Abstract
Pancreatic cancer is one of the most dangerous cancers and is associated with a grave prognosis. Despite increased knowledge of the complex signaling networks responsible for progression of pancreatic cancer, many challenging therapies have fallen short of expectations. In this study, we examined the anti-migratory effect of quercetin 3-O-glucoside in epidermal growth factor–induced cell migration by inhibiting EGF receptor (EGFR) signaling in several human pancreatic cancer cell lines. Treatment with quercetin, quercetin 3-O-glucoside, and quercetin 7-O-glucoside differentially suppressed epidermal growth factor–induced migration activity of human pancreatic cancer cells. In particular, quercetin 3-O-glucoside strongly inhibited the infiltration activity of pancreatic cancer cells in a dose-dependent manner. Furthermore, quercetin 3-O-glucoside exerted the anti-migratory effect even at a relatively low dose compared with other forms of quercetin. The anti-tumor effects of quercetin 3-O-glucoside were mediated by selectively inhibiting the EGFR-mediated FAK, AKT, MEK1/2, and ERK1/2 signaling pathway. Combinatorial treatment with quercetin 3-O-glucoside plus gemcitabine showed the synergistic anti-migratory effect on epidermal growth factor–induced cell migration in human pancreatic cancer cell lines. These results suggest that quercetin 3-O-glucoside has potential for anti-metastatic therapy in human pancreatic cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pancreatic cancer has the worst prognosis of all cancers and is the fourth leading cause of cancer mortality worldwide [1]. Most patients diagnosed with pancreatic cancer die, and the overall 5-year survival rate is <3 % [2]. The lethality of pancreatic cancer is caused by its aggressive biological features, including potential for metastasis and resistance to currently available anti-cancer drugs and/or radiation therapy. In particular, local metastasis to the surrounding structures makes surgical resection challenging because of unclear margins. Moreover, ~60 % of patients with this tumor have metastatic cancers at the time of diagnosis [3–5]. However, the molecular mechanisms responsible for these characteristics are unclear, and additional studies are needed to improve the treatment outcomes in patients with pancreatic cancer.
Natural products are an abundant source of active therapeutic reagents that exert anti-viral, anti-microbial, anti-inflammatory, anti-allergic, anti-thrombotic, anti-mutagenic, and anti-cancer effects on various cell types [6]. Intake of fruits and vegetables, which contain high amounts of phytochemicals, is associated with decreased risk of various cancers. Several different mechanisms have been suggested for phytochemicals, such as inhibiting oxidative DNA damage, altering gene expression, and modifying signaling pathways [7, 8]. One of the best known phytochemical groups is the flavonoids, which function as antioxidant compounds [9]. Among these, quercetin possesses potential chemopreventive effects via anti-oxidative activity and also modulates various proteins that are involved in carcinogenesis and signal transduction pathways [10–12].
O-Glucosidation of quercetin is a naturally occurring process, and quercetin glucosides exert high antioxidant effects in vitro [13]. However, little information is available regarding the functional effects of quercetin glucosides on tumor cells.
Epidermal growth factor receptor (EGFR) is a receptor tyrosine kinase that is activated when bound to EGF. Over-activation of EGFR is observed in various tumors and can be caused by gene over-expression, mutations that affect ligand-receptor interactions, and ligand-independent activation [14, 15].
In the present study, we investigated the functional effects of quercetin and its derivatives on cell migration and EFGR signaling in human pancreatic cancer cell lines. Our results suggest that quercetin 3-O-glucoside may block the infiltration of pancreatic cancer cells into surrounding structures.
Materials and methods
Cell culture and reagents
CFPAC-1 cells were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA), and SNU-213 and Panc-1 cells were obtained from the Korean Cell Line Bank (Seoul, Korea). The cells were cultured in DMEM (CFPAC-1 and Panc-1) or RPMI-1640 (SNU-213) medium supplemented with 10 % fetal bovine serum (Gibco-BRL, Gaithersburg, MD, USA), and 1 × 105 U/L penicilin-100 mg/L streptomycin (Invitrogen, Carlsbad, CA, USA) at 37 °C in a humidified atmosphere containing 5 % CO2. Human umbilical vein endothelial cells (HUVECs) were purchased from the ATCC (Manassa, VA, USA). HUVECs were grown in EGM-2 Bulletkit medium (Lonza Biologics, Hopkinton, MA, USA) at 37 °C in a humidified atmosphere containing 5 % CO2. All experiments were performed using HUVECs within three to six passages. Inhibitors of MEK1/2 (U0126), phosphatidylinositol 3-kinase (PI3K) (LY294002), and focal adhesion kinase (FAK) (SC203950) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Antibodies against phospho-EGFR (Tyr1068), EGFR, phospho-FAK (Tyr397), FAK, phospho-AKT (Ser473), AKT, phospho-MEK1/2 (S217/221), MEK1/2, phospho-ERK (Thr202/Tyr204), ERK, and GAPDH were obtained from Cell Signaling Technology (Beverly, MA, USA). Quercetin and quercetin-3-O glucoside were purchased from Sigma-Aldrich (St. Louis, MO, USA). Quercetin-7-O glucoside was obtained from Chem Faces (Dongfeng Rd, Wuhan, China). Recombinant EGF and platelet-derived growth factor (PDGF) were purchased from R&D Systems (Minneapolis, MN, USA).
Measurement of cell viability
Cell viability was determined using a WST-1 (2-(4-iodophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium) solution (Boehringer Mannheim, Mannheim, Germany) as described previously [16]. Briefly, CFPAC-1, Panc-1, and SNU-213 cells (5 × 103/well) were seeded in 96-well plates (Nunc, Roskilde, Denmark). The cells were maintained in culture medium for 24 h and treated with various doses of quercetin, quercetin-3-O-glucoside, and quercetin-7-O-glucoside. The cells were incubated at 37 °C for an additional 72 h. WST-1 solution (10 μL) was added to each well, and absorbance was measured at 450 nm using a microplate reader (Bio-Rad, Richmond, CA, USA) after a 10-min incubation at room temperature.
Migration assay
Cell migration assays were performed using 8.0-μm-pore-size Transwell permeable supports (Corning Costar, Lowell, MA, USA). Polycarbonate filters were pre-coated with 10 mg/L fibronectin (Sigma-Aldrich) in phosphate-buffered saline (PBS) for 30 min at room temperature. The lower chamber was filled with 500 μL of 10 % serum containing RPMI-1640 medium. After a 15-h starvation in RPMI-1640 medium with 0.2 % fetal bovine serum, cells (5 × 104 cells/well) were suspended in 100-μL serum-free medium and loaded into each of the upper chambers. The cells were then incubated for 6 h at 37 °C, after which cells on the upper surface of the filter were removed with a cotton swab. The filters were fixed and stained with 1 % crystal violet solution. The absorbance of the eluted dye was measured at 560 nm in an enzyme-linked immunosorbent assay reader (Bio-Rad, Richmond, CA, USA).
Western blot analysis
Western blotting was performed as previously described to evaluate phosphorylation of various molecules [17]. In brief, starved SNU-213 cells were stimulated with EGF for the indicated times and washed with pre-chilled PBS. For whole cell lysates, cells were lysed in M-PER lysis buffer (Thermo Scientific, Bonn, Germany) with protease and phosphatase inhibitors. Total protein quantity was determined by the BCA quantification method (Bio-Rad). Cell lysates were separated on sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to a nitrocellulose membrane (Amersham Bioscience, Little Chalfont, Buckinghamshire, UK). The membranes were blocked with 5 % bovine serum albumin and 2 % Tween in TBS. Antibodies specific for EGFR, p-FAK (Tyr397), p-FAK (Tyr576/577), FAK, p-AKT (Ser473), AKT, p-MEK1/2 (Ser217/221), MEK1/2, p-ERK (Thr202/204), ERK, and GAPDH were diluted in blocking buffer at 1:1000 and incubated overnight at 4 °C. Secondary antibodies included horseradish-peroxidase-conjugated donkey anti-rabbit or donkey anti-mouse antibodies (Santa Cruz Biotechnology) in conjunction with a Western blot detection reagent (iNtRON, Seoul, Korea) to visualize bands using X-ray film. The bands were measured by densitometry using ImageJ software (National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
Data are presented as means ± standard deviations. Student’s t test was used to compare two independent samples. Groups were also compared using a one-way analysis of variance with Tukey’s post hoc test for significant main effects (SPSS 12.0 K for Windows; SPSS, Inc., Chicago, IL, USA).
Results
Treatment with quercetin and its derivatives decreases EGF-induced migration of human pancreatic cancer cells
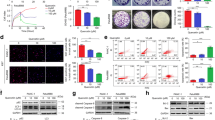
The CFPAC-1, Panc-1, and SNU-213 human pancreatic cancer cell lines were incubated in the absence or presence of different doses of quercetin and its derivatives, and cell viability was assessed. Treatment with quercetin and its derivatives had no significant affect on viability of CFPAC-1, Panc-1, or SNU-213 cells (Fig. 1a, b, c). Quercetin and its derivatives also had no effect on the migratory activity of CFPAC-1, Panc-1, and SNU-213 cells (Fig. 1d).
Sensitivity of human pancreatic cancer cells to quercetin and its derivatives: a CFPAC-1, Panc-1, and SNU-213 human pancreatic cancer cells were incubated with varying doses of quercetin, quercetin-3-O-glucoside, and quercetin-7-O-glucoside for 72 h. Viability was measured using the WST-1 assay (b, c). d CFPAC-1, Panc-1, and SNU-213 human pancreatic cancer cells were incubated with 100 nM quercetin, quercetin-3-O-glucoside, and quercetin-7-O-glucoside for 6 h. Migration was evaluated using the Transwell migration assay (n = 3; Tukey’s post hoc test was applied to detect significant group effects as determined by analysis of variance, p < 0.0001; asterisks indicate a significant difference vs. 0 % inhibition)
Previous reports suggest that EGF and EGFR signaling is important in pancreatic cancer metastasis [18, 19]. To elucidate the anti-cancer effects of quercetin and its derivatives in the presence of active EGF signaling, we examined their ability to prevent EGF-induced migration of human pancreatic cancer cells. Quercetin, quercetin 3-O-glucoside, and quercetin 7-O-glucoside all inhibited EGF-induced migration at 50 and 100 nM in SNU-213 cells (Fig. 2a). However, only 100 nM quercetin 3-O-glucoside inhibited migration significantly in CFPAC-1 cells (Fig. 2b). Panc-1 also showed the similar migration patterns to quercetin, quercetin-3-O glucoside, and quercetin-3-O glucoside treatment under EGF activation compared with SNU-213 and CFPAC-1 cells. However, Panc-1 was not statistically significant to quercetin, quercetin-3-O glucoside, and quercetin-3-O glucoside treatment (data not shown). These data suggest that quercetin 3-O-glucoside inhibits EGF-induced migration of both SNU-213 and CFPAC-1 cells, even after a relatively low dose treatment. In contrast, no significant inhibitory effect against PDGF-induced migration was detected under the same treatment conditions (Fig. 2c). Next, we examined the dose dependency of quercetin 3-O-glucoside to inhibit EGF-induced cell viability. EGF had little effect on SNU-213 and CFPAC-1 cell viability, and no significant inhibitory effects of quercetin 3-O-glucoside treatment were observed (Fig. 2d). These results suggest that quercetin and its derivatives inhibit EGF-induced migration of human pancreatic cancer cells and that quercetin 3-O-glucoside has particularly potent effects.
The effects of quercetin and its derivatives on epidermal growth factor (EGF)-induced migration and viability: a SNU-213 and CFPAC-1 human pancreatic cancer cells were pre-treated with different doses of quercetin, quercetin-3-O-glucoside, and quercetin-7-O-glucoside and then incubated with 50 μg/L EGF. Migration was evaluated using the Transwell migration assay (n = 3; Tukey’s post hoc test was applied to detect significant group effects as determined by analysis of variance, p < 0.0001; *p < 0.05, **p < 0.01 vs. 0 % inhibition) (b). c SNU-213 and CFPAC-1 human pancreatic cancer cells were pre-treated with different doses of quercetin-3-O-glucoside and then incubated with 50 μg/L exogenous platelet-derived growth factor (PDGF)-A. Migration was evaluated using the Transwell migration assay (n = 3; Tukey’s post hoc test was applied to detect significant group effects as determined by analysis of variance, p < 0.0001; *p < 0.05, **p < 0.01 vs. 0 % inhibition). d SNU-213 and CFPAC-1 human pancreatic cancer cells were pre-treated with different doses of quercetin-3-O-glucoside and then incubated with 50 μg/L EGF. Viability was measured using the WST-1 assay
Treatment with quercetin 3-O-glucoside decreases EGF-induced migration of human pancreatic cancer cells
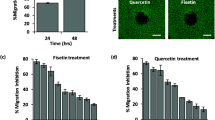
CFPAC-1 and SNU-213 cells were exposed dose dependently to quercetin 3-O-glucoside, and cell migration was assessed to further verify the anti-migratory effect of quercetin 3-O-glucoside. Quercetin-3-O-glucoside inhibited EGF-induced migration of SNU-213 cells in a dose-dependent manner. Treatment with quercetin-3-O-glucoside decreased EGF-induced migration of SNU-213 cells up to 45 % compared with that in exogenously EGF-treated cells in a dose-dependent manner (Fig. 3a, left). Representative images of the migration inhibition assays are shown in Fig. 3a, right. The same treatment caused an approximate 30 % decrease in migration of CFPAC-1 cells compared with that in exogenously EGF-treated cells (Fig. 3b). Next, we verified the basal toxicity of quercetin-3-O-glucoside using a representative normal cell line, HUVECs. As shown in Fig. 3c, d, treatments with various concentrations of quercetin-3-O-glucoside showed no change in the viability and migration of HUVECs. These results suggest that quercetin-3-O-glucoside effectively inhibits EGF-induced migration of human pancreatic cancer cells without significant toxicity.
Effects of quercetin-3-O-glucoside in epidermal growth factor (EGF)-induced migration: a Left, SNU-213 human pancreatic cancer cells were pre-treated with different doses of quercetin-3-O-glucoside and then incubated with 50 μg/L EGF. Migration was evaluated using the Transwell migration assay (n = 3; Tukey’s post hoc test was applied to detect significant group effects as determined by analysis of variance, p < 0.0001; *p < 0.05, **p < 0.01, ***p < 0.001 vs. 0 % inhibition). Right, representative image of the Transwell migration assay treated with 100 or 500 nM of quercetin-3-O-glucoside and EGF (scale bar = 50 μm). b CFPAC-1 human pancreatic cancer cells were pre-treated with different doses of quercetin-3-O-glucoside and then incubated with 50 μg/L EGF. Migration was evaluated using the Transwell migration assay (n = 3; Tukey’s post hoc test was applied to detect significant group effects as determined by analysis of variance, p < 0.0001; *p < 0.05, **p < 0.01, ***p < 0.001 vs. 0 % inhibition). c HUVECs were incubated with varying doses of quercetin-3-O-glucoside for 72 h. Viability was measured using the WST-1 assay (n = 3; Tukey’s post hoc test was applied to detect significant group effects as determined by analysis of variance, p < 0.0001; asterisks indicate a significant difference vs. 0 % inhibition). d Left, HUVECs were incubated with varying doses of quercetin-3-O-glucoside for 6 h. Migration was evaluated using the Transwell migration assay (n = 3; Tukey’s post hoc test was applied to detect significant group effects as determined by analysis of variance, p < 0.0001; asterisks indicate a significant difference vs. 0 % inhibition). Right, representative image of the Transwell migration assay (scale bar = 50 μm)
Treatment with quercetin 3-O-glucoside blocks EGF-induced migration by inhibiting EGFR signaling in human pancreatic cancer cells
We examined the signal transduction pathways that are modulated specifically by EGF stimulation and quercetin 3-O-glucoside treatment to understand the mechanism by which quercetin 3-O-glucoside inhibits the migration of human pancreatic cancer cells. Exogenous EGF treatment induced a dramatic increase in tyrosine phosphorylation of proteins 60, 120, 130, and 180 kDa in size in SNU-213 cells. However, pre-treatment with quercetin 3-O-glucoside inhibited these phosphorylation events in a dose-dependent manner, even in the presence of EGF (Fig. 4a). Similar results were also obtained in CFPAC-1 cells (data not shown). To further analyze the detailed mechanism of action of quercetin 3-O-glucoside in SNU-213 and CFPAC-1 cells, the levels of phosphorylated EGFR, FAK, AKT, MEK1/2, and ERK1/2 were examined under the same conditions. Exogenous EGF treatment increased phosphorylation of EGFR, FAK, AKT, MEK1/2, and ERK1/2, whereas pre-treatment with quercetin 3-O-glucoside inhibited these phosphorylation events in a dose-dependent manner (Fig. 4b).
Intracellular signaling in response to treatment with exogenous epidermal growth factor (EGF) and quercetin-3-O-glucoside a SNU-213 cells were pre-treated with different doses of quercetin-3-O-glucoside for 1 h and then incubated with 50 μg/L EGF for 10 min. The cell lysates were prepared and analyzed by Western blotting with antibodies specific for phospho-tyrosine kinases (Y99). b SNU-213 and CFPAC-1 cells were pre-treated with different doses of quercetin-3-O-glucoside for 1 h and then incubated with 50 μg/L EGF for 10 min. The cell lysates were prepared and analyzed by Western blotting with antibodies specific for phospho-epidermal growth factor receptor (EGFR) (Y1068), EGFR, phospho-focal adhesion kinase (FAK) (Y397), FAK, phospho-AKT (S473), AKT, phospho-MEK1/2 (S217/221), MEK1/2, phospho-ERK1/2 (T202/Y204), ERK1/2, and GAPDH. c SNU-213 cells were incubated with EGF (50 μg/L) in the absence or presence of various pharmacological inhibitors of FAK (SC294950), phosphatidylinositol 3-kinase (PI3K) (LY294002), and MEK1/2 (U0126). Migration was evaluated using the Transwell migration assay (n = 3; Tukey’s post hoc test was applied to detect significant group effects as determined by analysis of variance, p < 0.0001; *p < 0.05, **p < 0.01, ***p < 0.001 vs. 0 % inhibition). d SNU-213 cells were incubated with EGF (50 μg/L) in the absence or presence of various pharmacological inhibitors of FAK (SC294950), PI3K (LY294002), and MEK1/2 (U0126). The cell lysates were examined for phospho-FAK (Y397), FAK, phospho-AKT (S473), AKT, phospho-ERK1/2 (T202/Y204), ERK1/2, and GAPDH expression by Western blotting
Treatment with different doses of FAK, PI3K, and MEK1/2 pharmacological inhibitors inhibited migration of SNU-213 cells in a dose-dependent manner, even in the presence of exogenous EGF (Fig. 4c). In particular, inhibiting PI3K with LY294002 significantly inhibited cell migration to the maximum. In addition, inhibiting PI3K suppressed AKT and ERK1/2 phosphorylation, whereas specifically inhibiting FAK and MEK1/2 had no effect on phosphorylation of other molecules. This result suggests that PI3K is upstream of AKT and ERK1/2 and that it plays a pivotal role in this signaling pathway (Fig. 4d). Similar results were obtained in CFPAC-1 cells (data not shown). These results suggest that quercetin-3-O-glucoside inhibits the EGFR signaling pathway and that FAK, AKT, and ERK1/2 play roles in EGF-induced cell migration of pancreatic cancer cells.
Combined treatment of quercetin 3-O-glucoside and gemcitabine shows a synergistic anti-migration effect in EGF-treated pancreatic cancer cells
We further assessed whether quercetin-3-O-glucoside could have a synergistic anti-migratory effect when combined with gemcitabine, a conventional chemotherapeutic reagent. We first examined the anti-migratory effect of gemcitabine in EGF-activated SNU-213 cells. Gemcitabine alone had a weak anti-migratory effect on SNU-213 cells. Gemcitabine alone, at the highest concentration that we tested (1000 nM), reduced SNU-213 cell migration approximately 10 %; low doses of gemcitabine (10, 50, and 100 nM) had no effect on SNU-213 cells (data not shown). SNU-213 cells were incubated in the absence or presence of 50 or 100 nM gemcitabine with various doses of quercetin 3-O-glucoside (0, 200, 500, and 1000 nM) and EGF (50 μg/L). As shown in Fig. 5, migration of SNU-213 cells decreased significantly when the cells were treated with 200 or 500 nM quercetin-3-O-glucoside and gemcitabine; the 200 or 500 nM quercetin-3-O-glucoside and 100 nM gemcitabine co-treatment resulted in an approximate 17 and 18 % decrease in EGF-induced SNU-213 cell migration, respectively. In contrast, no synergistic effect was detected in the 1000-nM quercetin-3-O glucoside and gemcitabine co-treatment. These results indicate that the quercetin-3-O glucoside and gemcitabine co-treatment synergistically inhibits EGF-induced migration of human pancreatic cancer cells (Fig. 6).
Combined effects of quercetin-3-O-glucoside and gemcitabine in human pancreatic cancer cells: a SNU-213 human pancreatic cancer cells were pre-treated with different doses of quercetin-3-O-glucoside and gemcitabine and incubated with 50 μg/L epidermal growth factor (EGF). Migration was evaluated using the Transwell migration assay (n = 3; Tukey’s post hoc test was applied to detect significant group effects as determined by analysis of variance, p < 0.0001; *p < 0.05, **p < 0.01 vs. 0 % inhibition. n.s. non-significant value. b Representative image of a Transwell migration assay co-treated with 200 nM quercetin-3-O-glucoside, 50 nM gemcitabine, and EGF (scale bar = 50 μm)
Discussion
We demonstrated that quercetin-3-O-glucoside suppressed EGF-induced migration of human pancreatic cancer cells by inhibiting EGFR signaling, even at relatively low doses. We further showed that a quercetin 3-O-glucoside and gemcitabine co-treatment had a synergistic anti-migration effect in EGF-treated pancreatic cancer cells. This is the first report to reveal that quercetin 3-O-glucoside may have anti-migratory effects on human pancreatic cancer cells by inhibiting EGFR signaling.
Multiple functions have been attributed to various growth factor–related signaling pathways in almost every cancer, including metastasis, local infiltration, survival, and proliferation. In particular, EGF, which is a very effective oncogenic factor, promotes a variety of cancer behaviors, such as migration, invasion, and proliferation, in human pancreatic cancer [18, 19]. Accordingly, the viability of human pancreatic cancers increases in the presence of EGF, and EGF triggers migration of human pancreatic cancer cells.
Quercetin inhibits pancreatic cancer growth in vitro and in vivo through the apoptosis pathway. In particularly, quercetin was shown to have the anti-cancer effect even in oral administration in vivo [20]. Expression of the epithelial-mesenchymal transition-related proteins was inhibited by quercetin treatment [21]. In agreement with previous studies, quercetin and its derivatives quercetin 3-O-glucoside and quercetin 7-O-glucoside decreased EGF-induced migration of human pancreatic cancer cells. Our observation that inhibiting EGF-induced signaling with quercetin exerted prominent anti-cancer effects in vitro is consistent with a previous study [22]. However, we additionally found that quercetin 3-O-glucoside had strong anti-migratory effects even at lower doses compared to those of quercetin and quercetin 7-O-glucoside without significant toxicity. Low-dose treatments are attractive to avoid side effects, as high-dose treatment with anti-cancer drugs results in vomiting, anemia, diarrhea, and depilation. Quercetin 3-O-glucoside may be absorbed more efficiently through cell membranes than other forms of quercetin [23].
It is also possible that quercetin 3-O-glucoside inhibits EGFR signaling selectively, but not PDGF-A signaling, suggesting that quercetin 3-O-glucoside may block EGFR signaling effectively, despite being a phytochemical. We also showed that FAK, AKT, and ERK1/2 were critical signaling molecules in EGF-induced migration of pancreatic cancers cells, as described previously [24, 25]. Moreover, PI3K is suggested to be upstream of AKT and ERK1/2 and responsible for migration of human pancreatic cancers cells.
Numerous reports have suggested that a combined treatment of gemcitabine and a conventional chemotherapeutic reagent, such as erlotinib, folfirinox, and nab-paxitaxel, is effective in patients with pancreatic cancer [26–28]. Erlotinib, an EGF TKI, shows a synergistic effect when combined with gemcitabine compared with single gemcitabine treatment in a phase III trial of patients with pancreatic cancer [24]. Additionally, the gemcitabine and erlotinib combination therapy decreases the side effects of erlotinib in metastatic adenocarcinoma of the pancreas [29]. Our results suggest that the phytochemical quercetin 3-O-glucoside inhibits EGF-induced migratory activity effectively and has a synergistic anti-migratory effect with gemcitabine.
In summary, we demonstrated that a quercetin 3-O-glucoside and gemcitabine co- treatment had an additive or synergistic anti-migratory effect on human pancreatic cancer cells, suggesting that this drug combination could decrease the potential risk of side effects. Quercetin 3-O-glucoside may be a safe agent to inhibit infiltration of pancreatic cancer into surrounding structures.
References
Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA: Cancer J Clin. 2014;64:9–29.
Stathis A, Moore MJ. Advanced pancreatic carcinoma: current treatment and future challenges. Nat Rev Clin Oncol. 2010;7:163–72.
Bardeesy N, DePinho RA. Pancreatic cancer biology and genetics. Nat Rev Cancer. 2002;2:897–909.
Cano CE, Motoo Y, Iovanna JL. Epithelial-to-mesenchymal transition in pancreatic adenocarcinoma. Sci World J. 2010;10:1947–57.
Inoue S, Tezel E, Nakao A. Molecular diagnosis of pancreatic cancer. Hepato-Gastroenterology. 2001;48:933–8.
Formica JV, Regelson W. Review of the biology of quercetin and related bioflavonoids. Food Chem Toxicol : Int J Published British Ind Biol Res Assoc. 1995;33:1061–80.
Surh YJ. Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer. 2003;3:768–80.
Russo M, Spagnuolo C, Tedesco I, Bilotto S, Russo GL. The flavonoid quercetin in disease prevention and therapy: facts and fancies. Biochem Pharmacol. 2012;83:6–15.
Romagnolo DF, Selmin OI. Flavonoids and cancer prevention: a review of the evidence. J Nutr Gerontol Geriatr. 2012;31:206–38.
Yang JH, Hsia TC, Kuo HM, Chao PD, Chou CC, Wei YH, et al. Inhibition of lung cancer cell growth by quercetin glucuronides via g2/m arrest and induction of apoptosis. Drug Metab Disposition: Biol Fate Chem. 2006;34:296–304.
Gibellini L, Pinti M, Nasi M, Montagna JP, De Biasi S, Roat E, et al. Quercetin and cancer chemoprevention. Evid Based Complement Alternat Med: eCAM. 2011;2011:591356.
Murakami A, Ashida H, Terao J. Multitargeted cancer prevention by quercetin. Cancer Lett. 2008;269:315–25.
Razavi SM, Zahri S, Zarrini G, Nazemiyeh H, Mohammadi S. Biological activity of quercetin-3-o-glucoside, a known plant flavonoid. Bioorg Khim. 2009;35:414–6.
Mendelsohn J, Baselga J. Epidermal growth factor receptor targeting in cancer. Semin Oncol. 2006;33:369–85.
Oliveira-Cunha M, Newman WG, Siriwardena AK. Epidermal growth factor receptor in pancreatic cancer. Cancers. 2011;3:1513–26.
Lee J, Lee J, Yu H, Choi K, Choi C. Differential dependency of human cancer cells on vascular endothelial growth factor-mediated autocrine growth and survival. Cancer Lett. 2011;309:145–50.
Lee J, Ku T, Yu H, Chong K, Ryu SW, Choi K, et al. Blockade of vegf-a suppresses tumor growth via inhibition of autocrine signaling through fak and akt. Cancer Lett. 2012;318:221–5.
Korc M, Chandrasekar B, Yamanaka Y, Friess H, Buchier M, Beger HG. Overexpression of the epidermal growth factor receptor in human pancreatic cancer is associated with concomitant increases in the levels of epidermal growth factor and transforming growth factor alpha. J Clin Investig. 1992;90:1352–60.
Pryczynicz A, Guzinska-Ustymowicz K, Kemona A, Czyzewska J. Expression of egf and egfr strongly correlates with metastasis of pancreatic ductal carcinoma. Anticancer Res. 2008;28:1399–404.
Angst E, Park JL, Moro A, Lu QY, Lu X, Li G, et al. The flavonoid quercetin inhibits pancreatic cancer growth in vitro and in vivo. Pancreas. 2013;42:223–9.
Zhou W, Kallifatidis G, Baumann B, Rausch V, Mattern J, Gladkich J, et al. Dietary polyphenol quercetin targets pancreatic cancer stem cells. Int J Oncol. 2010;37:551–61.
Lee LT, Huang YT, Hwang JJ, Lee PP, Ke FC, Nair MP, et al. Blockade of the epidermal growth factor receptor tyrosine kinase activity by quercetin and luteolin leads to growth inhibition and apoptosis of pancreatic tumor cells. Anticancer Res. 2002;22:1615–27.
Morand C, Manach C, Crespy V, Remesy C. Quercetin 3-o-beta-glucoside is better absorbed than other quercetin forms and is not present in rat plasma. Free Radic Res. 2000;33:667–76.
Du XY, Huang J, Xu LQ, Tang DF, Wu L, Zhang LX, et al. The proto-oncogene c-src is involved in primordial follicle activation through the pi3k, pkc and mapk signaling pathways. Reprod Biol Endocrinol : RB&E. 2012;10:58.
Roby KF, Son DS, Taylor CC, Montgomery-Rice V, Kirchoff J, Tang S, et al. Alterations in reproductive function in src tyrosine kinase knockout mice. Endocrine. 2005;26:169–76.
Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase iii trial of the national cancer institute of canada clinical trials group. J Clin Oncol : Off J Am Soc Clin Oncol. 2007;25:1960–6.
Conroy T, Desseigne F, Ychou M, Bouche O, Guimbaud R, Becouarn Y, et al. Folfirinox versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–25.
Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691–703.
Aranda E, Manzano JL, Rivera F, Galan M, Valladares-Ayerbes M, Pericay C, et al. Phase ii open-label study of erlotinib in combination with gemcitabine in unresectable and/or metastatic adenocarcinoma of the pancreas: relationship between skin rash and survival (pantar study). Ann Oncol : Off J Europ Soc Med Oncol/ESMO. 2012;23:1919–25.
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2009-0094059).
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, J., Han, SI., Yun, JH. et al. Quercetin 3-O-glucoside suppresses epidermal growth factor–induced migration by inhibiting EGFR signaling in pancreatic cancer cells. Tumor Biol. 36, 9385–9393 (2015). https://doi.org/10.1007/s13277-015-3682-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-015-3682-x