Abstract
Pancreatic ductal adenocarcinoma (PDA) is an aggressive type of malignant tumor with a poor prognosis and high mortality. Aberrant activation of hedgehog signaling plays a crucial role in the maintenance and progression of PDA. Here, we report that the dietary bioflavonoid quercetin has therapeutic potential for PDA by targeting sonic hedgehog (SHH) signaling. The effects of quercetin on the proliferation, apoptosis, migration, and invasion of pancreatic cancer cells (PCCs) and tumor growth and metastasis in PDA xenograft mouse models were evaluated. Additionally, SHH signaling activity was determined. Quercetin significantly inhibited PCC proliferation by downregulating c-Myc expression. In addition, quercetin suppressed epithelial-mesenchymal transition (EMT) by reducing TGF-β1 level, which resulted in inhibition of PCC migration and invasion. Moreover, quercetin induced PCC apoptosis through mitochondrial and death receptor pathways. In nude mouse models, PDA growth and metastasis were reduced by quercetin treatment. Mechanically, quercetin exerts its therapeutic effects on PDA by decreasing SHH activity. Interestingly, quercetin-induced SHH inactivation is mainly dependent on Gli2, but not Gli1. Enhance SHH activity by recombinant Shh protein abolished the quercetin-mediated inhibition of PCC proliferation, migration, and invasion. Furthermore, Shh activated TGF-β1/Smad2/3 signaling and promoted EMT by inducing the expression of Zeb2 and Snail1 that eventually resulted in a partial reversal of quercetin-mediated inhibition of PCC migration and invasion. We conclude that quercetin inhibited the growth, migration, and invasion and induced apoptosis of PCCs by antagonizing SHH and TGF-β/Smad signaling pathways. Thus, quercetin may be a potential candidate for PDA treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pancreatic cancer is a highly malignant tumor of the digestive system with a very poor prognosis (Bray et al. 2018). According to the World Cancer Research Fund, 458,918 people were diagnosed with pancreatic cancer in 2018 (Bray et al. 2018). Only 8 percent of diagnosed patients are expected to survive more than 5 years after diagnosis. The main reason of the poor prognosis in patients with pancreatic cancer is that pancreatic cancer has a strong local invasion and early metastasis ability (Christenson et al. 2020). Furthermore, most patients with pancreatic cancer are unresectable due to local invasion or distant metastasis at the time of diagnosis. Therefore, elucidating the underlying mechanism of tumor metastasis and taking effective measures to curb metastasis are of utmost importance for improving the prognosis of patients with pancreatic cancer.
Pancreatic ductal adenocarcinoma (PDA) is a type of exocrine pancreatic cancer. It is the most common type of pancreatic cancer and accounts for 95% of all pancreatic cancers (Collisson et al. 2019). Due to the high degree of heterogeneity, pancreatic cancer cells (PCCs) have an abnormal growth, early dissemination, and metastasis and have some resistance to radiotherapy and chemotherapy (Nevala-Plagemann et al. 2019). Emerging evidence has shown that the epithelial-to-mesenchymal transition (EMT) program was considered a major driver of PDA progression from initiation to metastasis (Aiello et al. 2017; Rhim et al. 2012). In the EMT process, epithelial-derived tumor cells often lose the characteristics of epithelial cells during invasion and metastasis, reduce adhesion between cells, and express markers of mesenchymal cells and enzymes that decompose the extracellular matrix, eventually leading to increased invasive ability (Zhou et al. 2017). Thus, targeting EMT is one of the main strategies for the treatment of PDA metastasis.
Aberrant activation of the hedgehog pathway has been reported to be involved in the maintenance and progression of PDA (Hidalgo and Maitra 2009; Saini et al. 2019; Xu et al. 2009). The hedgehog signaling pathway consists of ligands, such as Sonic hedgehog (Shh), Indian hedgehog (Ihh), and two transmembrane receptors: Patched (Ptch) and Smoothened (Smo), and the downstream transcription factor Gli family (Gli1 and Gli2) (Amakye et al. 2013). In absence of the Shh ligand, the membrane receptor Ptch blocks the Smo receptor, thereby repressing its activity. Conversely, binding of the Shh ligand to the Ptch receptor diminishes its inhibitory effects on Smo, allowing signal transduction through the pathway that culminates in activation and nuclear translocation of Gli zinc finger transcription factors. Consequently, these transcription factors turn on genes in the nucleus to promote cellular proliferation and induce the activation of downstream cascade signals such as TGF-β/Smad2/3 to trigger EMT in PDA (Moutasim et al. 2014).
Quercetin is a natural flavonoid, also known as meletin, mainly found in five-color fruit and vegetables, such as cherries and cranberries (Kawabata et al. 2015). Quercetin has anti-platelet aggregation, anti-oxidation, anti-inflammatory, free radical scavenging, and other biological activities (Chen et al. 2016; Lu et al. 2018). In PDA, quercetin also has potential therapeutic activity. Quercetin sensitizes PCCs to tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis through JNK-mediated cFLIP turnover (Kim et al. 2016). In PCCs, quercetin facilitates cell death and chemosensitivity through the RAGE/PI3K/Akt/mTOR axis (Lan et al. 2019). Moreover, quercetin inhibited the EMT and regulated apoptosis and proliferative pathways by the suppression of sonic hedgehog (SHH) signaling (Liu et al. 2019; Salama et al. 2019). Considering the above, we hypothesized that quercetin might have therapeutic potential for the treatment of PDA by antagonizing SHH signaling.
In the present study, we investigated the effects of quercetin on the proliferation, migration, and invasion and apoptosis by real-time cell analysis, colony formation and cell migration assays, and flow cytometry analysis in PCCs, as well as tumor growth and metastasis via a nude mouse tumorigenicity assay. In addition, the activity of SHH signaling was determined both in vivo and in vitro. Taken together, our findings revealed that quercetin inhibited PCC growth, migration, and invasion and induced apoptosis by antagonizing SHH and TGF-β/Smad signaling pathways. Thus, quercetin may be a potential candidate for the treatment of PDA.
Materials and methods
Cell culture and drug treatment
Human PCC lines PANC-1 and Patu8988 were obtained from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). PANC-1 and Patu8988 cells were cultured at 37 °C and 5% CO2 in Dulbecco’s modified Eagle’s medium (DMEM; Invitrogen, Carlsbad, CA, USA), supplemented with 10% fetal bovine serum (FBS; Invitrogen), 100 μg/ml streptomycin, and 100 U/ml penicillin (Invitrogen). Cells were treated with 10 or 100 μM quercetin (CAS: 117-39-5, Yuanye Biotechnology, Shanghai, China) with or without recombinant Shh protein (PeproTech, Cranbury, NJ, USA).
Real-time cellular analysis
In this study, 10% FBS served as the stimulator and cells were subjected to serum-free medium for 24 h before experimentation. Real-time monitoring of cell proliferation was performed using an xCELLigence MP system (ACEA Biosciences, San Diego, CA, USA). Prior to seeding cells into the E-plate 96, background impedance was measured after 100 μl of medium was added to the wells for 30 min at room temperature. Cell density was determined by using a hemocytometer after methylene blue staining. Following seeding of the appropriate number of cells (6 × 103 cells) into each well, the plate was incubated at room temperature for 30 min to allow cells to settle. Cell proliferation was monitored every 30 min for 24 h.
CCK-8 assay
The CCK-8 cell proliferation and cytotoxicity test kit (Dojindo, Shanghai, China) was performed as per the manufacturer’s instructions to determine the viability of PANC-1 and Patu8988 cells that were treated with different concentrations of quercetin. At specified time points, 10 μl of the reagent at different concentrations was added to each pore containing 100 μl cell suspension (5 × 103 cells), incubated for 1 h, and the absorbance at 450 nm was determined. The cell viability was calculated by comparing the experimental cells with untreated control cells. All the experiments were repeated at least in triplicate.
Flow cytometry analysis
PANC-1 and Patu8988 cells treated with quercetin were incubated for 24 h and were collected by centrifugation. For apoptosis analysis, Annexin V-FITC (Multi Science, Hangzhou, China) was added to re-suspended cells at room temperature and incubated for 15 min in the dark. Next, propidium iodide (PI, Multi Science) was added to re-suspended cells and incubated for another 5 min in the dark. Finally, cell apoptosis was analyzed using flow cytometry (Ex = 488 nm; Em = 530 nm, BD FACSVerse™, BD Biosciences, San Jose, CA, USA).
Colony formation assay
Cells were incubated into six-well plates at 500~1000 cells per well. When cells grew into colonies that were visible to the naked eye, cells were treated with quercetin. At 24 h after treatment, colonies were fixed with formaldehyde and stained with crystal purple, and the number of clones was counted.
Cell migration assay
PANC-1 and Patu8988 cells were incubated in a 6-well plate at 37 °C for 48 h. Then, the culture area was scraped at the tip of the crystal pipette to form a linear gap in the confluent cell monolayer. Isolated cells were washed with PBS and added to DMEM containing quercetin. Cells would grow to fill the gaps, and an inverted microscope was used to capture images of the culture area every 24 h.
Transwell invasion assay
A transwell chamber (Costar, New York City, NY, USA) assay was used to determine the invasive ability of PANC-1 and Patu8988 cells in vitro. In brief, cells were incubated in 500 μl serum-free medium containing betulinic acid, coated with Matrigel® reduced by growth factor (invasion test), and 10% FBS was added to the lower chamber to serve as a chemical inducer. After incubation for 24 h, cells on the upper surface of the membrane were collected with a Q-TIP, whereas invaded cells were fixed with formaldehyde and stained with 0.5% crystal purple (Sigma). The number of invasive cells in five randomly selected areas was counted using a microscope (Leica Microsystems, Wetzlar, Germany).
Immunofluorescence staining
PANC-1 and Patu8988 cells were cultured with quercetin in six-well plates containing glass slides, washed in phosphate buffer saline (PBS), and fixed in 4% paraformaldehyde (Sigma-Aldrich, Shanghai, China) for 30 min at 4 °C. After permeating in 0.1%Triton X100 for 10 min, cells were washed in PBS and sealed with 10% FBS to eliminate non-specific fluorescence. Immunofluorescence staining was performed by incubating the cell sections containing cells overnight with the following primary antibodies: α-SMA (1: 200, Santa Cruz Biotechnology, Santa Cruz, CA, USA), Ptch1 (1: 200, Santa Cruz), and Smo (1: 200, Santa Cruz) at 4 °C (Additional file 1). After washing three times in PBS, cell preparations were incubated with DyLight 488 (green)- or 594 (red)-labeled secondary antibodies (Sigma-Aldrich) at room temperature for 1 h. After washing in PBS, a mounting media was dropped onto the cell preparations, which were mounted onto a slide. Immunofluorescence studies were quantitatively or semi-quantitatively evaluated by two independent investigators in a blinded manner.
Tumorigenicity assay in nude mice
Male nude mice (BALB/c), weighing 18–22 g, 6–8 weeks old, were purchased from the Experimental Animal Center of Wenzhou Medical University (Wenzhou, China). Mice were placed in an environment controlled by temperature, humidity, and light and were fed a standard mouse feed and water. The day before the experiment, mice were fasted. Prior to the experiment, a total of 0.2% pentobarbital sodium was injected intraperitoneally. The side of the left neck of experimental mice (n = 12) was injected subcutaneously with 5 × 106 PANC-1 cells in 10 μl of PBS after which mice received daily intragastric administration of quercetin (75 mg/kg day) for 30 days. Model mice (n = 12) received an injection of 5 × 106 PANC-1 cells and a daily gastric tube of solvent (PBS), whereas the healthy group (n = 6) was treated with PBS only. Tumor was monitored daily until the tumor grew too bulky or became necrotic. Based on the formula V = length2 × width, the tumor volume was measured every other day, in which the length was always the longest measurement. At the end of the experiment, all the mice were euthanized by CO2 asphyxiation. Animal protocols including the method of euthanasia were approved by the Institutional Animal Care and Use Committee of Wenzhou Medical University, China. All methods were performed according to the guidelines approved by the Institutional Review Board of the Key Laboratory of Diagnosis and Treatment of Severe Hepato-Pancreatic Diseases of the Zhejiang Province, China.
Histopathological examination and immunohistochemical staining
Tumor specimens from animals fixed in formalin and embedded in paraffin were cut into 4-μm sections and stained with hematoxylin and eosin (HE, Yuanye Biotechnology, Shanghai, China). Immunohistochemical (IHC) analysis was performed using 4-μm-thick sections that had been dewaxed with xylene and rehydrated in sequential ethanol. Sections were incubated in 0.1% sodium citrate buffer (pH 6.0) for antigen retrieval, and endogenous peroxidase activity was blocked with 3% hydrogen peroxide (Beyotime, Shanghai, China). IHC staining was performed using the following primary antibodies: anti-Ki67 (1:200), anti-E-cadherin (1:200), anti-α-SMA (1:200), and anti-Shh (1:200) (Additional file 1). Stained sections were examined and images were taken using a DM4000B LED Microscope System (Leica Microsystems) and a DFC 420C 5M Digital Microscope Camera (Leica Microsystems). The integrated optical density (IOD) was measured using Image-Pro Plus software (version 6.0, Media Cybernetics, Silver Spring, MD, USA). All samples were semi-quantitatively or quantitatively assessed by two independent investigators in a blinded manner.
Western blot analysis
Western blot analysis was performed according to a previous report (Zhang et al. 2019). Total proteins or nucleoprotein from PANC-1 and Patu8988 cells or tumor tissues were collected, and the protein concentration was determined by double star choline acid protein analysis kit (Beyotime Biotechnology). Total protein (20 μg) of each sample was separated by SDS-PAGE and transferred to polyvinylidene fluoride membrane (Solarbio, Beijing, China). After blocking with 5% skim milk that was dissolved in TBST, membranes were probed with a primary antibody overnight at 4 °C, and a horseradish peroxidase (HRP)-conjugated secondary antibody detection system was used for visualization. A SuperSignal West Pico Chemiluminescent Substrate (Thermo Fisher Scientific) was used for visualization. All primary antibodies listed in Additional file 1 were diluted to 1:1000. Protein bands were quantified by measuring the intensity of the signals using Image-Pro Plus (version 6.0) and normalized to the GAPDH or Histone H3 signals.
Quantitative reverse transcriptase-PCR (qRT-PCR)
Total RNA was extracted from PANC-1 and Patu8988 cells or tumor tissues using TRIzol reagent (Invitrogen) and reverse transcribed into cDNA template using an ReverTra Ace qPCR RT Kit (Toyobo, Japan). SYBR Green Real-Time PCR Master Mix Plus (Toyobo) was used for QRT-PCR. The cDNA was separated on an agarose gel, and the quantity was determined by Varioskan flash (Thermo Fisher Scientific, San Jose, CA, USA). Sequence-specific primers for Cdh1, Acta2, Vim, Tgfb1, Zeb2, Snail1, Shh, Ihh, Gli1, and Gli2, listed in Additional file 2, were synthesized by Invitrogen, and GAPDH was used as an endogenous reference gene. Samples were analyzed in triplicate and melting curves were examined to verify that a single product was amplified. For quantitative analysis, samples were analyzed by the ΔΔCT method.
Human PDA tissues and IHC staining
Human PDA tissues and paired non-tumorous pancreatic tissues were collected during surgical resection at The First Affiliated Hospital of Wenzhou Medical University (Wenzhou, China). The use of human samples was approved by the Institutional Review Board of The First Affiliated Hospital of Wenzhou Medical University (Wenzhou, China). For histopathological analysis, tissues were sectioned (4 μm), and HE staining was performed according to the manufacturer’s instructions.
Oncomine data analyses
Oncomine data analyses were carried out as previously described (Xu et al. 2015). Briefly, we searched for Shh or Gli2 using the following threshold values: P value of 0.05, fold-change of 2, and gene rank in the top 10% among all differentially expressed genes. Subsequently, Oncomine ordered all data sets by P value per published data set, and the values were linked to the graphical representations of the original microarray data set. Each sample value was log2 transformed. P values were calculated using Student’s t test.
RNA interference
Small interfering RNA (siRNA) targeting Gli2 were synthesized by GenePharma (Shanghai, China). The primer sequences of siRNAs were as follows: Gli2 siRNA, 5′-GGUUCGAGCAGCUCAAGAAGG-3′ (sense) and 5′-UUCUUGAGCUGCUCGAA CCGG-3′ (antisense). We transfected the PANC-1 cells with siRNAs using Lipofectamine 3000 (Life Technologies, CA, USA) as per the instructions of the manufacturer. The efficiency of siRNA transfection was assessed by qRT-PCR.
Statistical analysis
Data were presented as the mean ± standard error of the mean (SEM). All statistical analyses were performed using GraphPad Prism software (version 8.0, GraphPad Software, Inc., La Jolla, CA, USA). Two-sided Student’s t test was used to analyze differences between the two groups. A one-way analysis of variance was used when more than two groups were compared. A P value of less 0.05 was considered statistically significant.
Results
Quercetin inhibits proliferation and induces apoptosis of PCCs
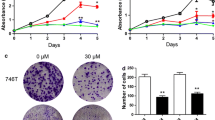
To investigate the effects of quercetin on the proliferation of PCCs, unlabeled real-time cell analysis (RTCA), CCK-8 assay, and colony formation assay were performed. As shown in Fig. 1a, as expected, the growth of PANC-1 and Patu8988 cells was markedly inhibited by quercetin treatment (0, 10, and 100 μM) and was concentration- and time-dependent. The CCK-8 results of PANC-1 and Patu8988 cells confirmed the concentration-dependent inhibitory effects of quercetin on cell viability (Fig. 1b). In addition, quercetin significantly reduced the colony formation in PANC-1 and Patu8988 cells (Fig. 1c). Ki67 (also known as MKI67) has been reported as a cellular marker for proliferation and is highly expressed in pancreatic cancer tissues (Panzuto et al. 2011). Ki67 is present during all active phases of the cell cycle (G1, S, G2, and M), but is absent in resting cells (G0). The cellular content of Ki67 protein markedly increased during PCC progression through the S phase of the cell cycle (Foltyn et al. 2012). In this study, the ratio of Ki67-positive cells in total cells was reduced in quercetin-treated PANC-1 and Patu8988 cells when compared with the control (Fig. 1d). Thus, these results suggested that quercetin has proliferation-inhibitory activities on PCCs and it is concentration- and time-dependent.
Quercetin inhibits proliferation and induces apoptosis of pancreatic cancer cells. a The growth of PANC-1 and Patu8988 cells with or without quercetin treatment assessed by real-time cellular analysis (RTCA). b The viability of PANC-1 and Patu8988 cells with or without quercetin treatment determined by CCK-8 assay. c The proliferation of PANC-1 and Patu8988 cells with or without quercetin treatment analyzed by colony formation assay. d Immunocytochemical staining of Ki67 in PANC-1 and Patu8988 cells with or without quercetin treatment. Bar = 100 μm. e Flow cytometry analysis revealing apoptotic and necrotic cells in PANC-1 and Patu8988 cells with or without quercetin treatment. f The effects of quercetin on the expression of p62, LC3-I, and LC3-II protein levels of PANC-1 and Patu8988 cells by Western blot analysis. g The effects of quercetin on the expression of cleaved caspase-8 and cleaved caspase-3 protein levels of PANC-1 and Patu8988 cells by Western blot analysis. h The effects of quercetin on the expression of Bcl-2 and Bax protein levels of PANC-1 and Patu8988 cells by Western blot analysis. Data were presented as the mean ± standard deviation in quintuplicate for the cell line experiment. *P < 0.05; **P < 0.01, ***P < 0.001
Next, we assessed the effects of quercetin on apoptosis of PCCs by flow cytometry analysis. Treatment with quercetin significantly increased the proportion of apoptosis and necrotic cells (Fig. 1e). Further studies showed that the expression levels of p62, LC3-I, and LC3-II did not alter in quercetin-treated PANC-1 and Patu8988 cells, thereby suggesting that quercetin did not induce autophagy in PCCs (Fig. 1f). Interestingly, upregulated levels of cleaved caspase-8 and cleaved caspase-3 were observed after quercetin treatment (Fig. 1g). In addition, quercetin increased Bax expression and decreased Bcl-2 expression (Fig. 1h). Taken together, quercetin induced death receptor- and mitochondrial-mediated apoptosis of PCCs.
Quercetin inhibits migration and invasion of PCCs by suppressing EMT
PCC migration and invasion are the main factors for PDA metastasis and poor prognosis (Christenson et al. 2020). Here, the invasion number of PANC-1 and Patu8988 cells using transwell chamber assay was markedly inhibited by quercetin treatment (Fig. 2a). Also, the migrated rate of PANC-1 and Patu8988 cells as determined by a wound healing assay was reduced by quercetin (Fig. 2b) and was concentration- and time-dependent. Further studies revealed that gene expression levels of Acta2 (encoding α-SMA) and Vim (encoding vimentin) were downregulated, and the gene expression of Cdh1 (encoding E-cadherin) was upregulated in quercetin-treated PANC-1 and Patu8988 cells (Fig. 2c, d). In addition, quercetin decreased the protein levels of α-SMA, N-cadherin, type I collagen, and vimentin and increased the protein level of E-cadherin in PANC-1 and Patu8988 cells (Fig. 2e, f). Moreover, immunofluorescence analysis showed suppressed expression of α-SMA and vimentin and enhanced expression of E-cadherin after quercetin treatment (Fig. 2g, h). Collectively, these results indicated that quercetin inhibits migration and invasion of PCCs by suppressing the EMT process as well as matrix production.
Quercetin inhibits migration and invasion of pancreatic cancer cells by suppressing the epithelial-mesenchymal transition. Bar = 100 μm. a The effects of quercetin on the invasion number of PANC-1 and Patu8988 cells analyzed by transwell chamber assay. b The effects of quercetin on the migrated rate of PANC-1 and Patu8988 cells determined by wound healing assay. c qRT-PCR analysis showing the mRNA expression of Cdh1 and Acta2 in quercetin-treated PANC-1 and Patu8988 cells. d qRT-PCR analysis for the mRNA expression of Vim in quercetin-treated PANC-1 and Patu8988 cells. e Western blot analysis showing the protein expression of α-SMA, N-cadherin, and E-cadherin in PANC-1 and Patu8988 cells. f Western blot analysis for the protein expression of type I collagen and vimentin in PANC-1 and Patu8988 cells. g Immunocytochemical staining of α-SMA and E-cadherin in quercetin-treated PANC-1 and Patu8988 cells. Bar = 25 μm. h Immunocytochemical staining of vimentin in quercetin-treated PANC-1 and Patu8988 cells. Bar = 25 μm. Data were presented as the mean ± standard deviation in quintuplicate for the cell line experiment. *P < 0.05; **P < 0.01, ***P < 0.001
Quercetin reduces c-Myc expression and TGF-β1/Smad2/3 signaling activity
Considering that quercetin inhibited cell proliferation and induced apoptosis in PCCs, we investigated the effect of quercetin on the expression of c-Myc, which plays an important role in apoptosis, cell cycle progression, and proliferation (McMahon 2014). As shown in Fig. 3a, quercetin treatment reduced c-Myc expression in a concentration-dependent manner in PANC-1 and Patu8988 cells, thereby suggesting that reduced c-Myc expression may be responsible for quercetin-mediated effects on proliferation and apoptosis of PCCs.
Quercetin reduces c-Myc expression and TGF-β1/Smad2/3 signaling activity. a Western blot analysis showing c-Myc expression in PANC-1 and Patu8988 cells. b ELISA analysis for TGF-β1 level in PANC-1 and Patu8988 cells. c Western blot analysis showing TGF-β1 expression in PANC-1 and Patu8988 cells. d Immunocytochemical staining of TGF-β1 in quercetin-treated PANC-1 and Patu8988 cells. Bar = 25 μm. e Western blot analysis showing the expression and phosphorylation of Smad2 and Smad3 in PANC-1 and Patu8988 cells. f Immunocytochemical staining of Smad2 and Smad3 in quercetin-treated PANC-1 and Patu8988 cells. Bar = 25 μm. g Western blot analysis showing the phosphorylation of Smad2 and Smad3 in the nucleus of PANC-1 and Patu8988 cells. h qRT-PCR analysis for the mRNA expression of Zeb2 and Snail1 in quercetin-treated PANC-1 and Patu8988 cells. i Western blot analysis showing the expression of Zeb2 and Snail1 in PANC-1 and Patu8988 cells. Data were presented as the mean ± standard deviation in quintuplicate for the cell line experiment. *P < 0.05; **P < 0.01, ***P < 0.001
Next, we analyzed the mechanism of quercetin-mediated EMT inhibition to reduce the migration and invasion of tumor cells. The TGF-β1/Smad pathway is a key regulator of proliferation and differentiation of tumor cells (Gaarenstroom and Hill 2014; Massague 2012). TGF-β1 from the tumor microenvironment may cause cancer cell apoptosis and tumor suppression or induce EMT that promotes cancer cell invasion and metastasis (David et al. 2016; Heldin et al. 2012). Evidence from qRT-PCR, Western blot analysis, and immunofluorescence staining showed that quercetin decreased TGF-β1 expression in PANC-1 and Patu8988 cells (Fig. 3b–d). In addition, quercetin inhibited the phosphorylation and nuclear translocation of Smad2 and Smad3 (Fig. 3e, f). Furthermore, quercetin reduced mRNA and protein expression of EMT-inducing transcription factors (EMT-TFs) Snail1 and Zeb2 (Fig. 3g, h), which are two key downstream target molecules of TGF-β1/Smad2/3 signaling that suppress E-cadherin expression (Thiery et al. 2009; Zheng et al. 2015). Thus, quercetin regulates EMT to inhibit migration and invasion of PCCs by reducing TGF-β1/Smad2/3 activity and EMT-TF expression.
Quercetin reduces SHH signaling activity dependent of Gli2 in PCCs
During embryonic development, the complex but delicate interactions between TGF-β1/Smad2/3, SHH, JAK2/STAT3, WNT/β-catenin, PI3K/Akt/mTOR, and other pathways are crucial for cell fate determination and organogenesis (Guo and Wang 2009). Aberrant activation of these pathways may result in the development of pathological consequences, including a variety of human tumors, such as PDA (Guo and Wang 2009; Hidalgo and Maitra 2009; Massague 2012). In this study, we investigated the effects of quercetin on the activities of SHH, JAK2, β-catenin, and mTOR in PANC-1 and Patu8988 cells. We found that quercetin did not inhibit the expression and phosphorylation of JAK2, β-catenin, and mTOR (Figure S1A-C). However, quercetin did reduce the mRNA expression of hedgehog ligand Shh, but did not affect the mRNA expression of the Ihh ligand (Fig. 4a), thereby indicating that SHH signaling may be the target of quercetin. In previous studies, it was shown that SHH signaling plays an important role in the induction of EMT through dialog with TGF-β1 pathway (Lu et al. 2016; Thiery et al. 2009). In our study, we identified upregulated Shh expression in human PDA tissues (Figure S2A), as well as PCCs (Figure S2B). These results were further confirmed by in silico analyses of three other independent data sets that were obtained from Oncomine (Figure S2C) (Rhodes et al. 2004). Thus, in addition to TGF-β1/Smad2/3 signaling, the activity of SHH signaling in PANC-1 and Patu8988 cells may be suppressed by quercetin. As expected, enhanced expression of Ptch1 and reduced expression of Smo were observed in quercetin-treated PANC-1 and Patu8988 cells (Fig. 4b, c). Interestingly, quercetin induced the mRNA and protein expression of nuclear transcription factor Gli2, but did not induce the expression of Gli1 (Fig. 4d–f). In hypoxic tumor microenvironment, in mammals, Gli2 can be activated to promote cancer cell stemness and resistance to chemotherapy (Tang et al. 2018). Here, we confirmed enhanced Gli2 expression in PDA tissues in silico analyses of two other independent data sets obtained from Oncomine (Figure S3A). Our findings obtained from TCGA data showed that patients with high Gli2 protein levels had shorter overall survival times compared to patients harboring tumors with low Gli2 levels (P < 0.001) (Figure S3B) and indicated that Gli2 as a nuclear transcription factor for SHH signaling was more important in tumor progression. Collectively, these results showed that quercetin exerted pancreatic anti-tumor activity by suppressing SHH signaling activity dependent of Gli2.
Quercetin reduces SHH signaling activity in pancreatic cancer cells. a qRT-PCR analysis showing the mRNA expression of Shh and Ihh in quercetin-treated PANC-1 and Patu8988 cells. b Western blot analysis showing the expression of Ptch1 and Smo in PANC-1 and Patu8988 cells. c Immunocytochemical staining of Ptch1 and Smo in quercetin-treated PANC-1 and Patu8988 cells. Bar = 25 μm. d qRT-PCR analysis showing the mRNA expression of Gli1 and Gli2 in quercetin-treated PANC-1 and Patu8988 cells. e Western blot analysis showing the expression of Gli1 and Gli2 in PANC-1 and Patu8988 cells. f Immunocytochemical staining of Gli1 and Gli2 in quercetin-treated PANC-1 and Patu8988 cells. Bar = 25 μm. Data were presented as the mean ± standard deviation in quintuplicate for the cell line experiment. *P < 0.05; **P < 0.01, ***P < 0.001
Quercetin inhibits the growth and metastasis of PDA in animal xenograft models
To evaluate whether similar anticancer effects also occur in vivo, quercetin (75 mg/kg day) was administered for 30 consecutive days to mice subjected to the injection of PCCs. Figure 5a shows morphologic changes in the experimental groups as a result of quercetin treatment. We found that quercetin administration significantly reduced the volume (45.49 mm3 vs 167.9 mm3, P < 0.001) and weight (206.5 mg vs 764.5 mg, P < 0.001) of the tumor (Fig. 5b, c). In addition, HE staining showed pathological results of PDA in tissues of model group (Fig. 5d). The downregulated proportion of Ki67-positive cells in total cells in quercetin-treated models showed the inhibition of tumor cell proliferation (Fig. 5e). Further studies showed that quercetin administration upregulated the expression of cleaved caspase-8 and cleaved caspase-3 (Fig. 5f). Furthermore, quercetin increased Bax expression and decreased Bcl-2 expression (Fig. 5g). Thus, our in vivo findings showed that quercetin induced apoptosis of PCCs via a death receptor- and mitochondrial-mediated manner.
Quercetin inhibits the growth and metastasis of pancreatic ductal adenocarcinoma in animal xenograft models. a Effect of quercetin on morphologic changes in experimental groups. b Effect of quercetin on the volume of tumor in animal xenograft models. c Effect of quercetin on tumor weight. d HE staining for pathological results of pancreatic ductal adenocarcinoma (PDA) in tissues of the model group. Bar = 100 μm. e Immunohistochemical (IHC) staining for Ki67 in quercetin-treated models. Bar = 100 μm. f Western blot analysis showing the expression of cleaved caspase-8 and cleaved caspase-3 in PDA tissues. g Western blot analysis showing the expression of Bcl-2 and Bax in PDA tissues. h Western blot analysis showing the expression of type I collagen and vimentin in PDA tissues. i IHC staining for E-cadherin and α-SMA in quercetin-treated models. Bar = 100 μm. j Western blot analysis showing the expression or phosphorylation of TGF-β1, Smad2, and Smad3 in PDA tissues. k IHC staining for Shh, Ptch1, Gli1, and Gli2 in PDA tissues. Bar = 100 μm. l Western blot analysis showing the expression of Ptch1, Smo, Gli1, and Gli2 in PDA tissues. m Effect of quercetin on hepatic metastasis in PDA tissues. Data were presented as the mean ± standard deviation for six rats per group. *P < 0.05; **P < 0.01, ***P < 0.001
To assess whether quercetin suppressed the metastasis of PDA, we firstly examined the expression of matrix proteins, which are produced in myofibroblasts that originated from activated pancreatic stellate cells (PSCs) or epithelial cells by phenotypic transformation when stimulated by TGF-β1. We found that quercetin administration significantly decreased the expression of type I collagen and vimentin (Fig. 5h). Quercetin also reduced the expression of α-SMA and increased E-cadherin expression (Fig. 5i). We further identified that downregulated TGF-β1 levels were responsible for quercetin-mediated effects and resulted in the inhibition in phosphorylation of Smad2 and Smad3 (Fig. 5j). Interestingly, quercetin administration induced inactivation of SHH signaling in tumor tissues dependent of Gli2, not Gli1 (Fig. 5k, l). As a result, quercetin suppressed PDA metastasis, including hepatic metastases (Fig. 5m). Taken together, these data suggested that quercetin inhibited the growth and metastasis of PDA in mouse xenograft models.
Activated SHH signaling abolishes quercetin-mediated anti-tumor effects
Given that quercetin exerts anti-tumor effects on PDA by suppressing SHH signaling, we next investigated whether activated SHH signaling impacted this effect of quercetin. In PANC-1 and Patu8988 cells, recombinant Shh protein was used to activate SHH signaling by enhancing Smo expression and reducing Ptch1 expression (Fig. 6a). Moreover, Shh treatment increased the expression of Gli2, as well as Gli1 (Fig. 6a), thereby suggesting that Shh-induced activation of SHH signaling in PCCs in despite of quercetin treatment. As a result, activated SHH signaling abolished the anti-proliferative effect of quercetin as determined by the colony formation assay (Fig. 6b). However, upregulated activity of SHH signaling did not alter the ratio of apoptotic cells caused by quercetin, thus indicating that the pro-apoptotic activity of quercetin was not affected by SHH signaling (Fig. 6c). Using a transwell chamber assay and wound healing assay, we confirmed that Shh treatment increased the invasion of PANC-1 and Patu8988 cells (Fig. 6d), as well as the migrated rate (Fig. 6e). Thus, activated SHH signaling abolished quercetin-induced inhibition of EMT, as indicated by reduced expression of E-cadherin and increased expression of N-cadherin, α-SMA, vimentin, and type I collagen (Fig. 6f). Combined, these findings confirmed a crucial role of SHH signaling in the anti-tumor effects of quercetin.
Activated SHH signaling abolished quercetin-mediated anti-tumor effects. a Western blot analysis showing the expression of Ptch1, Smo, Gli1, and Gli2 in quercetin-treated PANC-1 and Patu8988 cells with or without Shh. b Colony formation assay for cell proliferation in quercetin-treated PANC-1 and Patu8988 cells with or without Shh. c Flow cytometry analysis for cell apoptosis in quercetin-treated PANC-1 and Patu8988 cells with or without Shh. d Transwell chamber assay for the invasion of quercetin-treated PANC-1 and Patu8988 cells with or without Shh. Bar = 100 μm. e Western blot analysis showing the expression of α-SMA, N-cadherin, and E-cadherin in quercetin-treated PANC-1 and Patu8988 cells with or without Shh. f Western blot analysis showing the expression of N-cadherin, α-SMA, vimentin, and type I collagen in quercetin-treated PANC-1 and Patu8988 cells with or without Shh. Data were presented as the mean ± standard deviation in quintuplicate for the cell line experiment. *P < 0.05; **P < 0.01, ***P < 0.001
Activated SHH signaling rescues quercetin-mediated inhibition of TGF-β1/Smad2/3 signaling
To further elucidate the role of TGF-β1/Smad2/3 signaling in activation of SHH signaling to promote EMT and reduce anti-tumor effects of quercetin, we first examined the activity of TGF-β1/Smad2/3 signaling in SHH signaling activation. In quercetin-treated PANC-1 and Patu8988 cells, the inhibition of TGF-β1 expression as determined by Western blot analysis and immunofluorescence staining was abolished by Shh (Fig. 7a, b). Also, quercetin-mediated inhibition of the phosphorylation and nuclear translocation of Smad2 and Smad3 was rescued by Shh (Fig. 7c, d). Moreover, Shh induced the expression of Snail1 (Fig. 7e). However, Shh did not increase Zeb2 expression in quercetin-treated PANC-1 and Patu8988 cells (Fig. 7e). These results indicated TGF-β1/Smad2/3 signaling as a downstream target of SHH signaling to promote EMT by inducing Snail1 expression. Furthermore, recombinant TGF-β1 protein was used to assess the effect of TGF-β1/Smad2/3 signaling on SHH signaling. Our findings indicated that TGF-β1 increased the expression of Smo in PANC-1 and Patu8988 cells (Fig. 7f and Figure S4). However, TGF-β1 did not induce the expression and nuclear translocation of Gli1 and Gli2 (Fig. 7f and Figure S4), thereby indicating that TGF-β1 activates the SHH signaling pathway dependent of Gli1 and Gli2 in PCCs. Moreover, Gli2 siRNA significantly decreased the mRNA and protein expression of TGF-β1 (Figure S5A and B), revealing that SHH signaling and TGF-β1/Smad2/3 signaling may have a dialog, which affects the effects of quercetin on EMT and tumor growth and metastasis (Fig. 8). Thus, activated SHH signaling can rescue quercetin-mediated inhibition of TGF-β1/Smad2/3 signaling.
Activated SHH signaling rescued quercetin-mediated inhibition of TGF-β1/Smad2/3 signaling. a Western blot analysis showing the expression of TGF-β1 in quercetin-treated PANC-1 and Patu8988 cells with or without Shh. b Immunocytochemical staining of TGF-β1 in quercetin-treated PANC-1 and Patu8988 cells with or without Shh. Bar = 25 μm. c Western blot analysis for the expression and phosphorylation of Smad2 and Smad3 in quercetin-treated PANC-1 and Patu8988 cells with or without Shh. d Immunocytochemical staining of Smad2 and Smad3 in quercetin-treated PANC-1 and Patu8988 cells with or without Shh. Bar = 25 μm. e Western blot analysis showing the expression of Zeb2 and Snail1 in quercetin-treated PANC-1 and Patu8988 cells with or without Shh. f Immunocytochemical staining of Smo, Gli1, and Gli2 in TGF-β1-treated PANC-1 and Patu8988 cells. Bar = 25 μm. Data were presented as the mean ± standard deviation in quintuplicate for the cell line experiment. *P < 0.05; **P < 0.01, ***P < 0.001
Discussion
In the present study, we investigated the effects of quercetin, a natural flavonoid compound with various biological activities, on proliferation, apoptosis, migration, and invasion of PCCs and tumor growth and metastasis in PDA xenograft mouse models. Our data showed that quercetin treatment significantly inhibited PCC proliferation by downregulating c-Myc expression. In addition, quercetin suppressed EMT via reducing the TGF-β1 level, resulting in the inhibition of PCC migration and invasion. Moreover, quercetin induced PCC apoptosis through mitochondrial and death receptor pathways. In nude mouse models, pancreatic tumor growth and metastasis were reduced by quercetin administration. Our results provide a rational for the use of quercetin as a potential supplemental treatment for PDA.
Previous studies have shown that hedgehog signaling was involved in the occurrence and development of PDA (Hidalgo and Maitra 2009; Saini et al. 2019; Xu et al. 2009). Drug research and development based on hedgehog signaling has become a new hotspot in the treatment of PDA. Effective drugs could potentially include small-molecule inhibitors and natural compounds. IPI-926, a small-molecule inhibitor of Smo, significantly improved the survival rate of PDA when used in combination with gemcitabine in a mouse model (Olive et al. 2009). In addition, GANT-61, an inhibitor of Gli1 and Gli2, inhibited pancreatic cancer stem cell growth in vitro and in a NOD/SCID/IL2R gamma null mice xenograft (Fu et al. 2012). As a flavonol compound, quercetin is thought to antagonize the occurrence and development of tumors through targeting hedgehog signaling with no associated teratogenicity (Khaksary Mahabady et al. 2016; Sistani Karampour et al. 2014). Quercetin has the ability to inhibit the self-renewal capacity of pancreatic cancer stem cells (CSCs) via hedgehog signaling (Drenkhahn et al. 2013; Tang et al. 2011). In this study, we demonstrated that quercetin exerted a pancreatic anti-tumor activity by antagonizing hedgehog signaling. However, quercetin did not inhibit the activities of JAK2, β-catenin, and mTOR, thereby revealing that hedgehog signaling may be a potential target for quercetin. Interestingly, quercetin reduced the expression of hedgehog ligand Shh in two PCC lines, but not Ihh. In mammals, three related hedgehog ligand proteins exist, namely Sonic (Shh), Desert (Dhh), and Indian (Ihh). Dhh mainly exists in fruit flies and may be involved in both male gonadal differentiation and perineurial development (Kuspert et al. 2012). Ihh is a vertebrate homolog that may be related to cartilage differentiation in long bone growth (Probst et al. 2013). Shh plays a role in the growth and differentiation of various cells, which reveals that abnormal expression of Shh may be closely related to tumorigenesis (Xu et al. 2019). Shh-triggered signaling activation in turn affects the biological functions of tumor cells, which is mediated by its receptor Ptch1, as well as Dispatched, a protein with sequence similarity to Ptch1 (Cannac et al. 2020). In this study, our findings confirmed that Shh was overexpressed in human pancreatic tumor tissues and cell lines, and it was associated with poor prognosis (Haque et al. 2012). Moreover, when compared to Ihh, Shh plays a more important role in the anti-tumor effect of quercetin. Thus, Shh-triggered (SHH) downstream signal activation may be an important potential target for anticancer drugs, including quercetin.
Further studies have shown that the nuclear transcriptional regulator Gli (Gli1/Gli2) was responsible for the crucial role of SHH signaling in quercetin-mediated anti-tumor effects. In mammals, the activator form of Gli2, not Gli1, is required for SHH signaling, and Gli1-KO mice develop normally (Bai et al. 2002; Park et al. 2000), whereas Gli2-KO mice die at birth and have severe skeletal and neural defects (Ding et al. 1998). Gli2 can rescue most Gli1 functions, whereas Gli1 cannot rescue Gli2 function (Park et al. 2000). In the present study, we confirmed enhanced Gli2 expression in PDA tissues and that patients with high Gli2 expression had a shorter overall survival time. Moreover, quercetin reduced the expression of Gli2 and inhibited its nuclear translocation in vivo and in vitro. However, quercetin did not affect Gli1 expression, though Gli1 is involved in PDA development (Kasai 2016). Thus, quercetin induced inactivation of SHH signaling in PDA, which was dependent on Gli2, not Gli1.
In previous studies, it was shown that activation of TGF-β1/Smad2/3 signaling promoted the EMT transformation of tumor cells, which in turn aggravated the ability of tumor cells to invade and infiltrate (Heldin et al. 2012; Massague 2012). In PDA, TGF-β1 activates Smad2 and/or Smad3 through complexes of heteromeric transmembrane type I (TGβRI) and type II (TGβRII) receptors. Subsequently, phosphorylated Smad2 and Smad3 form heteromeric complexes with Smad4 and translocate to the nucleus to induce the expression of EMT-TFs (Snail1 and Zeb2). As a result, the expression of E-cadherin is downregulated, and tumor cells shed their differentiated epithelial characteristics, including polarity and cell-cell adhesion, and acquire mesenchymal abilities, including invasiveness and motility (Xu et al. 2015). In this study, we observed over-activation of TGF-β1/Smad2/3 signaling in PDA. Quercetin treatment can reduce TGF-β1 levels in two types of PCCs and block the phosphorylation and nuclear translocation of Smad2 and Smad3. Thus, these results supported that TGF-β1/Smad2/3 pathway, as SHH signaling, plays an important role in quercetin-mediated anti-tumor effects.
Since both SHH and TGF-β1/Smad2/3 signaling are involved in the occurrence and metastasis of PDA, it is of utmost importance to understand their interaction mechanism, including anti-tumor effects of quercetin. Recombinant Shh protein was used to enhance SHH activity in quercetin-treated PCCs, and thereby activated TGF-β1/Smad2/3 signaling and promoted the EMT process by inducing the expression of EMT-TFs Zeb2 and Snail1. Although exogenous TGF-β1 protein slightly increased the expression of Smo protein, the expression of Gli1 or Gli2 was not affected. In addition, Gli2 knockdown significantly decreased the mRNA and protein expression of TGF-β1, which indicated that TGF-β1/Smad2/3 was more likely to be a signaling pathway downstream of SHH. It has not yet been determined whether the underlying mechanism was caused by drugs or is a general phenomenon. Although most studies have shown that Smad-dependent TGF-β1 signaling during EMT may activate many signaling pathways (Zhang et al. 2016), the mechanism by which SHH affects the growth and metastasis of PDA through activation of TGF-β1 signaling requires more in-depth research.
This study has some limitations. First, a genetic approach and an in vivo experiment involved in the association between SHH and TGF-β1/Smad2/3 signaling in quercetin-treated PCCs need to be presented. In addition, the mechanism of action between TGF-β1 and hedgehog pathways should be clarified. Importantly, the precise target of the anticancer effect of quercetin needs to be further clarified and confirmed by pharmacological studies.
Taken together, quercetin inhibited PCC proliferation by downregulating c-Myc expression. In addition, quercetin suppressed EMT via reducing the TGF-β1 level, resulting in inhibition of PCC migration and invasion. Moreover, quercetin induced PCC apoptosis through mitochondrial and death receptor pathways, but did not induce autophagy. In nude mouse models, the growth and metastasis of PDA were deprived by quercetin administration. Mechanically, quercetin exerts its therapeutic effects on PDA by decreasing SHH activity dependent on Gli2, not Gli1. Activated SHH abolished quercetin-mediated inhibition of PCC proliferation, migration, and invasion. Furthermore, Shh activated TGF-β1/Smad2/3 signaling and promoted the EMT process by inducing the EMT-TFs Zeb2 and Snail1, which eventually led to a partial reversal of quercetin-mediated inhibition of migration and invasion of PCCs. Thus, quercetin may be a potential candidate for the treatment of PDA.
Abbreviations
- Bax:
-
Bcl-2-associated X protein
- Bcl-2:
-
B cell lymphoma 2
- CCK-8:
-
Cell counting kit 8
- DMEM:
-
Dulbecco’s modified Eagle’s medium
- ELISA:
-
Enzyme-linked immunosorbent assay
- EMT:
-
Epithelial-to-mesenchymal transition
- EMT-TFs:
-
EMT-inducing transcription factors
- FBS:
-
Fetal bovine serum
- GAPDH:
-
Glyceraldehyde 3-phosphate dehydrogenase
- IHC:
-
Immunohistochemical
- PBS:
-
Phosphate buffer saline
- PCCs:
-
Pancreatic cancer cells
- PDA:
-
Pancreatic ductal adenocarcinoma
- qRT-PCR:
-
Quantitative reverse transcriptase-PCR
- RTCA:
-
Real-time cell analysis
- Shh:
-
Sonic hedgehog
- Smo:
-
Smoothened
- TGF-β1:
-
Transforming growth factor-β1
References
Aiello NM, Brabletz T, Kang Y, Nieto MA, Weinberg RA, Stanger BZ. Upholding a role for EMT in pancreatic cancer metastasis. Nature. 2017;547:E7–8.
Amakye D, Jagani Z, Dorsch M. Unraveling the therapeutic potential of the Hedgehog pathway in cancer. Nat Med. 2013;19:1410–22.
Bai CB, Auerbach W, Lee JS, Stephen D, Joyner AL. Gli2, but not Gli1, is required for initial Shh signaling and ectopic activation of the Shh pathway. Development. 2002;129:4753–61.
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424.
Cannac F, Qi C, Falschlunger J, Hausmann G, Basler K, Korkhov VM. Cryo-EM structure of the Hedgehog release protein Dispatched. Sci Adv. 2020;6:eaay7928.
Chen S, Jiang H, Wu X, Fang J. Therapeutic effects of quercetin on inflammation, obesity, and type 2 diabetes. Mediators Inflamm. 2016;2016:9340637.
Christenson ES, Jaffee E, Azad NS. Current and emerging therapies for patients with advanced pancreatic ductal adenocarcinoma: a bright future. Lancet Oncol. 2020;21:e135–45.
Collisson EA, Bailey P, Chang DK, Biankin AV. Molecular subtypes of pancreatic cancer. Nat Rev Gastroenterol Hepatol. 2019;16:207–20.
David CJ, Huang YH, Chen M, Su J, Zou Y, Bardeesy N, et al. TGF-beta tumor suppression through a lethal EMT. Cell. 2016;164:1015–30.
Ding Q, Motoyama J, Gasca S, Mo R, Sasaki H, Rossant J, et al. Diminished Sonic hedgehog signaling and lack of floor plate differentiation in Gli2 mutant mice. Development. 1998;125:2533–43.
Drenkhahn SK, Jackson GA, Slusarz A, Starkey NJ, Lubahn DB. Inhibition of hedgehog/Gli signaling by botanicals: a review of compounds with potential hedgehog pathway inhibitory activities. Curr Cancer Drug Targets. 2013;13:580–95.
Foltyn W, Zajecki W, Marek B, Kajdaniuk D, Sieminska L, Zemczak A, et al. The value of the Ki-67 proliferation marker as a prognostic factor in gastroenteropancreatic neuroendocrine tumours. Endokrynol Pol. 2012;63:362–6.
Fu J, Rodova M, Roy SK, Sharma J, Singh KP, Srivastava RK, et al. GANT-61 inhibits pancreatic cancer stem cell growth in vitro and in NOD/SCID/IL2R gamma null mice xenograft. Cancer Lett. 2012;330:22–32.
Gaarenstroom T, Hill CS. TGF-beta signaling to chromatin: how Smads regulate transcription during self-renewal and differentiation. Semin Cell Dev Biol. 2014;32:107–18.
Guo X, Wang XF. Signaling cross-talk between TGF-beta/BMP and other pathways. Cell Res. 2009;19:71–88.
Haque I, De A, Majumder M, Mehta S, McGregor D, Banerjee SK, et al. The matricellular protein CCN1/Cyr61 is a critical regulator of Sonic Hedgehog in pancreatic carcinogenesis. J Biol Chem. 2012;287:38569–79.
Heldin CH, Vanlandewijck M, Moustakas A. Regulation of EMT by TGFbeta in cancer. FEBS Lett. 2012;586:1959–70.
Hidalgo M, Maitra A. The hedgehog pathway and pancreatic cancer. N Engl J Med. 2009;361:2094–6.
Kasai K. GLI1, a master regulator of the hallmark of pancreatic cancer. Pathol Int. 2016;66:653–60.
Kawabata K, Mukai R, Ishisaka A. Quercetin and related polyphenols: new insights and implications for their bioactivity and bioavailability. Food Funct. 2015;6:1399–417.
Khaksary Mahabady M, Gholami MR, Najafzadeh Varzi H, Zendedel A, Doostizadeh M. Protective effect of quercetin on skeletal and neural tube teratogenicity induced by cyclophosphamide in rat fetuses. Vet Res Forum. 2016;7:133–8.
Kim JH, Kim MJ, Choi KC, Son J. Quercetin sensitizes pancreatic cancer cells to TRAIL-induced apoptosis through JNK-mediated cFLIP turnover. Int J Biochem Cell Biol. 2016;78:327–34.
Kuspert M, Weider M, Muller J, Hermans-Borgmeyer I, Meijer D, Wegner M. Desert hedgehog links transcription factor Sox10 to perineurial development. J Neurosci. 2012;32:5472–80.
Lan CY, Chen SY, Kuo CW, Lu CC, Yen GC. Quercetin facilitates cell death and chemosensitivity through RAGE/PI3K/AKT/mTOR axis in human pancreatic cancer cells. J Food Drug Anal. 2019;27:887–96.
Liu X, Sun N, Mo N, Lu S, Song E, Ren C, et al. Quercetin inhibits kidney fibrosis and the epithelial to mesenchymal transition of the renal tubular system involving suppression of the Sonic Hedgehog signaling pathway. Food Funct. 2019;10:3782–97.
Lu H, Chen B, Hong W, Liang Y, Bai Y. Transforming growth factor-beta1 stimulates hedgehog signaling to promote epithelial-mesenchymal transition after kidney injury. FEBS J. 2016;283:3771–90.
Lu H, Wu L, Liu L, Ruan Q, Zhang X, Hong W, et al. Quercetin ameliorates kidney injury and fibrosis by modulating M1/M2 macrophage polarization. Biochem Pharmacol. 2018;154:203–12.
Massague J. TGFbeta signalling in context. Nat Rev Mol Cell Biol. 2012;13:616–30.
McMahon SB. MYC and the control of apoptosis. Cold Spring Harb Perspect Med. 2014;4:a014407.
Moutasim KA, Mellows T, Mellone M, Lopez MA, Tod J, Kiely PC, et al. Suppression of Hedgehog signalling promotes pro-tumourigenic integrin expression and function. J Pathol. 2014;233:196–208.
Nevala-Plagemann C, Hidalgo M, Garrido-Laguna I. From state-of-the-art treatments to novel therapies for advanced-stage pancreatic cancer. Nat Rev Clin Oncol. 2019;17:108–23.
Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457–61.
Panzuto F, Boninsegna L, Fazio N, Campana D, Pia Brizzi M, Capurso G, et al. Metastatic and locally advanced pancreatic endocrine carcinomas: analysis of factors associated with disease progression. J Clin Oncol. 2011;29:2372–7.
Park HL, Bai C, Platt KA, Matise MP, Beeghly A, Hui CC, et al. Mouse Gli1 mutants are viable but have defects in SHH signaling in combination with a Gli2 mutation. Development. 2000;127:1593–605.
Probst S, Zeller R, Zuniga A. The hedgehog target Vlk genetically interacts with Gli3 to regulate chondrocyte differentiation during mouse long bone development. Differentiation. 2013;85:121–30.
Rhim AD, Mirek ET, Aiello NM, Maitra A, Bailey JM, McAllister F, et al. EMT and dissemination precede pancreatic tumor formation. Cell. 2012;148:349–61.
Rhodes DR, Yu J, Shanker K, Deshpande N, Varambally R, Ghosh D, et al. ONCOMINE: a cancer microarray database and integrated data-mining platform. Neoplasia. 2004;6:1–6.
Saini F, Argent RH, Grabowska AM. Sonic Hedgehog Ligand: a role in formation of a mesenchymal niche in human pancreatic ductal adenocarcinoma. Cells. 2019;8:424.
Salama YA, El-Karef A, El Gayyar AM, Abdel-Rahman N. Beyond its antioxidant properties: quercetin targets multiple signalling pathways in hepatocellular carcinoma in rats. Life Sci. 2019;236:116933.
Sistani Karampour N, Arzi A, Najafzadeh Varzi H, Mohammadian B, Rezaei M. Quercetin preventive effects on theophylline-induced anomalies in rat embryo. Jundishapur J Nat Pharm Prod. 2014;9:e17834.
Tang SN, Fu J, Nall D, Rodova M, Shankar S, Srivastava RK. Inhibition of sonic hedgehog pathway and pluripotency maintaining factors regulate human pancreatic cancer stem cell characteristics. Int J Cancer. 2011;131:30–40.
Tang YA, Chen YF, Bao Y, Mahara S, Yatim S, Oguz G, et al. Hypoxic tumor microenvironment activates GLI2 via HIF-1alpha and TGF-beta2 to promote chemoresistance in colorectal cancer. Proc Natl Acad Sci U S A. 2018;115:E5990–9.
Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–90.
Xu FG, Ma QY, Wang Z. Blockade of hedgehog signaling pathway as a therapeutic strategy for pancreatic cancer. Cancer Lett. 2009;283:119–24.
Xu Y, Chang R, Peng Z, Wang Y, Ji W, Guo J, et al. Loss of polarity protein AF6 promotes pancreatic cancer metastasis by inducing Snail expression. Nat Commun. 2015;6:7184.
Xu L, Deng S, Xiong H, Shi W, Luo S, Chen L. GATA-6 transcriptionally inhibits Shh to repress cell proliferation and migration in lung squamous cell carcinoma. Int J Biochem Cell Biol. 2019;115:105591.
Zhang J, Tian XJ, Xing J. Signal transduction pathways of EMT induced by TGF-beta, SHH, and WNT and their crosstalks. J Clin Med. 2016;5:28.
Zhang X, Lu H, Xie S, Wu C, Guo Y, Xiao Y, et al. Resveratrol suppresses the myofibroblastic phenotype and fibrosis formation in kidneys via proliferation-related signalling pathways. Br J Pharmacol. 2019;176:4745–59.
Zheng X, Carstens JL, Kim J, Scheible M, Kaye J, Sugimoto H, et al. Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature. 2015;527:525–30.
Zhou P, Li B, Liu F, Zhang M, Wang Q, Liu Y, et al. The epithelial to mesenchymal transition (EMT) and cancer stem cells: implication for treatment resistance in pancreatic cancer. Mol Cancer. 2017;16:52.
Acknowledgments
This study was sponsored by Wenzhou Science and Technology Plan Project, China (Grant No. Y20180100) and Key Laboratory of Diagnosis and Treatment of Severe Hepato-Pancreatic Diseases of Zhejiang Province (2018E10008).
Author information
Authors and Affiliations
Contributions
YB and XL designed the research. YG, HZ, and YX performed the experiments, analyzed the data, and drafted the manuscript. YG, HG, SM, LS, and WZ performed the experiments and collected data for the revision. YT and YB edited the manuscript. YB, SM, and XL contributed to the discussion and review of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Declaration of transparency and scientific rigor
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigor of preclinical research as stated in the BJP guidelines for Design & Analysis, Immunoblotting and Immunochemistry, and Animal Experimentation, and as recommended by funding agencies, publishers, and other organizations engaged with supporting research.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Bullet point summary
What is already known?
Quercetin inhibits the growth, migration, and invasion and induced apoptosis of pancreatic cancer cells.
What does this study add?
Gli2-dependent sonic hedgehog signaling is critical for quercetin-mediated anticancer effects in pancreatic ductal adenocarcinoma.
Clinical significance
Quercetin has therapeutic potential for the treatment of pancreatic ductal adenocarcinoma by targeting SHH and TGF-β/Smad signaling pathways.
Highlights
1. Quercetin inhibited growth, migration, and invasion and induced apoptosis of pancreatic cancer cells.
2. Gli2-dependent SHH signaling pathway was involved in quercetin-mediated anticancer effects.
3. Quercetin reduced SHH activity, thereby inhibiting TGF-β1/Smad2/3 signaling and blocking the EMT process by regulating the expression of EMT-TFs Zeb2 and Snail1.
Pharmacological nomenclature
TARGETS
Smo
LIGANDS
Quercetin
Transforming growth factor beta-1
Electronic supplementary material
Figure S1
Effect of quercetin on the activities of JAK2, β-catenin and mTOR. (A) Western blot analysis showing the expression and phosphorylation of JAK2 in quercetin-treated PANC-1 and Patu8988 cells. (B) Western blot analysis showing the expression and phosphorylation of β-catenin in quercetin-treated PANC-1 and Patu8988 cells. (C) Western blot analysis for the expression and phosphorylation of mTOR in quercetin-treated PANC-1 and Patu8988 cells. Data were presented as the mean ± standard deviation in quintuplicate for the cell line experiment. (PNG 583 kb)
Figure S2
Shh expression in human pancreatic ductal adenocarcinoma tissues. (A) Immunohistochemical (IHC) staining for Shh in human pancreatic ductal adenocarcinoma (PDA) and adjacent normal tissues. Bar = 100 μm and 50 μm. (B) ELISA for Shh level in pancreatic ductal epithelial cells (HPDE6-C7 and hTERT-HPNE) and tumor cells (PANC-1 and Patu8988). c In silico analyses of three other independent data sets obtained from Oncomine. *P < 0.05; ***P < 0.001. (PNG 1560 kb)
Figure S3
Gli2 expression in pancreatic ductal adenocarcinoma data sets. (A) In silico analyses of two other independent data sets obtained from Oncomine. (B) Correlation between Gli2 expression level and survival curve of pancreatic ductal adenocarcinoma (PDA). (PNG 399 kb)
Figure S4
Effect of TGF-β1 on SHH signaling in pancreatic cancer cells. Western blot analysis showing the expression of Smo, Gli1, and Gli2 in TGF-β1-treated PANC-1 and Patu8988 cells. Data were presented as the mean ± standard deviation in quintuplicate for the cell line experiment.*P < 0.05. (PNG 392 kb)
Figure S5
Effect of Gli2 siRNA on TGF-β1 expression in pancreatic cancer cells. (A) Western blot analysis showing the expression of Gli2 and TGF-β1 in PANC-1 cells. (B) qRT-PCR analysis showing the mRNA expression of Gli2 and TGF-β1 in PANC-1 cells. Data were presented as the mean ± standard deviation in quintuplicate for the cell line experiment.***P < 0.001. (PNG 241 kb)
ESM.6
(DOCX 16 kb)
ESM 7
(DOCX 14 kb)
Rights and permissions
About this article
Cite this article
Guo, Y., Tong, Y., Zhu, H. et al. Quercetin suppresses pancreatic ductal adenocarcinoma progression via inhibition of SHH and TGF-β/Smad signaling pathways. Cell Biol Toxicol 37, 479–496 (2021). https://doi.org/10.1007/s10565-020-09562-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10565-020-09562-0