Abstract
To conduct a meta-analysis to assess the association between CD133 expression and clinicopathological significance and prognostic value in hepatocellular carcinoma patients. Studies were identified via an electronic comprehensive literature search through the Pubmed, Chinese CNKI, and Wanfang databases. This meta-analysis was performed using Stata statistical software version 12.0. The outcomes included various clinicopathological and survival parameters (P < 0.05 was consider to indicate a statistical significance). A total of 21 studies comprising 2592 patients were included in this meta-analysis. CD133 overexpression was significantly associated with a series of clinicopathological parameters, such as low tumor differentiation (pooled odds ratio (OR) = 2.26, 95% CI: 1.59–3.21, P < 0.00001), advanced tumor stage (pooled OR = 2.17, 95% CI: 1.70–2.77, P < 0.00001), vascular invasion (pooled OR = 2.06, 95% CI: 1.25–3.39, P = 0.005), and vascular thrombosis (pooled OR = 1.47, 95% CI: 1.08–1.99, P = 0.015). However, CD133 expression was not correlated with hepatitis, cirrhosis, α-fetoprotein level, tumor number, tumor size, encapsulation, or metastasis. Regarding survival outcome, CD133 overexpression was significantly correlated with poor overall survival (pooled hazard ratio (HR) = 2.01, 95% CI: 1.45–2.80, P = 0.00002) and poor disease-free survival (pooled HR = 1.82, 95% CI: 1.45–2.29, P < 0.00001). This meta-analysis indicated that CD133 overexpression is significantly associated with clinicopathological factors and poorer survival outcome.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common malignancy and the third leading cause of death worldwide, with nearly one million new cases diagnosed every year worldwide [1, 2]. Previous researches have indicated that hepatic resection is the most practical and effective treatment for HCC, and 5-year survival rates of up to 50 % can be achieved [3–5]. However, the prognosis for HCC patients remains unpredictable and unsatisfactory due to high rates of recurrence and metastasis, which can be as high as 45 % within 2 years of surgery [6–9]. The cellular and molecular mechanisms involved in the initiation and progression of HCC are still unclear. In recent studies, stem/progenitor cells have been associated with hepatocarcinogenesis in rodents and activated in HCC patients [10, 11]. Increasing evidence suggests that tumors can be initiated and maintained by cancer stem cells (CSCs) [12–14]. The cell surface marker CD133, also known as prominin-1, is widely used as a CSC marker in various tumors, including liver cancer, lung cancer, colon cancer, and gastric cancer [2, 15–18]. To our knowledge, many recent studies have attempted to determine whether CD133 overexpression is associated with clinicopathological factors and prognosis in different malignant tumors. However, the relationship between high CD133 expression and clinical outcome in HCC patients remains a controversial issue. Here, we performed a meta-analysis to assess whether CD133 overexpression was correlated with the clinicopathological factors and prognosis of HCC patients and provide a new insight into the potential treatment of HCC and molecular mechanisms involved in HCC.
Materials and methods
Literature search strategy
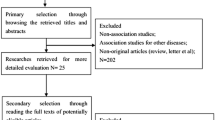
Studies were identified via an electronic comprehensive literature search using the Pubmed, Chinese CNKI, and Wanfang databases with the key words “CD133” OR “AC133” OR “prominin-1” AND “liver cancer” OR “liver carcinoma” OR “liver neoplasm” OR “liver tumor” OR “hepatic cancer” OR “hepatic carcinoma” OR “hepatic neoplasm” OR “hepatic tumor” OR “hepatocellular carcinoma”. This search was performed up to February 15, 2015. The title and abstract of each study were identified to exclude any irrelevant studies. The remaining articles were browsed to determine whether they contained information relating to the topic of interest. The reference lists of the relevant articles were also screened to further identify potential studies (Fig. 1).
Selection criteria
The inclusion criteria were showed to select literature as follows: (1) diagnosis of HCC was confirmed by histopathological methods; (2) studies of CD133 expression based on liver cancer tissue rather than serum or any other types of specimen were included; (3) CD133 expression was detected by the immunohistochemical staining method; (4) articles on the association of CD133 levels with overall survival (OS), disease-free survival (DFS) or clinicopathological features of HCC; (5) articles providing sufficient data to allow the estimation of an odds ratio (OR) with 95 % confidence interval (CI) of clinicopathological parameters or a hazard ratio (HR) with 95% CI of OS or DFS. There were no limits of the number of cases, gender ratio, or the cutoff values of immunohistochemical staining in this meta-analysis. If there were multiple articles by the same group based on similar patients in the searching results, only the most recent or most informative article was selected in this meta-analysis (Fig. 1). We assessed the quality of the primary studies by using Newcastle-Ottawa Quality Assessment Scale (case-control studies).
Data extraction
To minimize bias and improve reliability, two reviewers independently assessed all potentially relevant studies. The following characteristics were extracted from eligible studies and are illustrated in detail in Table 1: name of first author, year of publication, number of patients, and CD133+-group, age (mean/median), gender, cutoff values, tumor differentiation, TNM stage, follow-up (months), treatment, and quality score. ORs with 95% CI were evaluated the correlation between high CD133 expression and general clinicopathological parameters, including hepatitis B virus (HBV, negative vs. positive), hepatitis C virus (HCV, negative vs. positive), cirrhosis (absent vs. present), α-fetoprotein (AFP) level (≤400 vs. >400 ng/ml), tumor differentiation (I + II vs. III + IV) and tumor stage (I + II vs. III + IV), tumor number (single vs. multiple), tumor size (≤5 vs. >5 cm), vascular invasion (absent vs. present), vascular thrombosis (absent vs. present), encapsulation (absent vs. present), and metastasis (absent vs. present). HRs with 95% CI assessed the association between high CD133 expression and survival outcome, including OS and DFS (5 years of follow-up).
Statistical analysis
ORs with 95% CI were used to estimate the association between high CD133 expression and general clinicopathological parameters (P < 0.05 was considered to indicate a statistical significance). HRs with 95% CI were used to assess the correlation between high CD133 expression and survival outcome (P < 0.05 was representative of statistical significance). For articles that did not directly provide HR with 95% CI of OS and DFS, Engauge Digitizer 4.1 (Boston, USA) software was applied to digitize and extract the data from Kaplan-Meier curves. The lnHR and variance were calculated by the methods described by Tierney et al. [40]. Statistical heterogeneity within studies was tested with the chi-squared-based Q-test and I2 test (P < 0.10 or I 2 > 50 %, indicated the existence of heterogeneity among studies). The fixed-effects (Mantel–Haenszel method) model or random-effects (DerSimonian and Laird method) model was used depending on the heterogeneity analysis. Publication bias was estimated by the Begg’s and Egger’s funnel plot (P < 0.05 was considered of significant publication bias). In the sensitivity analyses, P < 0.05 was representative of significant differences. All statistics were calculated by Stata statistical software version 12.0 (StataCorp, College Station, TX, USA).
Results
Description of studies and quality assessment
A total of 21 studies comprising 2592 patients met our selection criteria in this meta-analysis. The sample sizes of the studies included ranged from 25 to 387, with a mean of 123.4. Most patients were male (83.4 % from 19 studies). No preoperative therapy was performed on patients in 14 studies. One study reported that preoperative therapy (transcatheter arterial chemoembolization or liver transplantation) was performed in some HCC patients. All these studies evaluated CD133 expression by immunohistochemical staining methods in liver cancer tissues. All patients were divided into either CD133+ or CD133− groups. Tumor differentiation was graded by Edmondson and Steiner, and tumor stage was classified by the International Union Against Cancer (UICC) 2002 issue of the TNM stage. Nine studies did not report tumor stage. All studies were graded by using Newcastle-Ottawa Quality Assessment Scale (case-control studies). More than five points meant a better quality. More detailed information was showed in Table 1.
CD133 overexpression and clinicopathological features in HCC patients
In our analyses, CD133 overexpression in HCC patients was associated with several clinicopathological parameters (Table 2), such as low tumor differentiation (pooled OR = 2.26, 95% CI: 1.59–3.21, P < 0.00001, random effect), advanced tumor stage (pooled OR = 2.17, 95% CI: 1.70–2.77, P < 0.00001, fixed effect), vascular invasion (pooled OR = 2.06, 95% CI: 1.25–3.39, P = 0.005, random effect), and vascular thrombosis (pooled OR = 1.47, 95% CI: 1.08–1.99, P = 0.015, fixed effect). However, as shown in Table 2, no correlations were observed between CD133 expression and HBV (pooled OR = 1.10, 95% CI: 0.86–1.42, P = 0.447, fixed effect), HCV (pooled OR = 0.80, 95% CI: 0.48–1.33, P = 0.379, fixed effect), cirrhosis (pooled OR = 1.28, 95% CI: 0.99–1.67, P = 0.059, fixed effect), elevated serum AFP level (pooled OR = 1.17, 95% CI: 0.95–1.44, P = 0.129, fixed effect), tumor number (pooled OR = 1.43, 95% CI: 0.80–2.54, P = 0.230, random effect), tumor size (pooled OR = 0.99, 95% CI: 0.81–1.20, P = 0.899, fixed effect), encapsulation (pooled OR = 0.80, 95% CI: 0.61–1.05, P = 0.110, fixed effect), or metastasis (pooled OR = 1.47, 95% CI: 0.90–2.39, P = 0.121, fixed effect). The results of the heterogeneity analyses indicated no statistically significant difference (P > 0.10 for Q-test, I 2 < 50 % for I2 test), except tumor differentiation (P = 0.001, I 2 > 56.7 %), tumor number (P < 0.010, I 2 > 70.1 %), and vascular invasion (P = 0.059, I 2 > 48.6 %) (Table 2).
CD133 overexpression and survival outcome in HCC patients
Ten studies (1950 patients) that had assessed the correlation between CD133 overexpression and OS and five studies (949 patients) containing information regarding the association between high CD133 expression and DFS could be obtained from published articles. The unadjusted HRs with 95% CI, which were not directly mentioned, were gained the assessment by the Kaplan-Meier method [19, 21, 24, 29–31, 33–35]. It showed that CD133 overexpression was significantly associated with poor OS (pooled HR = 2.01, 95% CI: 1.45–2.80, P = 0.00002, random effect) for a heterogeneity (P = 0.001 for Q-test, I2 = 67.4 % for I2 test) (Fig. 2) and poor DFS (pooled HR = 1.82 95% CI: 1.45–2.29, P < 0.00001, fixed effect) for no heterogeneity (P = 0.566 for Q-test, I 2 = 0.0 % for I2 test) (Fig. 3). These results indicated that CD133 is an important influencing factor of prognosis in HCC patients.
Publication bias and sensitivity analyses
The meta-analysis of observational studies has their inherent limitations. Several factors may influence the publication bias, such as the selection of materials, the methods of technique, and the ways of data extraction. Additionally, one of the important limitations is publication bias caused by failing to balance unknown confounders. In this meta-analysis, inclusion criteria were strictly formulated, and funnel plots (Begg’s and Egger’s test) were used to identify bias. In order to minimize publication bias, two reviewers independently estimated all potentially relevant studies. As to clinicopathological factors, there was no significant publication bias (Table 2, P > 0.05), except encapsulation (P = 0.466 and P = 0.029 in Begg’s and Egger’s test, respectively). As to survival outcome, the shapes of Begg’s and Egger’s funnel plots had no evidence of obviously asymmetrical patterns in the results of meta-analyses of OS (P = 0.371 and P = 0.362 in Begg’s and Egger’s test, respectively) and DFS (P = 0.462 and P = 0.201 in Begg’s and Egger’s test, respectively) (Fig. 4). In order to explain the stability of these results in this meta-analysis, we performed sensitivity analyses regarding of all sub-groups of clinicopathological features and survival outcome (Table 3). We found that OS, tumor differentiation, and tumor number had significant differences (P < 0.05). However, these differences had no effect on the final results of meta-analysis. Therefore, we did not exclude these studies from the comprehensive consideration.
Discussion
Recent studies have shown that some functional molecules could be an attractive inhibitor of the target gene CD133, which reactive anticancer mechanisms in targeted CSC therapy in HCC patients [41]. Therefore, the expression of CD133 has played an increasing important role of the evaluation of prognosis and may aid the improvement of diagnosis, treatments, and prevention of HCC. So, we did a comprehensive meta-analysis to systematically evaluate the correlation between the CD133 overexpression and clinical and prognostic outcome.
CD133 is expressed in normal fetal livers and cancerous livers but not in normal adult livers. Generally, chronic viral hepatitis leads to the regeneration of hepatocytes due to the chronic and continuous inflammatory stimulation caused by HBV or HCV. Gradually, cirrhosis and carcinogenesis can occur. However, our study showed that CD133 overexpression had no connection with hepatitis and cirrhosis. These results were the same as those reported by Ma et al. [2] and were against a CSC origin of hepatitis virus-related HCC. Meanwhile, elevated levels of AFP were no correlation with CD133 overexpression which differ from Ma et al. [2]. There were several reasons for this. First, we included more studies in this meta-analysis, both in English and in Chinese. Second, we established more stringent criteria for selecting articles, such as immunohistochemical staining methods. However, higher-quality studies and further researches are needed to clarify this problem.
CD133 is believed to play a key role in the occurrence of cancer, including promoting tumor angiogenesis and growth, activating self-renewal through the neurotensin/IL-8/CXCL1 signal pathway, initiating metastasis and recurrence of cancer, and influencing the prognosis of HCC patients [42]. Here, the CD133+ group had an increased incidence of vessel invasion and generation of embolism. However, no correlation was observed with tumor number, tumor size, encapsulation, or metastasis. Suetsugu et al. demonstrated that CD133+ cells from Huh-7 had a higher tumorigenic potential, greater proliferative ability, and lower differentiation status than CD133− cells [43]. This meta-analysis demonstrated significant differences between the CD133+ and CD133− groups in terms of tumor differentiation and tumor stage. Additionally, CD133 overexpression was significantly associated with poor OS and DFS. Thus, CD133 overexpression may be an important marker as the accurate assessment of clinical and prognostic outcome and a potential target to deeply explore the cellular and molecular mechanism for the treatment of HCC patients.
However, the results from our study should be interpreted cautiously due to some limitations that might have influenced the conclusions of this meta-analysis. First, the number of included articles was relatively small and included HCC patients from single studies. Second, although CD133 expression was uniformly detected by immunohistochemical staining method in all studies, the cutoff values of positive CD133 expression were not unified in each study. Third, the HRs of OS and DFS were indirectly extracted from Kaplan-Meier curves and calculated by the methods described by Tierney et al. [40]. Accordingly, the HRs with 95% CI may be less reliable that those obtained directly from assessing analysis of variance. These limitations might have partly influenced the significance of high CD133 expression in clinicopathological features and survival outcome observed in this study.
Using CD133 as a biomarker is also limited in terms of predicting clinicopathological significance and prognosis in HCC patients. In addition to CD133, some other cell surface molecules have been considered potential CSC markers in HCC patients, including CD90, CD44, and EpCAM [44, 45]. Ma et al. found that CD133+ALDH+ cells were significantly more tumorigenic than CD133+ALDH− or CD133−ALDH− cells both in vitro and in vivo [46]. Zhu et al. suggested that the CD133+CD44+ subpopulation might allow a better understanding of HCC initiation and progression and establish a precise target for the development of more effective therapies [47], which was consistent with the conclusion by Zheng et al. [48]. Thus, we speculated that the co-expression of markers for the identification of HCC may be more meaningful and efficient for clinicopathological and prognostic estimation.
In summary, this meta-analysis indicated that CD133 overexpression was significantly associated with several clinicopathological parameters, such as low tumor differentiation, advanced tumor stage, vascular invasion, and vascular thrombosis. However, no correlations were observed between high CD133 expression and hepatitis, cirrhosis, AFP, tumor number, tumor size, encapsulation, or metastasis. Regarding survival outcome, CD133 overexpression was significantly correlated with poor OS and poor DFS. In brief, this meta-analysis indicated that CD133 overexpression is significantly associated with clinicopathological factors and poorer survival outcome. Therefore, CD133 could be considered an important influencing factor of clinical application and prognostic estimation and a potential molecular target for the treatment of HCC patients.
References
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90.
Ma YC, Yang JY, Yan LN. Relevant markers of cancer stem cells indicate a poor prognosis in hepatocellular carcinoma patients: a meta-analysis. Eur J Gastroenterol Hepatol. 2013;25:1007–16.
Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–2.
Ho CM, Wu CY, Lee PH, Lai HS, Ho MC, Wu YM, et al. Analysis of the risk factors of untransplantable recurrence after primary curative resection for patients with hepatocellular carcinoma. Ann Surg Oncol. 2013;20:2526–33.
Poon RT, Fan ST, Lo CM, Liu CL, Lam CM, Yuen WK, et al. Extended hepatic resection for hepatocellular carcinoma in patients with cirrhosis: is it justified? Ann Surg. 2002;236:602–11.
Tung-Ping Poon R, Fan ST, Wong J. Risk factors, prevention, and management of postoperative recurrence after resection of hepatocellular carcinoma. Ann Surg. 2000;232:10–24.
Ding X, Yang LY, Huang GW, Yang JQ, Liu HL, Wang W, et al. Role of AFP mRNA expression in peripheral blood as a predictor for postsurgical recurrence of hepatocellular carcinoma: a systematic review and meta-analysis. World J Gastroenterol. 2005;11:2656–61.
Chun JM, Kwon HJ, Sohn J, Kim SG, Park JY, Bae HI, et al. Prognostic factors after early recurrence in patients who underwent curative resection for hepatocellular carcinoma. J Surg Oncol. 2011;103:148–51.
Hoshida Y, Villanueva A, Kobayashi M, Peix J, Chiang DY, Camargo A, et al. Gene expression in fixed tissues and outcome in hepatocellular carcinoma. N Engl J Med. 2008;359:1995–2004.
Hixson DC, Brown J, McBride AC, Affigne S. Differentiation status of rat ductal cells and ethionine-induced hepatic carcinomas defined with surface-reactive monoclonal antibodies. Exp Mol Pathol. 2000;68:152–69.
Libbrecht L, Roskams T. Hepatic progenitor cells in human liver diseases. Semin Cell Dev Biol. 2002;13:389–96.
Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–11.
Ailles LE, Weissman IL. Cancer stem cells in solid tumors. Curr Opin Biotechnol. 2007;18:460–6.
Wang K, Liu L, Zhang T, Zhu YL, Qiu F, Wu XG, et al. Oxaliplatin-incorporated micelles eliminate both cancer stem-like and bulk cell populations in colorectal cancer. Int J Nanomedicine. 2011;6:3207–18.
Wang W, Chen Y, Deng J, Zhou J, Zhou Y, Wang S, et al. The prognostic value of CD133 expression in non-small cell lung cancer: a meta-analysis. Tumor Biol. 2014;35:9769–75.
Wang K, Xu J, Zhang J, Huang J. Prognostic role of CD133 expression in colorectal cancer: a meta-analysis. BMC Cancer. 2012;12:573.
Chen S, Song X, Chen Z, Li X, Li M, Liu H, et al. CD133 expression and the prognosis of colorectal cancer: systematic review and meta-analysis. PLoS One. 2013;8, e56380. doi:10.1371/journal.pone.0056380.
Wen L, Chen XZ, Yang K, Chen ZX, Zhang B, Chen JP, et al. Prognostic value of cancer stem cell marker CD133 expression in gastric cancer: a systematic review. PLoS One. 2013;8, e59154. doi:10.1371/journal.pone.0059154.
Song W, Li H, Tao K, Li R, Song Z, Zhao Q, et al. Expression and clinical significance of the stem cell marker CD133 in hepatocellular carcinoma. Int J Clin Pract. 2008;62:1212–8.
Tsuchiya A, Kamimura H, Takamura M, Yamagiwa S, Matsuda Y, Sato Y, et al. Clinicopathological analysis of CD133 and NCAM human hepatic stem/progenitor cells in damaged livers and hepatocellular carcinomas. Hepatol Res. 2009;39:1080–90.
Yeh CT, Kuo CJ, Lai MW, Chen TC, Lin CY, Yeh TS, et al. CD133-positive hepatocellular carcinoma in an area endemic for hepatitis B virus infection. BMC Cancer. 2009;9:324.
Zhang H, Gao ZH, Xu TY, Zhu JF, Zhang Y. Expression of CD133 in hepatocellular carcinoma and its clinical significance. Cancer Res Clin. 2009;21:440–3.
Lingala S, Cui YY, Chen X, Ruebner BH, Qian XF, Zern MA, et al. Immunohistochemical staining of cancer stem cell markers in hepatocellular carcinoma. Exp Mol Pathol. 2010;89:27–35.
Sasaki A, Kamiyama T, Yokoo H, Nakanishi K, Kubota K, Haga H, et al. Cytoplasmic expression of CD133 is an important risk factor for overall survival in hepatocellular carcinoma. Oncol Rep. 2010;24:537–46.
Yang XR, Xu Y, Yu B, Zhou J, Qiu SJ, Shi GM, et al. High expression levels of putative hepatic stem/progenitor cell biomarkers related to tumour angiogenesis and poor prognosis of hepatocellular carcinoma. Gut. 2010;59:953–62.
Guo XD, Wang HB, Qu F, Liu LM, Zhang JL, Li XX, et al. Expression of CD133 in hepatocellular carcinoma and its relationship with clinical pathological features. Clin J Lab Diagn. 2011;15:78–80.
Wu XH, Wang SX, Cui DP, Li JK, Yang BM. Expression and significance of CD133 and CD90 in hepatocellular carcinoma. Clin J Hepatol. 2011;19:376–7.
Zen C, Zen Y, Mitry RR, Corbeil D, Karbanová J, O’Grady J, et al. Mixed phenotype hepatocellular carcinoma after transarterial chemoembolization and liver transplantation. Liver Transpl. 2011;17:943–54.
Pan QX, Su ZJ, Wang CR, Zhuang JN. Expression of tumor stem cell markers EpCAM and CD133 in human primary hepatocellular carcinoma and their value in prognostic prediction. Tumori. 2012;32:628–33.
Zeng XC, Zhang D, Fu BS, Chen W, Chen GZ, Li H, et al. Expression of CD133 as a tumor stem cell surface marker in hepatocellular and its significance in the prognosis of liver transplantation patients. Chin J Hepat Surg (Electronic Edition). 2012;1:51–6.
Liu LL, Li W, Li YJ. Expression of CD133 and CD90 in hepatocellular carcinoma tissue and their clinical significance. Acta Academiae Medicinae Qindao Universitatis. 2013;49:393–6.
Tian J, Gong XM. Expression and their relationship to clinicopathological significance of CD133 and maspin in hepatocellular carcinoma. Chinese Journal of Histochemistry and Cytochemistry. 2013;22:528–32.
Wu LM, Cheng CT, Chen XX, Wang JH, Liu HY, Tian L. Prognostic significance of expression of CD133, VEGF and CD34 in hepatocellular carcinoma. Clin J Cancer Prev Treat. 2013;20:998–1002.
Chan AW, Tong JH, Chan SL, Lai PB, To KF. Expression of stemness markers (CD133 and EpCAM) in prognostication of hepatocellular carcinoma. Histopathology. 2014;64:935–50.
Chen K, Li ZH, Jiang P, Zhang X, Zhang YJ, Jiang Y, et al. CD44, CD133 and TF correlate with formation of portal vein tumor thrombus and poor prognosis in patients with hepatocellular carcinoma. J Third Mil Med Univ. 2014;36:1068–73.
Guo Z, Li LQ, Jiang JH, Ou C, Zeng LX, Xiang BD. Cancer stem cell markers correlate with early recurrence and survival in hepatocellular carcinoma. World J Gastroenterol. 2014;20:2098–106.
Ye F, Jing YY, Guo SW, Yu GF, Fan QM, Qu FF, et al. Proliferative ductular reactions correlate with hepatic progenitor cell and predict recurrence in HCC patients after curative resection. Cell Biosci. 2014;4:50.
Yilmaz G, Akyol G, Cakir A, Ilhan M. Investigation of diagnostic utility and expression profiles of stem cell markers (CD133 and CD90) in hepatocellular carcinoma, small cell dysplasia, and cirrhosis. Pathol Res Pract. 2014;210:419–25.
Zhao MY. Immunohistochemical analysis of CD133 and CD90 expression in hepatocellular carcinoma and its clinicopathological significance. Med Lab Sci. 2014; 104–5.
Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16.
Zhang L, Li H, Ge C, Li M, Zhao FY, Hou HL, et al. Inhibitory effects of transcription factor Ikaros no the expression of liver cancer stem cell marker CD133 in hepatocellular carcinoma. Oncotarget. 2014;5:10621–35.
Tang KH, Ma S, Lee TK, Chan YP, Kwan PS, Tong CM, et al. CD133(+) liver tumor-initiating cells promote tumor angiogenesis, growth, and self-renewal through neurotensin/interleukin-8/CXCL1 signaling. Hepatology. 2012;55:807–20.
Suetsugu A, Nagaki M, Aoki H, Motohashi T, Kunisada T, Moriwaki H. Characterization of CD133+ hepatocellular carcinoma cells as cancer stem/progenitor cells. Biochem Biophys Res Commun. 2006;351:820–4.
Ji J, Wang XW. Clinical implications of cancer stem cell biology in hepatocellular carcinoma. Semin Oncol. 2012;39:461–72.
Armeanu-Ebinger S, Wenz J, Seitz G, Leuschner I, Handgretinger R, Mau-Holzmann UA, et al. Characterisation of the cell line HC-AFW1 derived from a pediatric hepatocellular carcinoma. PLoS One. 2012;7, e38223. doi:10.1371/journal.pone.0038223.
Ma S, Chan KW, Lee TK, Tang KH, Wo JY, Zheng BJ, et al. Aldehyde dehydrogenase discriminates the CD133 liver cancer stem cell populations. Mol Cancer Res. 2008;6:1146–53.
Zhu Z, Hao X, Yan M, Yao M, Ge C, Gu J, et al. Cancer stem/progenitor cells are highly enriched in CD133+CD44+ population in hepatocellular carcinoma. Int J Cancer. 2010;126:2067–78.
Zheng YW, Tsuchida T, Shimao T, Li B, Takebe T, Zhang RR, et al. The CD133+ CD44+ precancerous subpopulation of oval cells is a therapeutic target for hepatocellular carcinoma. Stem Cells Dev. 2014;23:2237–49.
Acknowledgments
This study was supported by National Natural Science foundation of China (81273261). The authors would like to thank Nature Publishing Group Language Editing for revising the English text.
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Additional information
Chen Zhong and Jin-Dao Wu contributed equally to this work.
Rights and permissions
About this article
Cite this article
Zhong, C., Wu, JD., Fang, MM. et al. Clinicopathological significance and prognostic value of the expression of the cancer stem cell marker CD133 in hepatocellular carcinoma: a meta-analysis. Tumor Biol. 36, 7623–7630 (2015). https://doi.org/10.1007/s13277-015-3487-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-015-3487-y