Abstract
Aberrant expression of the Cullin 4A (CUL4A) is found in many tumor types, but the functions and mechanism of CUL4A in prostate cancer (PCa) development and progression remain largely unknown. The aim of this study was to investigate the possible role of CUL4A in prostate tumorigenesis. Immunohistochemistry was used to examine CUL4A expression in human PCa tissues and BPH tissues. Cell proliferation was assessed by MTT, and migration and invasion were analyzed by Transwell and Matrigel assays after CUL4A knockdown in PCa in vitro. The results showed that CUL4A protein was overexpressed in 86.21 % of PCa tissues. CUL4A knockdown with siRNA in PCa cells decreased cell proliferation, migration, and invasion. Mechanistically, CUL4A could modulate the expression of P53 in PCa cells. Our results indicate that CUL4A overexpression play an oncogenic role in the pathogenesis of PCa, and CUL4A may be a potential therapeutic target for PCa.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Prostate cancer (PCa) remains one of the most common malignancies in men [1]. In the early stage, the initial treatment is surgical resection followed by androgen deprivation therapy [2]. However, the vast majority of PCa patients eventually develop to castrate resistant prostate cancer (CRPC), which limits the potential of conventional therapeutic approaches [3]. Therapeutic strategies that enhance the chemotherapy efficacy and target CRPC are urgently required.
CUL4A was discovered along with CRL1 E3 ligases, better known as the SCF (S-phase kinase-associated protein 1 (SKP1)–cullin 1 (CUL1)–F-box protein) complex that serves as the archetype for the CRL family. CUL4A is overexpressed during the progression of numerous aggressive and recurrent cancers, and represents a determinant factor associated with tumor growth, metastases, and treatment resistance [4]. Thus, CUL4A may act as an oncogene, but whether CUL4A plays a key role in PCa remains unclear. This study intends to investigate the expression of CUL4A and the function of the CUL4A in PCa.
Materials and methods
Patients and tissue specimens
This study included 116 patients who had undergone radical prostatectomy and bilateral lymphadenectomy at the Department of Urology, Affiliated Hospital of Weifang Medical University, between August 2005 and December 2013, and for whom archival tissues were available. No patient was managed preoperatively with either hormonal or radiation therapy, and no secondary cancers were observed. Ninety-six cases of benign prostate hyperplasia (BPH) were obtained from men undergoing suprapubic prostatectomy or transurethral plasmakinetic enucleation of prostate. Twenty-four cases of normal prostate tissue were obtained from bladder cancer patients who underwent radical cystoprostatectomy. The stages of cancer for all patients were determined by the American Joint Committee on Cancer (AJCC) 2002 system. The specimens were examined by two staff pathologists who were blinded to the clinical outcome and follow-up data. The evaluation of the specimen was performed according to the guidelines of the College of American Pathologists. This study was approved by the Ethics Committee of Weifang Medical University. All patients provided informed consent. All specimens were stored at −80 °C until the analysis. In addition, we still collected all of the above patients’ paraffin blocks to perform immunohistochemistry staining.
Cell lines and cell culture
The normal prostate epithelial cell line, RWPE2, and PCa cell lines LNCaP, DU145, and PC3 were obtained from the American Type Culture Collection. RWPE2 cells were maintained in keratinocyte serum-free growth media (K-SFM; Invitrogen), supplemented with bovine pituitary extract (0.05 mg/mL; Invitrogen) and human recombinant EGF (5 ng/mL; Invitrogen). LNCaP and PC3 cells were cultured in RPMI-1640 (Invitrogen) supplemented with 10 % FBS (Hyclone Laboratories). DU145 cells were cultured in Eagle’s Minimum Essential Medium (E-MEM; Quality Biological) with 10 % FBS. Infected cell lines were maintained in the appropriate medium with puromycin (0.5 mg/mL; Calbiochem, EMD Chemicals, Inc.). All cells were incubated at 37 °C in 5 % CO2. Androgen-deprivation experiments used RPMI-1640 supplemented with 10 % charcoal-stripped FBS (CS-FBS; Invitrogen). All experiments were carried out using cells harvested at low (<20) passages.
Immunohistochemistry
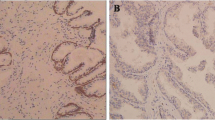
Immunostaining was performed using the avidin-biotinperoxidase complex method (UltrasensitiveTM, MaiXin, Fuzhou, China). The sections were deparaffinized in xylene, rehydrated with graded alcohol, and then boiled in 0.01 M citrate buffer (pH 6.0) for 2 min with an autoclave. Hydrogen peroxide (0.3 %) was applied to block endogenous peroxide activity, and the sections were incubated with normal goat serum to reduce nonspecific binding. Tissue sections were incubated with CUL4A rabbit polyclonal antibody (1:250 dilution). Mouse immunoglobulin (at the same concentration of the antigen specific antibody) was used as a negative control. Staining for both antibodies was performed at room temperature for 2 h. Biotinylated goat antimouse serum IgG was used as a secondary antibody. After washing, the sections were incubated with streptavidin-biotin conjugated with horseradish peroxidase, and the peroxidase reaction was developed with 3, 30-diaminobenzidine tetrahydrochloride. Two independent, blinded investigators examined all tumor slides randomly. Five views were examined per slide, and 100 cells were observed per view at 400× magnification. Given the homogenicity of the staining of the target proteins, tumor specimens were scored in a semi-quantitative manner. The percentage scoring of immunoreactive tumor cells was as follows: 0 (0 %), 1 (1–10 %), 2 (11–50 %), and 3 (>50 %). The staining intensity was visually scored and stratified as follows: 0 (negative), 1 (weak), 2 (moderate), and 3 (strong). A final immunoreactivity scores (IRS) was obtained for each case by multiplying the percentage and the intensity score. Protein expression levels were further analyzed by classifying IRS values as low (based on a IRS value less than 4) and as high (based on a IRS value greater than 4).
Reverse transcription polymerase chain reaction
Semi-quantitative RT-PCR was used in the measurement of CUL4A mRNA expression. Total RNA was extracted from three independent plates for each cell line using TRIzol reagent (Invitrogen, Carlsbad, California, USA). The reverse transcription reaction was performed with 5 mg of total RNA using M-MuLV reverse transcriptase (TaKaRa, Japan) at 42 °C for 60 min, and 0.5 mg cDNA was used for RT-PCR. The PCR step was performed using Taq DNA polymerase (TaKaRa, Japan). As an internal control, human GAPDH was amplified to ensure cDNA quality and quantity for each RT-PCR reaction. PCR primers were as follows: for human GAPDH, forward 5′-ATAGCACAGCCTGGATAGCAACGTAC-3′ and reverse 5′-CACCTTCTACAATGAGCTGCGTGTG-3′; for CUL4A, forward 5′-ATACTTCAGGACCCACGTTTGAT-3′ and reverse 5′-TCTCCAAGTACTAAAGCAGGAAAATCT-3′. Triplicate independent PCR reactions were carried out to ensure the reproducibility of expression. The result was analyzed by Quantity One 4.4.1 software (Bio-Rad Laboratories Inc, Hercules, California, USA).
Western blot analysis
Cells were harvested and lysed in RIPA buffer [50 mmol/L Tris-HCl, pH 8.0, with 150 mmol/L NaCl, 1.0 % Igepal CA-630 (NP-40), 0.5 % sodium deoxycholate, and 0.1 % SDS; Sigma-Aldrich, St. Louis, MO] and protease inhibitor cocktail (Roche Applied Science, Foster City, CA). The protein concentration of each sample was determined using a BCA Protein Assay Kit (Pierce Chemical Co., Rockford, Illinois, USA). Twenty micrograms of denatured total protein was subsequently applied to one end of 10 % SDS polyacrylamide gels submerged in a suitable buffer. Electrophoresis was terminated when the dyestuff had run to the edge of the other end of the gel. The proteins on the gel were then transferred onto a nitrocellulose membrane (Millipore, Temecular, California, USA). The membrane was blocked with 5 % defatted milk (in 25 mmol/L Tris, pH 8.0, 125 mmol/L NaCl, 0.1 % Tween 20) overnight at room temperature and incubated with anti-CUL4A (1:1000; CST), and anti-β-actin (1:2000, Santa Cruz Biotechnology) antibodies at room temperature for 1 h. After washing, the membrane was reacted with horse radish peroxidase-conjugated secondary antibodies at room temperature for 1 h and washed again. Finally, the immunoreactive bands were visualized using an ECL Western blotting system (GE, Fairfield, CT, USA).
Small interfering RNA transfection
For knockdown of CUL4A, small interfering RNA (siRNA) duplexes were used. The CUL4A and negative control siRNA duplexes were purchased from GenePharma, and the oligonucleotide sequences were as follows: CUL4A, 5′-CCAUGUAAGUAAACGCUUATT-3′; and negative control, 5′-UUCUCCGAACGUGUCACGUTT-3′. Transfection of siRNAs into PCa cells was carried out using Lipofectamine LTX and Lipofectamine 2000 CD (Invitrogen), respectively. For transient transfection, cells were transfected with plasmids or siRNA at different doses as indicated for 48 h before functional assays were carried out. PCa cells treated with transfection reagent alone were included as mock controls.
Cell survival assay
The effects of CUL4A on PCa cells survival were determined by MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazoliumbromide) assay. Four groups of cells were seeded into 96-well plates (5 × 103 cells/well) and cultured for 120 h. After treatments, cells were incubated with MTT (Sigma-Aldrich, St. Louis, MO) 20 μl/well at 37 °C for 4 h, and then 200 μl DMSO was added into each well. Cells were subjected to absorbance reading at 570 nm using a 96-well microplate reader. Percentage of residual cell viability was determined as (OD of experiment group − OD of blank group)/(OD of negative group − OD of blank group) × 100 %. Assays were performed three times.
Transwell migration assay
Cells were plated at a density of 105 cells in 24-well culture plates, and migration assays were done using a chemotaxis chamber (BD Biosciences, San Jose, CA) and transwell tissue culture plates (8 Am pore size). The bottom of the chamber was coated with either 10 Ag/mL fibronectin or Matrigel (BD Biosciences). One-hundred microliters of a 105-cells/mL suspension were introduced into each well and allowed to migrate for 6 h. Cells were then fixed with methanol and stained with crystal violet.
Statistical analysis
Data analyses were performed using SPSS statistical package 15.0 (SPSS Inc, USA). Patient characteristics were expressed as the mean ± SD for continuous variables, and as the count and percent for discrete variables. Data were analyzed using the Pearson’s chi-square test and Fisher’s exact test. Statistical significance was taken at the P < 0.05 level.
Results
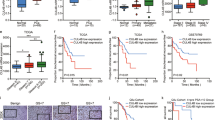
We first examined the expression of CUL4A in a panel of three human PCa cell lines and one normal human prostate epithelial cell line by QRT-PCR and Western blot. In both analyses, all of tumor cell lines were within the higher relative expression levels indicating that CUL4A is overall up-regulated in PCa cell lines compared with that in normal human prostate epithelial cells (Fig. 1a, b). We then determined CUL4A expression in clinical samples using QRT-PCR. Of 116 PCa patients, 93 (80.17 %) had higher CUL4A mRNA levels than BPH tissues (Fig. 2a). Overall, the average CUL4A mRNA levels in the cancer tissues were significantly higher than those in the BPH tissues and normal tissues. Moreover, we performed immunohistochemistry analysis in 116 PCa specimens and 96 BPH tissues and found that CUL4A level was overexpress in 86.21 % of tumor samples (100 of 116) (Fig. 2b). The CUL4A protein appeared to be expressed in both cytoplasmic and nuclear components of tumor cells with stronger signal observed in cytoplasm.
These results of clinical samples prompted us to examine whether targeted CUL4A inhibition would affect the proliferative and malignant properties of PCa. We then used PCa cell lines as a model to verify the function and underlying mechanisms of CUL4A in promoting PCa proliferation and metastasis. For this purpose, we specifically knocked down the CUL4A expression in PCa cell lines using RNA interference. We used two representative PCa cell lines, PC3 and LNCaP, in which CUL4A is highly overexpressed (Fig. 1a). The efficacy of CUL4A siRNA for knockdown of CUL4A protein was determined by Western blot analysis. As shown in Figs. 3a and 4a, CUL4A protein levels were significantly reduced in cells expressing CUL4A siRNA (>95 % reduction in endogenous levels) but not in cells expressing control siRNA.
We next evaluated the effects of CUL4A depletion on PCa cell growth and migration. Suppression of CUL4A by siRNA resulted in a substantial decrease in growth rate, but cells expressing control siRNA vectors grew normally in these PCa cells (Figs. 3b and 4b). We next investigated whether CUL4A knockdown modifies extracellular matrix interactions and causes an increase or a decrease in cell migration. We tested cellular migration and invasion levels using transwell chambers coated with Matrigel. As shown in Fig. 5, CUL4A siRNA shows a significant decrease in migration, whereas no effect was seen in PC3 cells expressing control siRNA. These results indicate that CUL4A inhibition suppresses cell migration and invasion.
Discussion
The overexpression of the CUL4A E3 ubiquitin ligase has been related to tumor aggressiveness and poor clinical outcome in various cancers. The present study demonstrates for the first time that CUL4A gene is amplified in human PCa cell lines. Consistent with gene amplification, overexpression of CUL4A protein was observed in PCa cell lines and human PCa tissues compared with BPH tissues. CUL4A gene silencing in PCa cells resulted in decreased proliferation, migration, and invasion in vitro. Thus, our results indicate that amplification of CUL4A gene may be an important oncogenic event in PCa development.
CUL4A employs the structurally distinct triple WD40 β-propeller domain-containing DDB1 adaptor to recruit members of the DDB1-CUL4A associated factor (DCAF) family of substrate receptors [5–7]. CUL4A was initially identified as amplified or overexpressed in various cancers [8–10]. CUL4A overexpression may contribute to tumorigenesis and cancer development in cancer cells, because CUL4A has been observed in the ubiquitination and proteolysis of tumor suppressors, such as p21, p27, and p53 [11]. Moreover, high CUL4A expression correlates with significantly shorter overall and disease-free survivals, indicating that dysregulation of CUL4A may play a role in promoting oncogenesis [12].
In this study, we examined the expression of CUL4A in clinical PCa tissues by immunohistochemistry, Western blotting, and qRT-PCR. The immunohistochemistry analysis showed that the positive rate of CUL4A staining was 86.21 % (100/116) in 116 cases of PCa. Furthermore, CUL4A was significantly elevated in all PCa cell lines when compared to the RWPE-1 cells at both the mRNA and protein levels. CUL4A silencing in CUL4A-overexpressing PCa cells induced a reduction of cell proliferation in MTT assay. Depletion of endogenous CUL4A attenuated proliferation of PCa cells in vitro; MTT cell proliferation assay showed that CUL4A siRNA significantly reduced the proliferation rate of siRNA cells compared with the control siRNA-transfected cells. Thereby CUL4A would constitute a promising target for therapeutic intervention and our data reinforce its clinical value in PCa.
CUL4A has been reported to be associated with malignant-cell behavior in human cancers. The evidence suggesting a role of CUL4A in tumor invasion and metastasis has increased. The effect of CUL4A on PCa cell migration and invasion were measured by Transwell and Matrigel. The results showed depletion of CUL4A could inhibit cell migration and invasion in vitro, suggesting that CUL4A expression can significantly promoted PCa cell proliferation, migration, and invasion. CUL4A complex has been known to target a multitude of regulatory proteins, thereby exerting its effect on important cellular processes. Cell growth-promoting and oncogenic activities of CUL4A proteins are based on their ability to regulate the expression of genes such as p53, p21, and p27 that have a crucial role in the control of cell proliferation [7, 13–15]. To determine whether p53 is a downstream target of CUL4A in PCa, expression of p53 in the cells with altered CUL4A expression was evaluated. The results showed that silencing of CUL4A expression in PCa cell showed dramatically increased p53 protein level. Our results indicate that CUL4A might play a central role in the oncogenic process by modulating the expression of p53 protein level.
In summary, we have shown for the first time that the CUL4A is overexpressed in PCa tissues and cell lines. Through siRNA knockdown of ectopic CUL4A studies, we also showed that CUL4A controls PCa cell proliferation, invasion, and migration. We propose CUL4A as an important contributor to the development and progression of PCa; however, additional studies are needed to elucidate the mechanisms of CUL4A related to its promoting effects in PCa.
References
Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9–29.
Zhang L, Yang BX, Zhang HT, Wang JG, Wang HL, et al. Prostate cancer: an emerging threat to the health of aging men in Asia. Asian J Androl. 2011;13:574–8.
Ma Y, Yang HZ, Dong BJ, Zou HB, Zhou Y, Kong XM, et al. Biphasic regulation of autophagy by miR-96 in prostate cancer cells under hypoxia. Oncotarget. 2014;5(19):9169–82.
Birner P, Schoppmann A, Schindl M, Dinhof C, Jesch B, Berghoff AS, et al. Human homologue for Caenorhabditis elegans CUL-4 protein overexpression is associated with malignant potential of epithelial ovarian tumours and poor outcome in carcinoma. J Clin Pathol. 2012;65(6):507–11.
Lee J, Zhou P. Pathogenic role of the CRL4 ubiquitin ligase in human disease. Front Oncol. 2012;2:21.
Sugasawa K. The CUL4 enigma: culling DNA repair factors. Mol Cell. 2009;34:403–4.
Nag A, Bagchi S, Raychaudhuri P. Cul4A physically associates with MDM2 and participates in the proteolysis of p53. Cancer Res. 2004;64:8152–5.
Liu L, Lee S, Zhang J, Peters SB, Hannah J, Zhang Y, et al. CUL4A abrogation augments DNA damage response and protection against skin carcinogenesis. Mol Cell. 2009;34:451–60.
Bondar T, Kalinina A, Khair L, Kopanja D, Nag A, Bagchi S, et al. Cul4A and DDB1 associate with Skp2 to target p27Kip1 for proteolysis involving the COP9 signalosome. Mol Cell Biol. 2006;26:2531–9.
Gupta A, Yang LX, Chen L. Study of the G2/M cell cycle checkpoint in irradiated mammary epithelial cells overexpressing Cul-4A gene. Int J Radiat Oncol Biol Phys. 2002;52:822–30.
Hung MS, Mao JH, Xu Z, Yang CT, Yu JS, Harvard C, et al. Cul4A is an oncogene in malignant pleural mesothelioma. J Cell Mol Med. 2011;15:350–8.
Schindl M, Gnant M, Schoppmann SF, Horvat R, Birner P. Overexpression of the human homologue for Caenorhabditis elegans cul-4 gene is associated with poor outcome in nodenegative breast cancer. Anticancer Res. 2007;27:949–52.
Li B, Jia N, Kapur R, Chun KT. Cul4A targets p27 for degradation and regulates proliferation, cell cycle exit, and differentiation during erythropoiesis. Blood. 2006;107:4291–9.
Nishitani H, Shiomi Y, Iida H, Michishita M, Takami T, Tsurimoto T. CDK inhibitor p21 is degraded by a proliferating cell nuclear antigen-coupled Cul4-DDB1Cdt2 pathway during S phase and after UV irradiation. J Biol Chem. 2008;283:29045–52.
El-Mahdy MA, Zhu Q, Wang QE, Wani G, Praetorius-Ibba M, Wani AA. Cullin 4A-mediated proteolysis of DDB2 protein at DNA damage sites regulates in vivo lesion recognition by XPC. J Biol Chem. 2006;281:13404–11.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
The Publisher and Editor retract this article in accordance with the recommendations of the Committee on Publication Ethics (COPE). After a thorough investigation we have strong reason to believe that the peer review process was compromised.
An erratum to this article is available at http://dx.doi.org/10.1007/s13277-017-5487-6.
About this article
Cite this article
Liu, G., Zhu, Z., Lang, F. et al. RETRACTED ARTICLE: Clinical significance of CUL4A in human prostate cancer. Tumor Biol. 36, 8553–8558 (2015). https://doi.org/10.1007/s13277-015-3580-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-015-3580-2