Abstract
CD133 has been identified as a potential cancer stem cell (CSC) marker in non-small cell lung cancer (NSCLC). However, the clinical and prognostic significance of CD133 in NSCLC remains controversial. In this study, a meta-analysis with a total number of 13 studies was performed to clarify the association between CD133 expression and clinical outcomes in publications up to June 2013. Odds ratios (ORs) and their 95 % confidence intervals (CIs) were used to assess the association between CD133 expression and the clinicopathological characteristics of NSCLC. Hazard ratios (HRs) and their 95 % CI were used to quantify the predictive ability of CD133 on NSCLC prognosis. Analysis of these data showed that CD133 expression was not associated with any clinicopathological parameters except for histology (pooled OR = 1.35, 95%CI = 1.04–1.76, P = 0.024) and tumor differentiation (pooled OR = 3.19, 95%CI = 1.10–9.21, P = 0.032). Simultaneously, we also found that positive CD133 expression was not associated with disease-free survival (DFS) (pooled HR = 1.76, 95 % CI = 0.87–3.57, P = 0.114, random-effect) but was associated with overall survival (OS) (pooled HR = 2.06, 95 % CI = 1.08–3.91, P = 0.027, random-effect). Overall, it is appropriate to regard CD133 expression as a potential prognostic factor for the OS of NSCLC patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lung cancer (LC) remains the most frequent and the most lethal human malignancy worldwide [1], and among all LC cases, NSCLC accounts for approximately 85 % [2]. Significant diagnostic and therapeutic improvements have been made for NSCLC; however, the prognosis is still suboptimal, with an overall five-year survival rate of less than 15 % [3]. CSCs are defined as a distinct subpopulation of cancer-initiating cells, possessing the properties of self-renewal, asymmetric cell division [4]. In the last decade, there has been increasing evidence that CSCs play a pivotal role in drug resistance, tumor regeneration, and metastasis [5, 6]. A correlation has identified that in terms of chemoresistance and bad prognosis of NSCLC patient, tumor samples enriched with CSCs [7]. Recently, specific biomarkers of CSCs have been identified by major efforts, such as CD44, aldehyde dehydrogenase, CD133 [8].
CD133, also known as prominin-1, is a cell-surface glycoprotein comprising five transmembrane domains and two large glycosylated extracellular loops [9]. CD133 has been used as a potential CSC marker to identify subpopulations of cells in brain [10], colon [11], and some lung tumors [12]. Some studies showed that CD133 positive is associated with poor prognosis of some cancers such as gastric cancer [13], colon cancer [14], and also NSCLC patients [15–17]. However, conflicting results on the detection, tumorigenicity, clinical pathologic features, and progression of CD133 positive tumor cells [9, 18] indicate limited availability of samples which has resulted in variations in the clinical significance of the results. The present meta-analysis was conducted to determine the association between CD133 and common clinical and pathologic features of NSCLC, considering the putative role of stem cells in the prediction of outcome in NSCLC.
Materials and methods
Search strategy, inclusion criteria, and data collection
The electronic database of PubMed was searched for studies that investigated the association of clinicopathological parameters and prognosis with CD133 expression in NSCLC to be included in the present meta-analysis. Studies were examined on papers imposed with an updating search on June 31, 2013. Search terms were (AC133 [all fields] OR AC-133 [all fields] OR (AC [all fields] AND 133 [all fields]) OR CD-133 [all fields] OR CD133 [all fields]) OR (CD [all fields] AND 133 [all fields]) OR “AC133 antigen” [supplementary concept] OR “AC133 antigen” [all fields] OR “prominin 1” [all fields] OR PROM1 [all fields] OR PROM-1 [all fields]) AND (“lung neoplasms” [MeSH terms] OR (“lung” [all fields] AND “neoplasms” [all fields]) OR “lung neoplasms” [all fields] OR (“lung” [all fields] AND “cancer” [all fields]) OR “lung cancer” [all fields]). The published studies that were eligible for inclusion in this meta-analysis should meet the following criteria: (1) the histological type of the tumors was NSCLC, and (2) it assesses the relationship between CD133 expression and clinical parameters or/and survival, and has been published as an English full paper. Studies that did not meet all inclusion criteria were excluded from our analysis.
Data extraction
To minimize the bias and to improve the reliability, two reviewers checked all potentially relevant studies independently. The following characteristics were extracted from eligible studies: name of first author, name of journal, year of publication, sample size, test method, cutoff value, gender, smoking status, histological type, depth of invasion, lymph node metastasis, metastasis, tumor grade, stage, differentiation, and HR estimation and its 95 % CI. When HR and its 95 % CI were described in original articles, these values were obtained directly. When these statistical variables were not provided, they were extracted and calculated from Kaplan-Meier survival curve by the HR digitizer software Engauge 4.0.
Statistical methods
Odds ratios (ORs) with 95 % CI were used to estimate the association between CD133 expression and the general prognostic markers for NSCLC, including gender, smoking status, histological type, depth of invasion, lymph node metastasis, metastasis, tumor grade, stage, and differentiation. Pooled HR with 95 % CI was used to estimate the relationship of CD133 expression with survival of NSCLC. Based on the sample size of the DFS (762 cases) and OS (1065 cases), 1.76 and 2.06 difference HRs between CD133 low and high expression levels with both power of 1.0 could be determined, respectively. The statistical heterogeneity within studies was tested with the chi-squared-based Q-test (P > 0.10) and I 2 (I 2 < 50 %, no heterogeneity); fixed-effects model was used with absence of heterogeneity across studies. Otherwise, the random-effects model was used. Evidence of publication bias was sought by Egger’s and Begg’s test. The significance of the intercept was determined by the t test suggested by Egger and Begg (P < 0.05 was considered representative of statistically significant publication bias). All the statistical analyses were performed using Stata/SE 10.0 for Windows (Stata Corporation, College Station, TX, USA).
Results
Description of studies
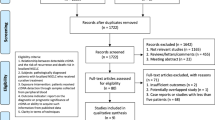
Combined search in PubMed on the terms indicated in Materials and methods retrieved 181 hits, and when excluding animal experiments, non-NSCLC-related studies, non-original English articles, or lack of data on the association of CD133 expression with clinicopathological features and/or overall survival, only 13 publications met the inclusion criteria for the present analysis (Fig. 1). Ten studies reported the association between CD133 expression and the different clinicopathological parameters. When assessing the pooled HR and its 95%CI, two studies were excluded because of insufficient information to calculate the HR. Of these studies, the sample sizes ranged from 29 to 305 patients. Expression of CD133 was evaluated by Immunohistochemistry (IHC) in 11 studies, by quantitative real-time polymerase chain reaction (qPCR) in 2 studies. In 9 of the 11 studies, the useful data for calculation of overall HR were obtained directly from the original articles, and in 2 studies, HRs and 95%CIs were extrapolated from Kaplan-Meier survival curves. Table 1 showed all the studies included in the meta-analysis in detail.
Correlation of CD133 expression with clinicopathological characteristics
The main results of the meta-analysis were summarized in Table 2. The results showed that CD133 was not associated with clinicopathological parameters such as sex, smoking status, depth of invasion, lymph node metastasis, metastasis, tumor stage, and grade, except for histology (pooled OR = 1.35, 95%CI = 1.04–1.76, P = 0.024) and tumor differentiation (pooled OR = 3.19, 95%CI = 1.10–9.21, P = 0.032 ).
Impact of CD133 expression on DFS and OS of NSCLC
The different results obtained from previous studies on the impact of CD133 expression on DFS and OS. The pooled HR of the DFS was 1.76 (95 %CI = 0.87–3.57, P = 0.114, random-effect, Fig. 2), with an I 2 of 62.9 %. When limiting the terminal event to OS, pooled HR was 2.06 (95 %CI = 1.08–3.91, P = 0.027 random-effect, Fig. 3), with an I 2 of 87.6 %.
Test for heterogeneity
There was significant heterogeneity for survival evaluation (I 2 = 62.9 % for DFS; I 2 = 87.6 % for OS). Then, we assessed the source of heterogeneity for additive model by population (Asian vs. the others) and CD133 cutoff value (IHC < =1 % vs. IHC <10 %). The results were shown in Table 3. For the DFS, cutoff value (χ 2 = 8.64, P = 0.003), but not population (χ 2 = 0.11, P = 0.736), was found to contribute to substantial heterogeneity. Moreover, high CD133 expression with <10 % cutoff level seemed to be a worse prognosis factor compared with low CD133 expression (pooled HR = 4.27, 95%CI = 2.08–8.79). Nevertheless, these significant difference was not found between the CD133 high or low when the cutoff value was IHC < =1 % (pooled HR = 0.97, 95%CI = 0.57–1.64). Simultaneously, population (χ 2 = 46.00, P < 0.001) and cutoff value (χ 2 = 16.19, P < 0.001) were found to associated with heterogeneity for OS. Moreover, high CD133 expression on the subgroups of non-Asian population (pooled HR = 4.93, 95%CI = 3.63–6.69) and IHC <10 % cutoff level (pooled HR = 2.81, 95%CI = 1.10–7.19) was more significantly prognostic parameter than low CD133 expression for OS. However, no difference had been found in the other corresponding subgroups.
Publication bias
Begg’s funnel plot with pseudo 95 % confidence limits and Egger’s publication bias plot were performed to estimate the publication bias of the included literature about DFS (Fig. 4a) and OS (Fig. 4b). Begg’s and Egger’s test did not reveal any evidence of obvious asymmetry in the overall meta-analysis of all studies.
Discussion
Knowledge regarding altered expression of genes during carcinogenesis may result in the discovery of biological markers for the evaluation of cancer diagnosis and prognosis, which may aid the improvement of diagnosis, treatment, and prevention of malignancies [19]. Recent studies have showed that some functional molecules may modulate response to platinum-based chemotherapy and serve as promising biomarkers for individualized chemotherapy of NSCLC patients [20, 21]. There has been great interest in identifying prognostic and predictive markers for patients with NSCLC helping guide clinical decision-making regarding therapy and outcomes.
CD133 has been used as a potential CSC marker. Recently, it has been reported that CD133 expression may serve as a promising biomarker in prognosis of colorectal and gastric cancers [22, 23]. Liu et al found that CD133 positive cells of NSCLC displayed CSCs characteristics of greater sphere-forming ability, cisplatin resistance, and migration ability [24]. Recent study also shows that NSCLC contains CD133 expressing cells, which is essential for tumor cell propagation and metastasis [25]. Moreover, CD133 expression was positively associated with poor prognosis [15, 26]. However, the conflicting results were also reported [9, 18]. Therefore, we performed this meta-analysis to identify the association between CD133 and clinicopathological outcomes, which showed that CD133 high expression was not positively correlated with any clinicopathological parameters except for adenocarcinoma (ADC) and tumor poor differentiation, which was accordant with recent founding that CD133 expression is more frequently upregulated in ADC than squamous cell carcinoma (SCC) patients [18]. Simultaneously, we also found that CD133 high expression was not associated with DFS but significantly associated with a worse OS compared with low CD133 expression, which was supported by the notions of the previous studies [15, 27].
This systematic review has some limitations. Firstly, the number of included studies, as well as the included NSCLC patients in each study, was relatively small. Secondly, though no significant heterogeneity across studies was detected in our study, we could not fully neglect potential heterogeneity. The subgroup analysis was used to assess the sources of heterogeneity. We found cutoff value was more contributed to heterogeneity both in DFS and OS. Thirdly, the methods used for the assessment of the level of CD133 expression and the cutoff values of defining the specimens as positive CD133 expression in NSCLC patients differed among these studies. The results changed according to the cutoff value. Therefore, some new studies with same cutoff values must be recruited and combined for further evaluation. Fourthly, non-original English articles were excluded in our analysis, which may introduce bias. However, articles published in other languages can be more difficult to locate, and may require translation, which will delay the conclusion of a review. Therefore, additional studies with the larger sample sizes, high quality, and different ethnic background are needed to make a more definitive conclusion.
In summary, our meta-analysis showed that high CD133 expression was not correlated with clinicopathological parameters except for histology and tumor differentiation. Simultaneously, CD133 overexpression predicted a poor overall survival. Therefore, it is appropriate to regard CD133 expression as a potential prognostic factor for NSCLC patients.
References
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90.
Esposito L, Conti D, Ailavajhala R, Khalil N, Giordano A. Lung cancer: are we up to the challenge? Curr Genomics. 2010;11:513–8.
Alberg AJ, Ford JG, Samet JM. Epidemiology of lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition). Chest. 2007;132:29S–55.
Dontu G, Liu S, Wicha MS. Stem cells in mammary development and carcinogenesis: implications for prevention and treatment. Stem Cell Rev. 2005;1:207–13.
Ribatti D. Cancer stem cells and tumor angiogenesis. Cancer Lett. 2012;321:13–7.
Baccelli I, Trumpp A. The evolving concept of cancer and metastasis stem cells. J Cell Biol. 2012;198:281–93.
Perona R, Lopez-Ayllon BD, de Castro Carpeno J, Belda-Iniesta C. A role for cancer stem cells in drug resistance and metastasis in non-small-cell lung cancer. Clin Transl Oncol. 2011;13:289–93.
Kitamura H, Okudela K, Yazawa T, Sato H, Shimoyamada H. Cancer stem cell: implications in cancer biology and therapy with special reference to lung cancer. Lung Cancer. 2009;66:275–81.
Salnikov AV, Gladkich J, Moldenhauer G, Volm M, Mattern J, Herr I. Cd133 is indicative for a resistance phenotype but does not represent a prognostic marker for survival of non-small cell lung cancer patients. Int J Cancer J Int Cancer. 2010;126:950–8.
Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, et al. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401.
Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, et al. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445:111–5.
Eramo A, Lotti F, Sette G, Pilozzi E, Biffoni M, Di Virgilio A, et al. Identification and expansion of the tumorigenic lung cancer stem cell population. Cell Death Differ. 2008;15:504–14.
Arne G, Kristiansson E, Nerman O, Kindblom LG, Ahlman H, Nilsson B, et al. Expression profiling of GIST: CD133 is associated with KIT exon 11 mutations, gastric location and poor prognosis. Int J Cancer J Int Cancer. 2011;129:1149–61.
Boegl M, Prinz C. Cd133 expression in different stages of gastric adenocarcinoma. Br J Cancer. 2009;100:1365–6. author reply 1367.
Wu S, Yu L, Wang D, Zhou L, Cheng Z, Chai D, et al. Aberrant expression of CD133 in non-small cell lung cancer and its relationship to vasculogenic mimicry. BMC Cancer. 2012;12:535.
Woo T, Okudela K, Mitsui H, Yazawa T, Ogawa N, Tajiri M, et al. Prognostic value of CD133 expression in stage i lung adenocarcinomas. Int J Clin Exp Pathol. 2010;4:32–42.
Mizugaki H, Sakakibara-Konishi J, Kikuchi J, Moriya J, Hatanaka KC, Kikuchi E, Kinoshita I, Oizumi S, Dosaka-Akita H, Matsuno Y, Nishimura M. Cd133 expression: A potential prognostic marker for non-small cell lung cancers. Int J Clin Oncol. 2013.
Sullivan JP, Spinola M, Dodge M, Raso MG, Behrens C, Gao B, et al. Aldehyde dehydrogenase activity selects for lung adenocarcinoma stem cells dependent on notch signaling. Cancer Res. 2010;70:9937–48.
Yang X, Takano Y, Zheng HC. The pathobiological features of gastrointestinal cancers (review). Oncol Lett. 2012;3:961–9.
Liu L, Wu C, Wang Y, Zhong R, Duan S, Wei S, et al. Combined effect of genetic polymorphisms in p53, p73, and mdm2 on non-small cell lung cancer survival. J Thorac Oncol. 2011;6:1793–800.
Liu L, Yuan P, Wu C, Zhang X, Wang F, Guo H, et al. Assessment of XPD Lys751gln and XRCC1 t-77c polymorphisms in advanced non-small-cell lung cancer patients treated with platinum-based chemotherapy. Lung Cancer. 2011;73:110–5.
Chen S, Song X, Chen Z, Li X, Li M, Liu H, et al. CD133 expression and the prognosis of colorectal cancer: a systematic review and meta-analysis. PLoS One. 2013;8:e56380.
Wen L, Chen XZ, Yang K, Chen ZX, Zhang B, Chen JP, et al. Prognostic value of cancer stem cell marker cd133 expression in gastric cancer: a systematic review. PLoS One. 2013;8:e59154.
Liu YP, Yang CJ, Huang MS, Yeh CT, Wu AT, Lee YC, et al. Cisplatin selects for multidrug-resistant CD133+ cells in lung adenocarcinoma by activating notch signaling. Cancer Res. 2013;73:406–16.
Lundin A, Driscoll B. Lung cancer stem cells: Progress and prospects. Cancer Lett. 2012.
Okudela K, Woo T, Mitsui H, Tajiri M, Masuda M, Ohashi K. Expression of the potential cancer stem cell markers, CD133, CD44, ALDH1, and beta-catenin, in primary lung adenocarcinoma-their prognostic significance. Pathol Int. 2012;62:792–801.
Xu YH, Zhang GB, Wang JM, Hu HC. B7-H3 and CD133 expression in non-small cell lung cancer and correlation with clinicopathologic factors and prognosis. Saudi Med J. 2010;31:980–6.
Acknowledgments
Research supported by National Natural Science Foundation of China (#81370078, #30930080, #81161120537, and #81001231), malignant tumor biomarkers in Jiangsu Province Key Laboratory (#11ZLKF09), the Natural Science Foundation of Jiangsu Province (#BK2012840), the Postdoctoral Science Foundation of China (#20100481165), Undergraduates Practice and InnovationTraining Project of Jiangsu Higher Education Institutes (#2012JSSPITP1021), and the Priority Academic Program Development of Jiangsu Higher Education Institutions (Public Health and Preventive Medicine).
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding authors
Additional information
Weimin Wang, Yansu Chen, and Jianliang Deng contributed equally to this work.
Rights and permissions
About this article
Cite this article
Wang, W., Chen, Y., Deng, J. et al. The prognostic value of CD133 expression in non-small cell lung cancer: a meta-analysis. Tumor Biol. 35, 9769–9775 (2014). https://doi.org/10.1007/s13277-014-2270-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-014-2270-9