Abstract
The aim of the present study was to determine the most meaningful preoperative prognostic factor of cancer-related death in ovarian cancer patients by comparing potentially prognostic systemic inflammatory response (SIR) markers. The levels of fibrinogen, albumin, C-reactive protein (CRP), and serum cancer antigen-125 (CA-125) and the neutrophil/lymphocyte ratio (NLR) and platelet/lymphocyte ratio (PLR) were evaluated in 190 ovarian cancer patients to identify predictors of overall survival (OS) and progression-free survival (PFS) using univariate and multivariate analyses. Patients with a PLR >203 had a shorter PFS and OS than the patients in PLR ≤203 group (11 vs. 24 months and 28 vs. 64 months). Univariate analyses revealed that tumor stage, postoperative residual tumor mass, ascites, and the levels of all SIR markers were associated with PFS and OS. Multivariate analysis revealed that PLR was independently associated with PFS (hazard ratio [HR] 1.852, 95 % confidence interval [CI] 1.271–2.697, P = 0.001) and OS (HR 2.158, 95 %CI 1.468–3.171, P < 0.001), as well as tumor stage and postoperative residual tumor mass. In contrast, fibrinogen remained significant only for PFS (HR 1.724, 95 %CI 1.197–2.482, P = 0.003). Patients with a PLR >203 were more prone to have advanced tumor stage (P = 0.002), postoperative residual tumor mass >2 cm (P = 0.032), malignant ascites (P < 0.001), and all the other elevated SIR markers (P < 0.001). Preoperative PLR is superior to other SIR markers (CA-125, NLR, fibrinogen, CRP, and albumin) as a predictor of survival in ovarian cancer patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ovarian cancer is the primary cause of death in females with gynecological malignancies worldwide, due to late detection, tumor heterogeneity, and a high rate of metastasis [1]. It is estimated that there will be ∼21,980 new cases and 14,270 deaths due to ovarian cancer in the USA in 2014 [2]. Primary cytoreductive surgery followed by adjuvant chemotherapy, if required, remains the standard treatment for ovarian cancer patients. However, the survival rate varies considerably in individuals with the same pathological stage and treatment. These survival differences might be caused by host-related factors, such as systemic inflammatory response (SIR) markers. Over the last 10 years, laboratory SIR markers such as hypoalbuminemia, hyperfibrinogenemia, C-reactive protein (CRP), absolute white blood cell count, neutrophil/lymphocyte ratio (NLR), and platelet/lymphocyte ratio (PLR) have been investigated as prognostic factors in patients with various types of cancer [3–8].
In recent years, chronic inflammation was identified as a key factor in the pathogenesis of ovarian cancer [9]. Furthermore, ovulation itself is a potentially inflammatory and mutagenic process [10]. Inflammation influences all stages of cancer formation, including initiation, promotion, and progression [9]. Various inflammatory mediators are induced by inflammatory or tumor cells, and they then participate in cancer formation by acting as growth or angiogenic factors. In addition, immune function is compromised by SIR mediators, which then increases the levels of leukocytes, neutrophils, platelets, CRP, and fibrinogen and also decreases lymphocyte concentrations. Although the number of circulating platelets can be significantly increased by cancer-induced thrombocytosis, the mechanisms responsible remained poorly understood until recently [11]. A multicenter study involving 619 ovarian cancer patients not only found that thrombocytosis was significantly associated with the poor prognosis and survival of the patients, but also discovered that inflammatory cytokines interleukin-6 (IL-6) can influence thrombocytosis in ovarian cancer by stimulating hepatic thrombopoietin synthesis and paraneoplastic induction of thrombocytosis in mouse models of ovarian cancer [12]. So, it is easy to think that use of anti-IL-6 antibody to halve platelet counts can significantly inhibit the tumor growth. Fortunately, all these have been confirmed in tumor-bearing mice and ovarian cancer patients by Stone and his colleagues [12].
To better estimate the survival of ovarian cancer patients, many laboratory SIR markers such as serum cancer antigen-125 (CA-125) [13], albumin [14], CRP [15], NLR [16], PLR [17], and fibrinogen [18] have been investigated as prognostic and predictive markers in patients with ovarian cancer. However, the association between PLR and survival is controversial. Some previous studies suggested that NLR is a superior prognostic factor to PLR in cancer patients [19, 20]. Currently, there are no established preoperative markers, including CA-125, which could predict cancer-related overall survival (OS) in ovarian cancer patients. Therefore, it is interesting that combining clinical preoperative systemic inflammatory markers and intrinsic tumor cell properties might yield useful prognostic indictors for survival.
Materials and methods
Patients
Between January 2000 and December 2012, 190 patients were enrolled in this study at Nanfang Hospital of Southern Medical University (Guangzhou, Guangdong Province, China). The ethics committee of Southern Medical University approved the study protocol. The inclusion criteria were new diagnosis and treatment with cytoreductive surgery followed by platinum-based chemotherapy in our hospital. The exclusion criteria included the presence of active infection, coexisting hematological malignancies, other hematological disorders, or autoimmune disorders.
All ovarian cancer patients were followed up every 2–4 months for the first 2 years and every 3–6 months thereafter until December 2013. At each visit, the patients were assessed by clinical, imaging examinations, and the serum level of CA-125. The median follow-up time was 43 months (range 2–164 months).
Clinicopathological data such as age, surgical International Federation of Gynecologists and Obstetricians (FIGO) stage (2010), the presence of ascites, postoperative residual tumor mass, histological grade, and subtype were obtained. Optimal surgery was defined as the size of each foci of residual disease after surgery was ≤2 cm [21]. Data regarding the levels of preoperative SIR markers, including serum albumin and CRP (AU800, Olympus, Japan), plasma fibrinogen (Sysmex CA-1500, TOA Medical Electronics, Kobe, Japan), and complete blood cell count (platelet, neutrophil, and lymphocyte counts) (CELL-DYN3500, Abbott, Chicago, USA), as well as serum CA-125 (ACS-A80, Bayer, Germany) were also obtained. The NLR was defined as the absolute neutrophil count divided by the absolute lymphocyte count, and the PLR was defined as the absolute platelet count divided by the absolute lymphocyte count. The normal reference range for plasma fibrinogen levels was 2–4 g/L, and a plasma fibrinogen level >4 g/L was defined as hyperfibrinogenemia. A serum albumin level <40 g/L was defined as hypoalbuminemia, and a serum CA-125 level >35 U/mL was used to diagnose ovarian cancer. A serum CRP level lower than 10 mg/L was regarded as normal. The primary endpoint of the study was progression-free survival (PFS), which was calculated from the date of operation to the date of the first tumor recurrence. OS was defined as the time from operation to death or the last follow-up.
Statistical analysis
Statistical analyses were performed using the SPSS 13.0 statistical software (SPSS, Chicago, IL, USA). Variables are presented as the means (standard deviation [SD]). A receiver operating characteristic (ROC) curve was constructed to estimate the optimal cutoff values for preoperative NLR and PLR. The associations between preoperative PLR and clinicopathological characteristics were evaluated using Pearson’s correlation coefficient, unpaired t tests, and one-way analysis of variance, as appropriate. Univariate and multivariate Cox regression models for PFS and OS were performed, comprising tumor stage (stage I, II, III, or IV), postoperative residual tumor mass (≤2 vs. >2 cm), histological grade (G1, G2, or G3), histological subtype (serous, mucinous, clear cell, endometrioid, mixed type, or adenocarcinoma/not otherwise specified), ascites (yes or no), patient age (≤50 vs. >50 years), fibrinogen levels (≤4 vs. >4 g/L), serum albumin levels (≤40 vs. >40 g/L), CRP levels (≤10 vs. >10 mg/L), CA-125 levels (≤35 vs. >35 U/mL), NLR (≤3.4 vs. >3.4), and PLR (≤203 vs. >203). The differences in survival among groups were analyzed using Kaplan-Meier curves and log rank tests. P values <0.05 were considered statistically significant.
Results
Patient characteristics
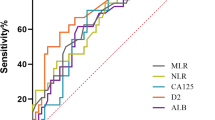
The mean patient age was 50.6 ± 11.1 years (range 24–76 years). The mean (SD) levels of albumin, fibrinogen, CRP, NLR, and PLR were 37.54 g/L (5.26), 4.11 g/L (1.49), 28.72 mg/L (44.58), 3.87 (2.66), and 234.50 (140.037), respectively. For the purpose of analysis, patients were then separated into elevated and non-elevated subgroups according to the NLR or PLR using the cutoff values derived from ROC curves (NLR, 3.4; PLR, 203). The areas under the curve (AUC) for NLR and PLR for OS were 0.650 (95 % confidence interval (CI) 0.566–0.735) and 0.737 (95 %CI 0.548–0.726), with a sensitivity (specificity) of 49.3 % (74.1 %) and 56 % (67 %), respectively. Patients were also divided into subgroups for fibrinogen (≤4 vs. >4 g/L), CRP (≤10 vs. >10 mg/L), and albumin (≤40 vs. >40 g/L) according to the upper limit levels of other SIR markers.
The relationship between preoperative PLR and the clinicopathological characteristics of patients with ovarian cancer is shown in Table 1. Patients with a PLR >203 were more prone to have advanced tumor stage (P = 0.032), postoperative residual tumor >2 cm (P = 0.002), massive ascites (P < 0.001), higher CA-125 (P < 0.001), higher CRP (P < 0.001), hyperfibrinogenemia (P < 0.001), hypoalbuminemia (P < 0.001), and an elevated NLR (P < 0.001). But, there is no statistic difference between high PLR and histological subtype or histological grade.
Prognostic factors
Univariate analyses revealed that tumor stage, postoperative residual tumor mass, ascites, CA-125 levels, fibrinogen, albumin, CRP, NLR, and PLR were significantly associated with both PFS and OS (Tables 2 and 3). Multivariate analysis demonstrated that tumor stage (hazard ratio (HR) 1.909, 95 %CI 1.414–2.579, P < 0.001), postoperative residual tumor mass (HR 2.486, 95 %CI 1.638–3.774, P < 0.001), fibrinogen levels (HR 1.724, 95 %CI 1.197–2.482, P = 0.003), and PLR (HR 1.852, 95 %CI 1.271–2.697, P = 0.001) were significantly associated with PFS (Table 2). Tumor stage (HR 2.161, 95 %CI 1.532–3.047, P < 0.001), postoperative residual tumor mass (HR 2.175, 95 %CI 1.406–3.365, P < 0.001), and PLR (HR 2.158, 95 %CI 1.468–3.171, P < 0.001) were also independently and significantly associated with OS (Table 3).
When patients were grouped according to the optimal cutoff for PLR determined using Kaplan-Meier survival analysis (203), PFS (P < 0.001) and OS (P < 0.001) were significantly shorter in patients with a PLR >203 compared with those with a PLR ≤203 (Fig. 1a, b). The median PFS and OS in patients with a PLR >203 were 11 and 28 months, respectively, compared with 24 and 64 months in those with a PLR ≤203. In both groups, patients with an optimal surgery had a longer PFS and OS than the patients with a suboptimal surgery (16 vs. 8 months and 43 vs. 23 months, all P < 0.001, respectively, in the PLR >203 group and 30 vs. 16 months and 70 vs. 43 months, all P < 0.001, respectively, in the PLR ≤203 group). In the optimal surgery group, the median PFS and OS were shorter in patients with a PLR >203 than those with a PLR ≤203 (8 vs. 16 months, P = 0.003 and 43 vs. 70 months, P = 0.004, respectively). In the suboptimal surgery, the median PFS and OS were shorter in patients with a PLR >203 than those with a PLR ≤203 (16 vs. 30 months and 23 vs. 43 months, all P < 0.001). It is the PFS (P = 0.037) not the OS (P = 0.288) that is significantly shorter in patients who had a PLR >203 and optimal surgery than the patients with a PLR ≤203 and suboptimal surgery.
Discussion
The aim of the present study was to identify a clinically useful prognostic factor among preoperative host factors and tumor factors in ovarian cancer patients who underwent cytoreductive surgery followed by platinum-based chemotherapy. This is the first study to show that PLR is a superior independent prognostic factor compared with other SIR markers in patients with ovarian cancer.
Since Virchow first described the presence of leukocytes in neoplastic tissue in 1863 [22], increasing evidence has revealed that many SIR markers, except for tumor-related factors, are associated with survival in patients with various cancers. SIR markers are host-related factors that are predominantly biochemical or hematological in nature, including CA-125, albumin, CRP, white blood cell counts, neutrophils, platelets, fibrinogen, and a combination thereof. Consequently, many studies have attempted to identify an independent SIR-related prognostic factor in various cancers. However, few studies combined these potential prognostic SIR markers to obtain an optimal prognostic factor that could better guide individualized treatment strategies and predict patient prognosis and survival. Therefore, we compared the prognostic significance of the SIR markers that were reported to be prognostic factors in ovarian cancer to determine the most meaningful predictor of PFS and OS.
Consistent with previous studies, tumor stage and residual tumor mass were the most significant predictors of patient survival [23]. Unlike some previous reports [13, 14, 16, 18], the current study demonstrated that CA-125, albumin, CRP, fibrinogen, and NLR were not independent prognostic indicators of survival in ovarian cancer patients. Petri et al., who studied serum CA-125 in 118 FIGO I ovarian cancer patients, found that elevated levels of CA-125 were significantly associated with shorter survival [13]. A multicenter study by Polterauer et al. determined that pretherapeutic hyperfibrinogenemia was associated with shorter survival in 422 patients with epithelial ovarian cancer [18]. Hefler et al. [15] also demonstrated that CRP was a novel and an independent prognostic variable in ovarian cancer. In addition, Asher et al. showed that both preoperative serum albumin and PLR were independent prognostic factors in 235 ovarian cancer patients [14, 17]. Although Asher et al. [17] and Raungkaewmanee et al. [24] both reported that PLR was an independent prognostic factor in patients with ovarian cancer, they did not assess the combination of PLR with other prognostic markers such as ascites, CA-125, fibrinogen, CRP, and albumin. Nevertheless, the prognostic value of PLR in cancer is controversial. Some previous studies demonstrated that NLR was a superior independent predictor of survival, as compared to PLR in various cancers [25–28]. Thus, we conducted the current study to determine whether preoperative PLR was the most meaningful SIR marker to predict the survival of ovarian cancer patients.
PLR is a reproducible, inexpensive, and widely available laboratory hematological marker that was suggested recently to be a marker of thrombotic and inflammatory conditions, mainly in patients with malignancies [29, 30]. Preoperative thrombocytosis was an unfavorable predictor of survival in ovarian cancer patients [31]. The activation and aggregation of platelets occur in response to the release of inflammatory cytokines and ADP from tumor cells [32, 33]. Thrombocytosis not only promotes tumor cell invasion and metastasis [33] but could also reflect a state of systemic inflammation [32].
Stone et al. [12] also demonstrated that anti-IL-6 antibody treatment can suppress the tumor growth by decreasing the platelet counts in ovarian cancer patients. Since Riesco reported that peripheral lymphocytes were positively associated with the “curability” of a variety of cancers [34], many studies have suggested that lymphocytes are predictors of survival in ovarian cancer patients [35, 36]. T lymphocytes form the major component of the cellular immune response and are essential for anti-tumor immunity [37]. Lymphopenia also correlates strongly with increased serum levels of IL-6, as well as the TNF receptor in soft tissue sarcomas [38]. In addition to inflammatory cytokines, the secretion of vascular endothelial growth factor (VEGF) from ovarian cancer cells could inhibit T cell development [39, 40]. In the present study, Kaplan-Meier analysis and log-rank tests determined that patients with a PLR >203 had a shorter PFS and OS compared with those with a PLR ≤203. In addition, a PLR >203 was not only associated with other SIR markers (CA-125, fibrinogen, albumin, and NLR) but was also related to tumor biological characteristics such as advanced tumor stage. Furthermore, the outcomes in the suboptimal surgery group were shorter in patients with a PLR >203 than PLR ≤203. So, if we combine the chemotherapy and anti-IL-6 antibody in such patients who have a suboptimal surgery and a PLR ≤203, it can significantly prolong the survival. These results suggest that PLR should be included in the routine assessment of patients with ovarian cancer.
This study has some limitations. First, it was a retrospective study based in a single institution. Second, all of the included patients underwent cytoreductive surgery followed by platinum-based chemotherapy. In addition, the patient sample size was relatively small. Thus, additional studies are needed to reach an international consensus and determine the prognostic value of PLR in combination with different morphological and biological parameters in patients with ovarian cancer.
In conclusion, this study demonstrates that the SIR marker PLR is an independent prognostic factor in patients with ovarian cancer.
References
Cohen CA, Shea AA, Heffron CL, Schmelz EM, Roberts PC. The parity-associated microenvironmental niche in the omental fat band is refractory to ovarian cancer metastasis. Cancer Prev Res (Phila). 2013;6:1182–93.
Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29.
Hirashima K, Watanabe M, Shigaki H, Imamura Y, Ida S, Iwatsuki M, et al. Prognostic significance of the modified Glasgow prognostic score in elderly patients with gastric cancer. J Gastroenterol. 2014;49:1040–6.
Wu N, Chen G, Hu H, Pang L, Chen Z. Low pretherapeutic serum albumin as a risk factor for poor outcome in esophageal squamous cell carcinomas. Nutr Cancer. 2015;67:481–5.
Oh BS, Jang JW, Kwon JH, You CR, Chung KW, Kay CS, et al. Prognostic value of C-reactive protein and neutrophil-to-lymphocyte ratio in patients with hepatocellular carcinoma. BMC Cancer. 2013;13:78.
Chua W, Charles KA, Baracos VE, Clarke SJ. Neutrophil/lymphocyte ratio predicts chemotherapy outcomes in patients with advanced colorectal cancer. Br J Cancer. 2011;104:1288–95.
Chen Q, Yang LX, Li XD, Yin D, Shi SM, Chen EB, et al. The elevated preoperative neutrophil-to-lymphocyte ratio predicts poor prognosis in intrahepatic cholangiocarcinoma patients undergoing hepatectomy. Tumour Biol. 2015. doi:10.1007/s13277-015-3188-6.
Bishara S, Griffin M, Cargill A, Bali A, Gore ME, Kaye SB, et al. Pre-treatment white blood cell subtypes as prognostic indicators in ovarian cancer. Eur J Obstet Gynecol Reprod Biol. 2008;138:71–5.
Kisielewski R, Tolwinska A, Mazurek A, Laudanski P. Inflammation and ovarian cancer—current views. Ginekol Pol. 2013;84:293–7.
Freedman RS, Deavers M, Liu J, Wang E. Peritoneal inflammation—a microenvironment for Epithelial Ovarian Cancer (EOC). J Transl Med. 2004;2:23.
Honn KV, Tang DG, Crissman JD. Platelets and cancer metastasis: a causal relationship? Cancer Metastasis Rev. 1992;11:325–51.
Stone RL, Nick AM, McNeish IA, Balkwill F, Han HD, Bottsford-Miller J, et al. Paraneoplastic thrombocytosis in ovarian cancer. N Engl J Med. 2012;366:610–8.
Petri AL, Hogdall E, Christensen IJ, Kjaer SK, Blaakaer J, Hogdall CK. Preoperative CA125 as a prognostic factor in stage I epithelial ovarian cancer. APMIS. 2006;114:359–63.
Asher V, Lee J, Bali A. Preoperative serum albumin is an independent prognostic predictor of survival in ovarian cancer. Med Oncol. 2012;29:2005–9.
Hefler LA, Concin N, Hofstetter G, Marth C, Mustea A, Sehouli J, et al. Serum C-reactive protein as independent prognostic variable in patients with ovarian cancer. Clin Cancer Res. 2008;14:710–4.
Williams KA, Labidi-Galy SI, Terry KL, Vitonis AF, Welch WR, Goodman A, et al. Prognostic significance and predictors of the neutrophil-to-lymphocyte ratio in ovarian cancer. Gynecol Oncol. 2014;132:542–50.
Asher V, Lee J, Innamaa A, Bali A. Preoperative platelet lymphocyte ratio as an independent prognostic marker in ovarian cancer. Clin Transl Oncol. 2011;13:499–503.
Polterauer S, Grimm C, Seebacher V, Concin N, Marth C, Tomovski C, et al. Plasma fibrinogen levels and prognosis in patients with ovarian cancer: a multicenter study. Oncologist. 2009;14:979–85.
Azab B, Shah N, Radbel J, Tan P, Bhatt V, Vonfrolio S, et al. Pretreatment neutrophil/lymphocyte ratio is superior to platelet/lymphocyte ratio as a predictor of long-term mortality in breast cancer patients. Med Oncol. 2013;30:432.
He W, Yin C, Guo G, Jiang C, Wang F, Qiu H, et al. Initial neutrophil lymphocyte ratio is superior to platelet lymphocyte ratio as an adverse prognostic and predictive factor in metastatic colorectal cancer. Med Oncol. 2013;30:439.
Piver MS, Lele SB, Marchetti DL, Baker TR, Tsukada Y, Emrich LJ. The impact of aggressive debulking surgery and cisplatin-based chemotherapy on progression-free survival in stage III and IV ovarian carcinoma. J Clin Oncol. 1988;6:983–9.
Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–45.
Makar AP, Baekelandt M, Trope CG, Kristensen GB. The prognostic significance of residual disease, FIGO substage, tumor histology, and grade in patients with FIGO stage III ovarian cancer. Gynecol Oncol. 1995;56:175–80.
Raungkaewmanee S, Tangjitgamol S, Manusirivithaya S, Srijaipracharoen S, Thavaramara T. Platelet to lymphocyte ratio as a prognostic factor for epithelial ovarian cancer. J Gynecol Oncol. 2012;23:265–73.
Yildirim M, Yildiz M, Duman E, Goktas S, Kaya V. Prognostic importance of the nutritional status and systemic inflammatory response in non-small cell lung cancer. J Buon. 2013;18:728–32.
Dirican A, Kucukzeybek Y, Somali I, Erten C, Demir L, Can A, et al. The association of hematologic parameters on the prognosis of patients with metastatic renal cell carcinoma. J Buon. 2013;18:413–9.
Hong C, Wei Y, Jiang J, Zhao C, Liang G, Wang G, et al. Associations between lifestyles and neutrophil-lymphocyte and platelet-lymphocyte ratios in colorectal cancer. Asia Pac J Clin Oncol. 2013;10:168–74. doi: 10.1111/ajco.12080.
Garcea G, Ladwa N, Neal CP, Metcalfe MS, Dennison AR, Berry DP. Preoperative neutrophil-to-lymphocyte ratio (NLR) is associated with reduced disease-free survival following curative resection of pancreatic adenocarcinoma. World J Surg. 2011;35:868–72.
Smith RA, Ghaneh P, Sutton R, Raraty M, Campbell F, Neoptolemos JP. Prognosis of resected ampullary adenocarcinoma by preoperative serum CA19-9 levels and platelet-lymphocyte ratio. J Gastrointest Surg. 2008;12:1422–8.
Wang D, Yang JX, Cao DY, Wan XR, Feng FZ, Huang HF, et al. Preoperative neutrophil-lymphocyte and platelet-lymphocyte ratios as independent predictors of cervical stromal involvement in surgically treated endometrioid adenocarcinoma. Onco Targets Ther. 2013;6:211–6.
Allensworth SK, Langstraat CL, Martin JR, Lemens MA, McGree ME, Weaver AL, et al. Evaluating the prognostic significance of preoperative thrombocytosis in epithelial ovarian cancer. Gynecol Oncol. 2013;130:499–504.
Alexandrakis MG, Passam FH, Moschandrea IA, Christophoridou AV, Pappa CA, Coulocheri SA, et al. Levels of serum cytokines and acute phase proteins in patients with essential and cancer-related thrombocytosis. Am J Clin Oncol. 2003;26:135–40.
Suzuki K, Aiura K, Ueda M, Kitajima M. The influence of platelets on the promotion of invasion by tumor cells and inhibition by antiplatelet agents. Pancreas. 2004;29:132–40.
Riesco A. Five-year cancer cure: relation to total amount of peripheral lymphocytes and neutrophils. Cancer-Am Cancer Soc. 1970;25:135–40.
Cho H, Hur HW, Kim SW, Kim SH, Kim JH, Kim YT, et al. Pre-treatment neutrophil to lymphocyte ratio is elevated in epithelial ovarian cancer and predicts survival after treatment. Cancer Immunol Immunother. 2009;58:15–23.
Wolff JP, de Oliveira CF. Lymphocytes in patients with ovarian cancer. Obstet Gynecol. 1975;45:656–8.
Yigit R, Massuger LF, Figdor CG, Torensma R. Ovarian cancer creates a suppressive microenvironment to escape immune elimination. Gynecol Oncol. 2010;117:366–72.
Ruka W, Rutkowski P, Kaminska J, Rysinska A, Steffen J. Alterations of routine blood tests in adult patients with soft tissue sarcomas: relationships to cytokine serum levels and prognostic significance. Ann Oncol. 2001;12:1423–32.
Shen GH, Ghazizadeh M, Kawanami O, Shimizu H, Jin E, Araki T, et al. Prognostic significance of vascular endothelial growth factor expression in human ovarian carcinoma. Br J Cancer. 2000;83:196–203.
Huang Y, Chen X, Dikov MM, Novitskiy SV, Mosse CA, Yang L, et al. Distinct roles of VEGFR-1 and VEGFR-2 in the aberrant hematopoiesis associated with elevated levels of VEGF. Blood. 2007;110:624–31.
Acknowledgments
Wei-wei Zhang and Ke-jun Liu contributed equally to this work. The authors would like to thank doctors, nurses, patients, and their family members for their kindness to support our study.
Grant support
The Department of the Science and Technology Planning Project Fund of Guangdong Province in China (2011B031800042).
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, Ww., Liu, Kj., Hu, Gl. et al. Preoperative platelet/lymphocyte ratio is a superior prognostic factor compared to other systemic inflammatory response markers in ovarian cancer patients. Tumor Biol. 36, 8831–8837 (2015). https://doi.org/10.1007/s13277-015-3533-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-015-3533-9