Abstract
A cohort study of patients included in the Basque Country colorectal cancer (CRC) screening programme was carried out to assess the risk of adenomatous polyps and CRC (P-CRC) associated with HFE gene mutations, with gender and with iron biomarkers (serum ferritin (SF), iron (Fe) and transferrin saturation index (TSI)). Among 432 included patients (mean age 59.8 years), 263 were men (60.9 %) and 169 women (39.1 %). P-CRC were identified in 221 patients (51.2 %) and no polyps (NP) in 211 patients (48.8 %). HFE mutations were identified in 43.8 % of the patients. C282Y/wt genotypic frequency was 6.8 % in the P-CRC group and 1.4 % in the NP group (p < 0.05). The allelic frequency was 3.8 versus 1.2 % (p < 0.05). For laboratory, all three iron biomarkers showed a statistically significant difference: mean Fe, 91.29 ± 34 for P-CRC and 80.81 ± 30.59 for NP group. Mean TSI for P-CRC was 24.95 ± 8.90 and 22.74 ± 8.79 for NP group. Mean SF 308.09 ± 536.32 for P-CRC and 177.55 ± 159.95 for NP group. In a multivariate logistic regression analysis, only male gender (odds ratio (OR) = 2.04, 1.29–3.22), SF (OR = 1.001, 1.0004–1.003) and Fe (OR = 1.01, 1.004–1.02) were related with the presence of CRC and adenoma. Men gender and raised serum iron biomarkers increase the risk of P-CRC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colorectal cancer (CRC) is a very important public health problem. It is the second most common cancer in developed countries [1], and there are over 400,000 cases per year in the European countries [2]. Multiple factors are implicated in the development of the disease, which is seriously influenced by gene mutations and environmental factors [3].
The possible role of iron in cancer has been extensively debated, and its implication as a causative factor in carcinogenesis remains in doubt. Results from animal and human clinical studies have been limited by the low number of subjects [4]. Iron overload that is caused by hereditary mutations or excess dietary iron uptake has been identified as a risk factor for CRC [2, 4]. A recent meta-analysis indicates that higher intake of heme iron has a positive association with cancer risk [5].
Raised plasma iron levels increase the presence of carcinogenesis promoters (reactive oxygen species (ROS)). ROS produced by iron have been shown to specifically target some tumour suppressor genes [6]. CRC arises from benign neoplasms and evolves into adenocarcinoma through a stepwise histological progression sequence, proceeding from either adenomas or hyperplastic/serrated adenomas [7]. Iron has been implicated in intestinal carcinogenesis in rodent models of CRC [1, 6].
Large prospectively collected epidemiological data sets have suggested that iron may confer an increased risk for CRC [2, 7]. Numerous population studies support the hypothesis that dietary iron [1, 5] and/or elevated iron biomarkers increase the risk of suffering some cancers, including CRC, hepatocellular carcinoma (HCC), lung cancer and breast cancer [1, 8–20].
Hereditary hemochromatosis patients have a higher incidence of CRC than normal individuals. Raised transferrin saturation index levels in women and the C282Y/C282Y mutation in men are associated with an increased risk for CRC [10]. A recent meta-analysis supported a positive association between C282Y/wt polymorphism with CRC [3].
The CRC screening of the population of Basque Country revealed a high incidence of polyps and CRC [21]. The H63D mutation in the HFE gene study contributes to iron overload, especially the H63D/H63D mutation, but with considerable variability in the phenotypic expression. Individuals in Basque Country have this mutation with a higher prevalence than other countries in the world (30 vs. 15–20 %) [22].
In this study, we present the results from a prospective study of patients included in the Basque Country CRC screening. Our purpose was to evaluate if the risk of CRC and adenoma is associated with the HFE gene mutations, with gender and with serum iron biomarkers.
Methods
Study population
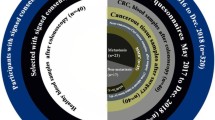
This was a prospective study of the Gipuzkoa population included in the CRC screening programme [21]. The inclusion criteria were patients from Gipuzkoa, asymptomatic, age range 50–69 years old, with positive faecal occult blood testing (FOBT)-OC Sensor® and complete colonoscopy [21]. Patients were excluded if they previously had the following: CRC, FOBT in the last 2 years, sigmoidoscopy in the last 5 years, complete colonoscopy (caecum reached) in the last 10 years [21], another digestive pathology currently being studied (IBD, etc.), serious disease or incapacity, no complete colonoscopy, non-recuperated polyp and absence of analytical parameters (incomplete data).
Endoscopy
The colonoscopy was performed under propofol sedation with an anaesthetist’s assistance. The patient was previously evaluated by the anaesthetist and received all of the required information about the procedure and the risks that were involved. The patients signed an informed consent form that was approved by the ethics council of Gipuzkoa.
Adult outpatients undergoing the CRC screening colonoscopy received a low-fibre diet, liquids and sodium picosulphate/magnesium citrate (CitraFleet®) for colon cleansing. The dosing and timing were previously described [23].
Laboratory measurements
Serum ferritin (SF), iron (Fe) and transferrin saturation index (TSI) were obtained from fasting blood samples from all of the patients. All were found to have raised SF values that were >300 μg/L. The laboratory normal range for SF is 15 to 200 μg/L in women and 30 to 300 μg/L in men [1, 3]. The ranges considered to be normal for Fe and TSI were 50–145 μg/dL and 15–45 %, respectively.
HFE mutation analysis
DNA was extracted from the blood samples, and HFE gene analysis was performed by multiplex real-time PCR using LightCycler technology (LC 1.0). Simultaneous detection of the HFE C282Y, H63D and S65C mutations was carried out in a single capillary using LC-Red 640, LC-Red 705 and fluorescein-labelled hybridisation probes (Tibmolbiol, Berlin, Germany). We used melting curve analysis to distinguish wild-type and mutant alleles in each sample [22].
Histologic findings
We classified the histologic findings of the resected lesions and of the biopsy samples taken in the colonoscopies as polyps and CRC (P-CRC), when we diagnosed adenomatous polyps or colorectal carcinoma (in situ, advanced carcinoma), and as no polyps (NP), when no colonic lesions were present (no polyps or isolated hyperplastic polyps with published criteria of no risk) [24].
Statistics
We described the variables as mean values with range and standard deviation for continuous variables and frequencies and percentages for categorical variables. To assess if the risk of CRC and adenoma was associated with the variables collected, we compare the distribution of each variable between patients with (P-CRC group) and without CRC and adenomas (NP group) using chi-square and Fisher’s exact tests. In the case of continuous variables, the Student t test was performed. The magnitude of the association was estimated by odds ratio (OR) and its 95 % confidence interval (binomial distribution). After the univariate analysis, we constructed a multivariable logistic regression model to assess the adjusted magnitude of the association of each one of the variables collected. In all the analyses, a p < 0.05 value was considered statistically significant, and STATA 12.1 (StataCorp LP, College Station, TX, USA) software was used.
Ethics
The study conformed to the principles of the Helsinki Declaration and was approved by the Ethics Committee of Clinical Investigation, Gipuzkoa, Spain (Acta no. 2/2011). The patients signed the informed consent form.
Results
Subjects
There were 450 consecutive patients during the period July 2011–May 2012. We excluded 18 of them due to non-recuperated polyp in seven cases, six for no complete colonoscopy and five had an absence of laboratory data. Nearly two thirds of the 432 patients with complete data were men (263, 60.9 %). Mean age was 59.8 years (SD, 5.66).
Histologic findings
We found P-CRC in 221 patients (51.2 %), which included 37 patients with cancer (16.7 %), 12 patients with adenocarcinoma and 25 with in situ carcinoma, and 211 NP patients (48.8 %).
HFE mutations
The study revealed 189 patients with HFE mutations (43.8 %): C282Y/wt mutation was detected in 18 patients (15 P-CRC group, 3 NP group), H63D/wt mutation was detected in 140 (76 P-CRC group, 64 NP group), H63D/H63D was detected in 16 (6 P-CRC group, 10 NP group), C282Y/H63D was detected in two patients (2 P-CRC group) and C282Y/C282Y was detected only in one patient (1 NP group). Among the 12 patients with the S65C mutation, nine were S65C/wt (6 in NP group), two H63D/S65C (1 in each group) and one C282Y/S65C (1 P-CRC group). The remaining 56.2 % of the patients were wt/wt (Table 1).
Only one HFE mutation presented statistically significant differences. The C282Y/wt genotypic frequency was 6.8 % in the P-CRC group and 1.4 % in the NP group (p < 0.05). Its allelic frequency was 3.8 versus 1.2 % (p < 0.05). The C282Y mutation presented an OR = 3 (95 % confidence interval (CI) 1.07–8.40) for P-CRC. The ORs for H63D and S65C, both non-significant, were respectively 1.26 (95 % CI 0.83–1.90) and 1.48 (95 % CI 0.40–6.02).
Laboratory
The distribution of the three serum iron biomarkers differed between the two groups, p < 0.05 (Table 2). Only SF and not the two other iron biomarkers was different between men and women, 330.35 ± 498 and 111.65 ± 75, respectively (Student’s t test, p < 0.001). The frequency of CRC was almost three times higher in men, OR = 2.75 (95 % CI 1.81 to 4.19; chi-square test, p < 0.001). Age was similar in both groups: 60.2 ± 5.50 and 59.3 ± 5.79 for P-CRC and NP, respectively (Student’s t test, p = 0.09). We introduced age, sex, C282Y and serum iron biomarkers in a multivariate logistic regression model. Only three of these variables showed a relation with the presence of CRC: male sex (OR = 2.04, 1.29–3.22), SF (OR = 1.001, 1.0004–1.003) and Fe (OR = 1.01, 1.004–1.02).
Discussion
The CRC screening programme in the Basque Country revealed a high prevalence of polyps and CRC [21]. The causes are not known, but iron has been implicated as a factor for CRC carcinogenesis. In the Basque Country, there is a high prevalence of the H63D mutation [22]. The causative role of a H63D/H63D mutation in hereditary hemochromatosis or iron overload has been demonstrated [22] but with less penetrance and a considerable variation of phenotypic expression. We have studied the prevalence of iron biomarkers and the different HFE mutations, with a special interest in the H63D/H63D mutation, in a CRC screening population set and compared the results between patients with or without polyps/CRC.
Previous studies have generally been performed retrospectively on groups of CRC patients, or in some prospective sets of patients with CRC or polyps, but this is the first work performed in a CRC screening prospective work. Recently, Fonseca-Nunes et al. [5] published a meta-analysis of the epidemiological evidence about iron and cancer risk. They concluded that more prospective studies were needed that combined results from research on dietary iron intake, iron biomarkers and genetic susceptibility.
The role of genetic risk factors for CRC has been studied by different groups [6–20]. HFE mutations have been implicated in the risk for developing CRC and polyps.
In some works, no association was reported, but in others, HFE mutations were associated with a risk for CRC, but with different mutations implicated [6–20].
A recent meta-analysis by Chen et al. [3] revealed that HFE gene C282Y variant is associated with colorectal cancer in Caucasians. They concluded that further large-scale studies taking gene/gene-environment interactions into consideration should be conducted to investigate the association.
In two works, one from Australia [8], with populations from very different origins, and another from Norway [9], it was determined that C282Y homozygosity increased the risk of CRC. An association between the C282Y allele (homozygosis and heterozygosis) in combination with transferrin receptor (TFR) Ser 142 allele homozygosity and the risk for CRC was reported in a study from Sweden [12]. Shaheen et al. [14], in the USA, determined that individuals with any of the HFE mutations were at an increased risk for CRC when compared to individuals without HFE mutations.
In contrast, some groups have found no relationship. A group from the UK [16] found that individuals with a single HFE mutation (C282Y or H63D) were unlikely to be predisposed to CRC. However, the risk of CRC was increased in the small number of individuals studied when there were compound heterozygotes (C282Y/H63D) for the HFE mutations. In the Netherlands [18], it was found that the C282Y mutation is not associated with an increased risk of CRC in postmenopausal women. In a prospective nested case-referent study in Sweden, Ekblom et al. [6] determined that HFE genotypes had no effect on CRC risk. There were too few homozygotes for HFE C282Y to make conclusions for this group.
There are three studies that did not detect C282Y homozygotes in both cases and controls and were omitted from the meta-analysis by Chen et al. [3]. A study conducted in Spain [11] did not report an association between HFE gene mutations and CRC. The authors suggested that the epidemiologic relationship between CRC and increased body iron probably results from dietary and environmental factors. Another study from Australia [13] did not report a relationship between the C282Y/wt mutation and the risk of CRC. Shi et al. [17] found a relationship between homozygosity for the H63D polymorphism and CRC risk in individuals from Australia and Poland.
Mc Glynn KA et al. [19] studied the risk of distal adenomatous colorectal polyps in association with HFE mutations. They did not find a relationship between HFE heterozygosity and the risk of advanced distal adenoma. Stratification of HFE genotypes by TFRC genotype did not change the results. In another study, the risk of colorectal adenoma in women was studied in the USA by Chan et al. [20], who did not find a relationship between HFE mutations and colorectal adenomas.
In our study, the C282Y/wt mutation genotypic frequency (6.79 %) was significantly higher in the P-CRC group than in the NP group (1.42 %) (p < 0.05). The C282Y allelic frequency was significantly higher as well (3.85 versus 1.18 %; p < 0.05). The other HFE mutations did not present significant differences between the two groups. The C282Y mutation had an OR = 2.99 (95 % CI 1.07–8.04). When we analysed the study’s significant variables by multivariate logistic regression, the C282Y/wt mutation did not give a significant predictive value (p = 0.126).
Iron biomarkers seem to be implicated in the risk for CRC. The relative risk for developing CRC in subjects with moderately high levels of serum transferrin saturation and high serum ferritin is three times higher than in the normal population [11]. Ekblom et al. [6] found that high iron levels do not increase the risk of CRC. Asberg et al. [9] determined that in the general population, individuals with very low or very high serum transferrin saturation may have increased risk for cancer. Chan et al. [20] did not observe a role for iron in the pathogenesis of CRC in women. Ellervik et al. [10] demonstrated that elevated transferrin saturation levels in women and a C282Y homozygous genotype are associated with an increased risk for cancer. In our study, the differences of the means of the three iron biomarkers (SF, TSI and Fe) between the two groups were statistically significant (p < 0.05). When we studied iron biomarkers and gender, only SF, Fe and male sex showed an independent association with P-CRC after multivariate logistic regression with odds ratios of 1.001 (95 % CI 1.0004–1.003), 1.01 (95 % CI 1.004–1.02) and 2.04 (95 % CI 1.29–3.22), respectively.
So gender seems to be an important factor for an increased risk for CRC and adenomas [21]. In our study, male gender more than doubled the risk compared with women.
We have not studied dietary factors in this work but rather focused on HFE gene mutations and iron biomarkers. A recent meta-analysis supports a paper of both dietary haemoglobin and red meat in colorectal carcinogenesis [25].
The age was homogenous, as they were patients from general population CRC screening study (50–69 years old). There were more men in the study (61 versus 39 %) as normally occurs in CRC screening studies [21].
We conclude that iron biomarkers and being male, but not HFE mutations, increased the risk for CRC and polyps in this prospective setting. Men with raised serum iron and ferritin may be a target risk group to be screened due to their higher risk for developing CRC.
References
Chua ACG, Klopcic B, Lawrance IC, Olynyk JK, Trinder D. Iron: an emerging factor in colorectal carcinogenesis. World J Gastroenterol. 2010;16:663–72.
Xue X, Shah YM. Intestinal iron homeostasis and colon tumorigenesis. Nutrients. 2013;5:2333–51.
Chen W, Zhao H, Li T, Yao H. HFE gene C282Y variant is associated with colorectal cancer in Caucasians: a meta-analysis. Tumor Biol. 2013;34:2255–9.
Fargion S, Valenti L, Fracanzani AL. Hemochromatosis gene (HFE) mutations and cancer risk: expanding the clinical manifestations of hereditary iron overload. Hepatology. 2010;51:1119–21.
Fonseca-Nunes A, Jakszyn P, Agudo A. Iron and cancer risk—a systematic review and meta-analysis of the epidemiological evidence. Cancer Epidemiol Biomarkers Prev. 2014;23:12–31.
Ekblom K, Marklund SL, Palmqvist R, Van Guelpen B, Hallmans G, Weinehall L, et al. Iron biomarkers in plasma, HFE genotypes, and risk for colorectal cancer in a prospective setting. Dis Col Rectum. 2012;55:337–44.
Butterworth JR. Another important function for an old friend! The role of iron in colorectal carcinogenesis. Gut. 2006;55:1384–86.
Osborne NJ, Gurrin LC, Allen KJ, Constantine CC, Delatycki MB, McLaren CE, et al. HFE C282Y homozygotes are at increased risk of breast and colorectal cancer. Hepatology. 2010;51:1311–18.
Asberg A, Thorstensen K, Irgens WO, Romundstad PR, Hveem K. Cancer risk in HFE C282Y homozygotes: results from the HUNT 2 study. Scand J Gastroenterol. 2013;48:189–95.
Ellervik C, Tybjaerg-Hansen A, Nordestgaard BG. Risk of cancer by transferrin saturation levels and haemochromatosis genotype: population-based study and meta-analysis. J Intern Med. 2012;27:51–63.
Altés A, Gimferrer E, Capella G, Barceló MJ, Baiget M. Colorectal cancer and HFE mutations. Haematologica. 1999;84:479–80.
Beckman LE, Van Landenghem GF, Sikstrom C, Wahlin A, Markevarn B, Hallmans G, et al. Interaction between haemochromatosis and transferrin receptor in different neoplastic disorders. Carcinogenesis. 1999;20:1231–3.
MacDonald GA, Tarish J, Whitehall VJL, McCann SJ, Mellick GD, Buttenshaw RL, et al. No evidence of increased risk of colorectal cancer in individuals heterozygous for the cys282Tyr haemochromatosis mutation. J Gastroenterol Hepatol. 1999;14:1188–91.
Shaheen NJ, Silverman LM, Keku T, Lawrence LB, Rohlfs EM, Martin CF, et al. Association between hemochromatosis (HFE) gene mutation carrier status and the risk of colon cancer. J Natl Cancer Inst. 2003;95:154–9.
Sullivan JL. Re: association between hemochromatosis (HFE) gene mutation carrier status and the risk of colon cancer. J Natl Cancer Inst. 2003;95:829–30.
Robinson JP, Johnson VL, Rogers PA, Houlston RS, Maher ER, Bishop DT, et al. Evidence for an association between compound heterozygosity for germ line mutations in the hemochromatosis (HFE) gene and increased risk of colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:1460–3.
Shi Z, Johnstone D, Talseth-Palmer B, Evans T-J, Spigelman AD, Groombridge C, et al. Haemochromatosis HFE gene polymorphisms as potential modifiers of hereditary nonpolyposis colorectal cancer risk and onset age. Int J Cancer. 2009;125:78–83.
DL V d A, van der Hel O, Roest M, van der Schouw YT, van Gils CH, Marx JJM, et al. Heterozygosity for the Cys282Tyr mutation in the HFE gene and the risk of colorectal cancer (Netherlands). Cancer Causes Control. 2003;14:541–5.
McGlynn KA, Sakoda LC, Hu Y, Schoen RE, Bresalier RS, Yeager M, et al. Hemochromatosis gene mutations and distal adenomatous colorectal polyps. Cancer Epidemiol Biomarkers Prev. 2005;14:158–63.
Chan AT, Ma J, Tranah GJ, Giovannucci EL, Rifai N, Hunter DJ, et al. Hemochromatosis gene mutations, body iron stores, dietary iron and risk of colorectal adenoma in women. J Natl Cancer Inst. 2005;97:917–26.
Portillo I, Idigoras I, Ojembarrena E, Arana E, Hurtado JL, Basurko R, et al. Lesions detected in a colorectal cancer screening program in the Basque Country: first round (2009–2011). Gastroenterol Hepatol. 2013;36:301–8.
De Juan MD, Reta A, Castiella A, Pozueta J, Prada A, Cuadrado E. HFE gene mutation analysis in Basque hereditary hemochromatosis patients and controls. Eur J Hum Genet. 2001;9:961–4.
Parra-Blanco A, Nicolás-Pérez D, Gimeno-García A, Grosso B, Jimenez A, Ortega J, et al. The timing of bowel preparation before colonoscopy determines the quality of cleansing, and is a significant factor contributing to the detection of flat lesions: a randomized study. World J Gastroenterol. 2006;14:6161–66.
Lambert R, Kudo SE, Vieth M, Allen JI, Fujii H, Fujii T, et al. Pragmatic classification of superficial neoplastic colorectal lesions. Gastrointest Endosc. 2009;70:1182–99.
Bastide NM, Pierre FHF, Corpet DE. Heme iron from meat and risk of colorectal cancer: a meta-analysis and a review of the mechanisms involved. Cancer Prev Res. 2011;4:177–84.
Acknowledgments
This work was supported by a grant from the Health Department of the Basque Country (no. 2010111125).
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Additional information
Agustin Castiella and Fernando Múgica contributed equally to this work.
Rights and permissions
About this article
Cite this article
Castiella, A., Múgica, F., Zapata, E. et al. Gender and plasma iron biomarkers, but not HFE gene mutations, increase the risk of colorectal cancer and polyps. Tumor Biol. 36, 6959–6963 (2015). https://doi.org/10.1007/s13277-015-3406-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-015-3406-2