3):60.
Scheifele C, Reichart PA. Is there a natural limit of the transformation rate of oral leukoplakia. Oral Oncol. 2003;39(5):470–5.
Bhatnagar R, Dabholkar J, Saranath D. Genome-wide disease association study in chewing tobacco associated oral cancers. Oral Oncol. 2012;48(9):831–5.
Chien MH, Yang JS, Chu YH, Lin CH, Wei LH, Yang SF, et al. Impacts of CA9 gene polymorphisms and environmental factors on oral-cancer susceptibility and clinicopathologic characteristics in Taiwan. PLoS One. 2012;7(12):5–12.
Ignatova Z, Martínez-Pérez I, Zimmermann K-H. DNA computing models. Springer Science & Business Media, 2008; 288.
Satagopan JM, Verbel DA, Venkatraman ES, Offit KE, Colin B, Verbel DA, et al. Two-stage designs for gene-disease association studies. 2015;58(1):163–70.
Pilato B, Martinucci M, Danza K, Pinto R, Petriella D, Lacalamita R, et al. Mutations and polymorphic BRCA variants transmission in breast cancer familial members. Breast Cancer Res Treat. 2011;125(3):651–7.
Erichsen HC, Chanock SJ. SNPs in cancer research and treatment. Br J Cancer. 2004;90(4):747–51.
Sever R, Brugge JS. Signal transduction in cancer. Cold Spring Harb Perspect Med. 2015;5(4):a006098.
Easton DF, Eeles R a. Genome-wide association studies in cancer. Hum Mol Genet. 2008;17(R2):R109–15.
Yang D, Tao J, Li L, Kedei N, Tóth ZE, Czap A, et al. RasGRP3, a Ras activator, contributes to signaling and the tumorigenic phenotype in human melanoma. Oncogene. 2011;30(45):4590–600.
Rosenfeldt H, Vázquez-Prado J, Gutkind JS. P-REX2, a novel PI-3-kinase sensitive Rac exchange factor. FEBS Lett. 2004;572(1–3):167–71.
Wertheimer E, Gutierrez-Uzquiza A, Rosemblit C, Lopez-Haber C, Sosa MS, Kazanietz MG. Rac signaling in breast cancer: a tale of GEFs and GAPs. Cell Signal. 2012;24(2):353–62.
Wu CS, Lu YJ, Li HP, Hsueh C, Lu CY, Leu YW, et al. Glutamate receptor, ionotropic, kainate 2 silencing by DNA hypermethylation possesses tumor suppressor function in gastric cancer. Int J Cancer. 2010;126(11):2542–52.
Kim MS, Yamashita K, Baek JH, Park HL, Carvalho AL, Osada M, et al. N-methyl-d-aspartate receptor type 2B is epigenetically inactivated and exhibits tumor-suppressive activity in human esophageal cancer. Cancer Res. 2006;66(7):3409–18.
Zheng D, Sun Y, Gu S, Ji C, Zhao W, Xie Y, et al. LNX (ligand of Numb-protein X) interacts with RhoC, both of which regulate AP-1-mediated transcriptional activation. Mol Biol Rep. 2010;37(5):2431–7.
Azad AK, Chakrabarti S, Xu Z, Davidge ST, Fu Y. Coiled-coil domain containing 3 (CCDC3) represses tumor necrosis factor-α/nuclear factor κB-induced endothelial inflammation. Cell Signal. 2014;26(12):2793–800.
Xavier S, Elisabet G, Joan V, Raquel I, and Victor M. Bioinformatics. 2006; 22: 1928–1929.
Bland M, Altman D. Statistics notes: the odds ratio. BMJ. 2000;320:1468.
Hirschhorn JN, Lohmueller K, Byrne E, Hirschhorn K. A comprehensive review of genetic association studies. Genet Med. 2002;4(2):45–61.
Berger MF, Hodis E, Heffernan TP, Deribe YL, Lawrence MS, Protopopov A, et al. Melanoma genome sequencing reveals frequent PREX2 mutations. Nature. 2012;485(7399):502–6.
Salanti G, Amountza G, Ntzani EE, Ioannidis JP a. Hardy-Weinberg equilibrium in genetic association studies: an empirical evaluation of reporting, deviations, and power. Eur J Hum Genet. 2005;13(7):840–8.
National Center for biotechnology information. http://www.ncbi.nlm.nih.gov/snp (Accessed 26th September, 2015)
Yang D, Kedei N, Li L, Tao J, Velasquez JF, Michalowski AM, et al. RasGRP3 contributes to formation and maintenance of the prostate cancer phenotype. Cancer Res. 2010;70(20):7905–17.
Komkov AY, Maschan MA, Shvets VI, Lebedev YB. Functional analysis of polymorphic insertions of Alu retroelements in acute lymphoblastic leukemia patients. Russ J Bioorganic Chem. 2012;38(3):306–18.
Li M, Gardiner JC, Breslau N, Anthony JC, Lu Q. A non-parametric approach for detecting gene-gene interactions associated with age-at-onset outcomes. BMC Genet. 2014;15(1):79.
Suzuki A, Mimaki S, Yamane Y, Kawase A, Matsushima K, Suzuki M, et al. Identification and characterization of cancer mutations in Japanese lung adenocarcinoma without sequencing of normal tissue counterparts. PLoS One. 2013;8(9):1–11.
Tsai M-H, Chen W-C, Tsai C-H, Hang L-W, Tsai F-J. Interleukin-4 gene, but not the interleukin-1 beta gene polymorphism, is associated with oral cancer. J Clin Lab Anal. 2005;19(3):93–8.
Vairaktaris E, Yannopoulos A, Vassiliou S, Serefoglou Z, Vylliotis A, Nkenke E, et al. Strong association of interleukin-4 (−590 C/T) polymorphism with increased risk for oral squamous cell carcinoma in Europeans. Oral Surg Oral Med Oral Pathol Oral Radiol Endodontol. 2007;104(6):796–802.
Yang L, Zhu X, Liang X, Ling Z, Li R. Association of IL-8-251A > T polymorphisms with oral cancer risk: evidences from a meta-analysis. Tumor Biol. 2014;35(9):9211–8.
Gaur P, Mittal M, Mohanti BK, Das SN. Functional genetic variants of TGF-β1 and risk of tobacco-related oral carcinoma in high-risk Asian Indians. Oral Oncol. 2011;47(12):1117–21.
Hsu H, Yang Y, Shieh T, Chen C. TGF-β1 and IL-10 single nucleotide polymorphisms as risk factors for oral cancer in Taiwanese. Kaohsiung J Med Sci. 2014;100:1–7.
Lin CW, Chuang CY, Tang CH, Chang JL, Lee LM, Lee WJ, et al. Combined effects of ICAM-1 single-nucleotide polymorphisms and environmental carcinogens on oral cancer susceptibility and clinicopathologic development. PLoS One. 2013;8(9):1–8.
Jha R, Gaur P, Sharma SC, Das SN. Single nucleotide polymorphism in hMLH1 promoter and risk of tobacco-related oral carcinoma in high-risk Asian Indians. Gene. 2013;526(2):223–7.
Anantharaman D, Chaubal PM, Kannan S, Bhisey R a, Mahimkar MB. Susceptibility to oral cancer by genetic polymorphisms at CYP1A1, GSTM1 and GSTT1 loci among Indians: tobacco exposure as a risk modulator. Carcinogenesis. 2007;28(7):1455–62.
Shukla D, Kale AD, Hallikerimath S, Vivekanandhan S, Venkatakanthaiah Y. Genetic polymorphism of drug metabolizing enzymes (GSTM1 and CYP1A1) as risk factors for oral premalignant lesions and oral cancer. Biomed Pap. 2012;156(3):253–9.
Masood N, Kayani MA, Malik FA, Mahjabeen I, Baig RM, Faryal R. Genetic variation in carcinogen metabolizing genes associated with oral cancer in Pakistani population. Asian Pac J Cancer Prev. 2011;12(2):491–5.
Singh R, Haridas N, Shah F, Patel J, Shukla S, Patel P. Gene polymorphisms, tobacco exposure and oral cancer susceptibility: a study from Gujarat, West India. Oral Dis. 2014;20(1):84–93.
Acknowledgments
The authors gratefully acknowledge the support of Cancer Patients Aids Association (CPAA), Foundation of Medical Research (FMR), and Balabai Nanavati Hospital, Mumbai, India, for the project.
Role of the funding source
Funding was provided by the affiliated university for procurement of reagents for the project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None
Ethics approval and consent to participate
The study was approved by the Institute Ethics Committees of NMIMS (deemed-to-be) University, Mumbai; Cancer Patients Aid Association, Mumbai; and Prince Aly Khan Hospital, Mumbai. All subjects gave written informed consent for voluntary participation in the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
Primer sequences for allelic discrimination PCR and sequencing primers. (DOCX 14 kb)
Rights and permissions
About this article
Cite this article
Multani, S., Pradhan, S. & Saranath, D. Gene polymorphisms and oral cancer risk in tobacco habitués. Tumor Biol. 37, 6169–6176 (2016). https://doi.org/10.1007/s13277-015-4448-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-015-4448-1