Abstract
Interleukin (IL)-16 plays a fundamental role in inflammatory diseases, as well as in the development and progression of tumors. Genetic variation in DNA sequence of IL16 gene may lead to altered cytokine production and/or activity, and this variation may modulate an individual's susceptibility to nasopharyngeal carcinoma (NPC). To test this hypothesis, we investigated the association of IL16 gene polymorphisms and serum IL-16 levels with NPC risk in a Chinese population. We analyzed IL16 gene rs11556218 T/G, rs4778889 T/C, and rs4072111 C/T polymorphisms using PCR-RFLP and DNA sequencing, and serum IL-16 levels were measured by ELISA. The IL16 rs11556218 T/G polymorphism was significantly associated with the susceptibility to NPC patients. The TG genotype was associated with a significantly higher risk of NPC as compared with the TT genotype (OR = 2.05, 95 % CI 1.04–4.01; p = 0.037). Patients carrying the G allele had a significantly higher risk for developing NPC compared with individuals carrying the T allele (OR = 1.79, 95 % CI 1.07–3.01; p = 0.027). The serum IL-16 levels were increased in NPC patients compared with controls (p < 0.01); the genotypes carrying the IL16 rs11556218 G variant allele were associated with increased serum IL-16 levels compared with the homozygous wild-type genotype in NPC patients (all p values <0.01). Our data suggested that IL16 rs11556218 T/G polymorphism was associated with increased susceptibility to NPC through increasing the production of serum IL-16 levels.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nasopharyngeal carcinoma (NPC) is an epithelial malignancy with strikingly variable racial and geographic distribution. It is rarely seen in most regions of the world but is prevalent among populations from Southeast Asia [1], especially in Guangdong and Guangxi provinces of southern China. Etiologically, carcinogenesis of NPC is a complex, multistep, and multifactor process, in which many factors are implicated. It was reported that major risk factors for the development of NPC are Epstein-Barr virus infection, long-term tobacco smoking, occupational exposure to formaldehyde, and various dietary factors [2]. However, even if a group of people are exposed to the same hazard in areas where NPC is endemic, only a few people develop the disease, suggesting that genetic differences such as single-nucleotide polymorphisms (SNP) may contribute to NPC occurrence.
Interleukin (IL)-16 is one of the potent pro-inflammatory cytokines which has a wide array of biological functions and was initially identified as lymphocyte chemoattractant factor in 1982 [3]. The IL16 gene is located on chromosome 15q26.3 in humans and encodes two distinct isoforms, leukocyte IL-16 (isoform 1) and neuronal IL-16 (isoform 2), which is derived from two different transcription variants [4]. IL-16 is a precursor protein consisting of 631 amino acids that is cleaved by caspase-3 to form the active C-terminal domain containing 121 amino acids [5, 6]. IL-16 is produced by activated CD8+ T cells, mast cells, and B cells [7–9] and can selectively activate CD4+ T cells, monocytes, macrophages, eosinophils, and dendritic cells by binding the CD4 receptor [10, 11]. Furthermore, IL-16 can promote the secretion of tumor-associated inflammatory cytokines TNF-α, IL-1β, IL-6, and IL-15 by monocytes [12]. All these cytokines have been demonstrated to play a critical role in the pathogenesis of human cancers [13–16]. Furthermore, the high levels of IL-16 also have been demonstrated in several malignant cancers both in vitro and in vivo [17–23]. Hence, it is biologically reasonable to hypothesize a potential relationship between the IL16 gene polymorphisms and cancer risk.
Recently, several studies have revealed that the genetic variants of the IL16 gene were associated with cancers, including prostate cancer, colorectal, and gastric cancer [23–25]. In previous study, we also identified that the rs11556218 T/G and rs4072111 C/T polymorphisms of the IL16 gene were significantly associated with the susceptibility to HBV-related hepatocellular carcinoma in a Chinese population [26]. However, few studies, to date, have examined the association between IL16 polymorphisms and NPC; the relationship between IL16 gene polymorphisms and the IL-16 serum levels remains unknown. Therefore, the aim of this study was to investigate the relationship between IL16 gene polymorphisms and the incidence of NPC and the influence of SNPs on IL-16 serum levels in patients with NPC versus healthy controls in a Chinese population.
Materials and methods
Study population
The case–control population contained 150 unrelated adult Chinese who were selected from the same population living in China between October 2009 and April 2010 (Table 1). A total of 75 NPC patients were recruited from the Department of Otolaryngology, First Affiliated Hospital of Guangxi Medical University. The only selection criterion for patients was that their NPC diagnosis had been pathologically confirmed. The patients (55 men; 20 women) had a mean (standard deviation) age of 46.03(9.05) years. The control group comprised of 75 healthy volunteers who visited the general health checkup division at the First Affiliated Hospital of Guangxi Medical University. Selection criteria for controls were no evidence of any personal or family history of cancer or other serious illness. The mean age of the control group (50 men and 25 women) was 44.04(8.33) years. There was no significant difference between patients and control subjects in terms of sex and age distribution. Written informed consent was obtained from all of the included subjects, and the study was performed with the approval of the ethics committee of the First Affiliated Hospital of Guangxi Medical University.
DNA extraction and genotyping
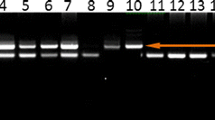
Genomic DNA was extracted from 200 μl EDTA anticoagulated peripheral blood using QIAamp DNA blood mini kit (QIAGEN GmbH, Hilden, Germany) according to the manufacturer's instructions. IL16 gene polymorphisms (rs11556218T/G, rs4072111C/T, and rs4778889T/C) were identified by performing polymerase chain reaction–restriction fragment length polymorphism analysis (Fig. 1). Primer sequences, reaction conditions, restriction enzymes used, and length of resulting polymerase chain reaction products are listed in Table 2. To confirm the genotyping results, polymerase chain reaction-amplified DNA samples were examined by DNA sequencing. The results were 100 % concordant.
PCR-RFLP assay for analyzing the rs11556218 T/G, rs4778889 T/C, and rs4072111 C/T polymorphisms of the IL16 gene. PCR product was digested by restriction enzyme and separated on 8 % polyacrylamide gels electrophoresis. a Lanes 1, 2, and 3 show rs11556218 TT genotypes; lanes 4, 5, and 6 show rs11556218 TG genotypes; lanes 7 and 8 show rs11556218 GG genotypes. b Lanes 1, 2, 3, and 4 show rs4778889 TT genotypes; lanes 5, 6, and 7 show rs4778889 TC genotypes. c Lanes 1, 3, 4, and 5 show rs4072111 CC genotypes; lanes 2, 6, 7, and 8 show rs4072111 TC genotypes
Serum IL-16 levels
Serum samples were available for all included subjects. When blood samples were obtained, the serum was allowed to clot for 30 min at 4 °C before centrifugation at 2,000 rpm for 10 min at 4 °C. Serum was isolated and stored at −80 °C before use. We detected IL-16 concentration by using a sandwich ELISA (Quantikine EGF immunoassay kit, R&D Systems, Minneapolis, MN, USA) according to the manufacturer's instructions. The minimum level of detection for IL-16 was 5 pg/mL. No cross-detection of other cytokines was observed. Each sample was assayed in duplicate, and the intra-assay coefficient of variation was 10 %.
Statistical analysis
Genotype and allele frequencies of IL16 were compared between NPC patients and controls using the χ 2 test and Fisher's exact test when appropriate. Odds ratio (OR) and 95 % confidence intervals (CIs) were calculated by binary logistic regression and adjusted for age, gender, smoking, and drinking status to assess the relative risk conferred by a particular allele and genotype. Demographic and clinical data among groups were compared using the χ 2 test and the Student's t test. Hardy–Weinberg equilibrium (HWE) was tested with a goodness of fit χ 2 test with 1° of freedom to compare the observed genotype frequencies among the subjects with the expected genotype frequencies. Differences in IL-16 serum levels among patients with NPC versus control subjects were examined using the one-way analysis of variance, and if significant, followed by multiplied paired analyses. To elucidate the interaction of the factors studied (genotype and/or severity) on IL-16 serum levels, the data were analyzed by two-way analysis of variance for independent samples. Statistical significance was assumed at the p < 0.05 level. The SPSS statistical software package version 13.0 was used for all of the statistical analyses.
Results
Characteristics of the study subjects
Table 1 summarizes the characteristics of the 75 cancer patients and 75 control subjects included in this study. No significant differences in age, gender distribution, or smoking and alcohol consumption status were identified between cancer patients and control subjects, suggesting that subject matching based on these variables were adequate.
Genotype and allele distribution of IL16 polymorphisms
The genotype and allele frequencies of IL16 gene polymorphisms among NPC patients and controls are summarized in Table 3. The genotype distributions of these three polymorphisms in control subjects were presented consistent with HWE. For the rs11556218 T/G, there was a significant difference in the genotype and allele frequencies of this polymorphism between NPC patients and control subjects. The frequencies of the TT, TG, and GG genotypes of rs11556218 T/G were 61.3, 34.7, and 4.0 % in controls, and 42.7, 49.3, and 8.0 % in NPC patients, respectively. The TG genotype was associated with a significantly increased risk of NPC as compared with the TT genotype (OR = 2.05, 95 % CI 1.04–4.01; p = 0.037). The G allele was associated with a significantly increased risk of NPC as compared with the T allele (OR = 1.79, 95 % CI 1.07–3.01; p = 0.027). Under the dominant model, genotype TG + GG appeared to be associated with an increased risk of NPC when compared with TT genotype (OR = 2.13, 95 % CI 1.11–4.09; p = 0.022). However, genotype and allele frequencies of the IL16 gene rs4778889 T/C and rs4072111 C/T polymorphisms in NPC patients were not significantly different from those in healthy controls (p > 0.05).
The genotype and allele frequencies of IL16 polymorphisms in relation to pathological indices of NPC severity
No significant association was found between IL16 gene rs11556218 T/G, rs4778889 T/C, and rs4072111 C/T polymorphisms and different clinical stage as shown in Table 4. Genotype and allele frequencies of the IL16 gene rs11556218 T/G, rs4778889 T/C, and rs4072111 C/T polymorphisms in stages I and II were not significantly different from that in stages III and IV of PNC patients (all p values are larger than 0.05).
Association between IL16 gene polymorphisms and serum IL-16 levels
The serum IL-16 concentration was significantly higher in the NPC patients (n = 75, 40.22 ± 9.09 pg/mL) than that in the controls (n = 75, 28.31 ± 5.28 pg/mL; p < 0.01). As shown in Table 5, we found no association between the serum IL-16 concentration and the IL16 gene polymorphisms in the controls. However, the genotypes of the rs11556218 T/G polymorphism were significantly associated with serum IL-16 levels in NPC patients. The serum IL-16 levels were significantly higher in individuals with homozygous GG genotypes (45.91 ± 8.31 pg/mL, n = 6) or heterozygous TG genotypes (43.12 ± 9.98 pg/mL, n = 37) than homozygous TT genotypes (35.80 ± 5.82 pg/mL, n = 32; p < 0.05, respectively); however, there were no significant differences in the serum IL-16 levels between GG and TG genotypes (Fig. 2). In addition, there were no significant associations of the IL16 gene rs4778889 T/C and rs4072111 C/T polymorphisms with serum levels of IL-16 in patients with NPC.
Association between serum IL-16 levels and IL16 rs11556218T/G polymorphism in NPC patients. Serum IL-16 level with TT homozygous was significantly lower than that of the GG homozygous or TG heterozygotes, respectively. However, there were no significant differences in the serum IL-16 levels between GG and TG genotypes
Discussion
In the present study, we investigated whether IL16 gene polymorphisms are related to the occurrence of NPC and whether IL16 gene polymorphisms correlate with serum levels of IL-16 in a Chinese population. Our results showed that the rs11556218 T/G polymorphism of IL16 gene and the serum levels of IL-16 were significantly associated with the presence of NPC. The rs11556218 T/G polymorphism may affect the serum levels of IL-16. People carrying the IL16 gene rs11556218 G variant allele had an increased ability to produce IL-16, which may contribute to NPC susceptibility. Our results suggest that IL16 gene rs11556218 T/G may play an important role in the development of NPC. Thus, IL16 gene rs11556218 T/G polymorphism may serve as the novel genetic marker of susceptibility to NPC in Chinese population.
In most parts of the world, NPC is rare, but it occurs at a high frequency in Southeast Asia and China and at a somewhat lower but still elevated rate in North and East Africa. Microscopically, one striking feature of NPC is a heavy infiltration of nonmalignant lymphocytes, and most of these lymphocytes have been shown to be T cells. Another important feature of NPC is its association with the EBV infection. Studies have shown that the expression of EBV gene products is involved in the latent and lytic cycles in NPC specimens, and some of these viral gene products might have the capacity to induce or influence inflammatory cytokine production, such as IL-10, IL-6, and TGF-β [27, 28]. The contribution of inflammation and inflammatory cells to the process of tumor development and progression has been increasingly recognized [29, 30]. Elevated expression of cytokines is a common phenomenon of tumor cell lines derived from many cancers, such as melanomas, leukemias, and gastric and ovarian carcinoma [31–34]. Evidence has shown that a substantial proportion of malignant tumors worldwide arise from infection and chronic inflammation [35]. Both inflammatory and tumor cells produce an assorted array of cytokines and chemokines, which mediate all aspects of inflammation and profoundly affect the development and progression of cancer [36, 37]. Previous studies have shown that NPC is associated with overexpression of numerous cytokines in NPC biopsies. EBV infection is ubiquitous in the world, but NPC incidence differs according to geographic region, and the reasons for this selective susceptibility are not fully understood. However, it is clear that environmental, viral, and host factors play a role.
As a multifunctional cytokine, IL-16 is an important mediator in inflammatory diseases as well as tumor growth and progression. Passam et al. [21] reported that non-Hodgkin's lymphoma patients who responded to standard treatment had lower levels of IL-16 compared with pretreatment levels. In human astrocytic brain tumors and rat C6 gliomas, Liebrich et al. [18] reported higher levels of IL-16 present. Similarly, Blaschke et al. [22] reported that IL-16 messenger RNA expression increased with the stage of cutaneous T cell lymphoma diagnosed. A few studies have shown that higher serum levels of IL-16 can be associated with advanced stages of cancer [17] and a worse patient outcome depending on the type of tumor [20]. Recently, IL-16 has been reported to be a candidate susceptibility gene to colorectal and gastric cancer [23]. Our previous study also has shown that the rs11556218 T/G and rs4072111C/T polymorphisms of the IL16 gene were significantly associated with the susceptibility to hepatocellular carcinoma in a Chinese population [26]. The mechanisms by which IL16 gene polymorphisms affect cancer risk remain unknown, but evidence shows that chronic inflammation pathogenesis is the underlying pathological event in these malignancies. NPC is an epithelial cancer that is causally associated with EBV infection; studies have shown that cytokines synthesis might contribute to lymphocyte infiltration and/or tumor growth during NPC development. Therefore, it is plausible that the various phenotypes of IL-16 may result in individuals with various inflammatory response and NPC risk.
Our study shows that the IL16 gene polymorphisms probably play a major role in susceptibility to NPC. The rs11556218 TG genotype was associated with a significantly increased risk of NPC when compared with the TT genotypes (OR = 2.05, 95 % CI 1.04–4.01; p = 0.037). Patients carrying the rs11556218 G allele had a significantly higher risk for developing NPC compared to individuals carrying the T allele (OR = 1.79, 95 % CI 1.07–3.01; p = 0.027). A possible mechanism for IL16 rs11556218 T/G polymorphism in modulating NPC susceptibility and tumor development is that the rs11556218 T/G polymorphism regulate the expression of serum IL-16 levels; the overexpressed IL-16 activates CD4+ T lymphocytes, monocyte macrophages, eosinophils, and dendrite cells by binding to CD4 (the main receptor of IL-16). The binding of IL-16 stimulates the monocytes to secrete multiple cytokines, including TNF-α, IL-1β, IL-6, and IL-15 [14, 16, 38], which have been shown to play an important role in tumorigenesis of NPC.
To our knowledge, the influence of IL16 polymorphisms on severity to NPC has not been previously studied. Moreover, no studies have been performed to investigate the association between serum IL-16 levels and NPC. In this study, our results showed that there were no significant differences when patients with the stages I and II were compared with patients with stages III and IV. Based on our results, we suggest that the IL16 polymorphisms may not have an influence on the severity of NPC. However, we observed that the serum IL-16 levels were significantly higher in individuals with homozygous rs11556218 GG genotypes or heterozygous rs11556218 TG genotypes than homozygous rs11556218 TT genotypes in NPC patients. Our results suggested that the IL16 gene rs11556218 T/G polymorphism may regulate expression of the serum IL-16 levels and associate with an increased risk of NPC. Thus, genotypes carrying the IL16 rs11556218 G variant allele may contribute to NPC susceptibility through increasing the ability to produce IL-16, which validated our hypothesis above.
There are several limitations in this study. First, detection of serum IL-16 concentration with the method of ELISA cannot completely and directly reflect the IL16 gene expression, and mRNA expression evaluated in peripheral blood mononuclear cells by RT-PCR may be helpful. Second, we do not identify whether IL16 gene polymorphism plays an important role in protein posttranslational modification. Further study will be required to explore the exact mechanism on how the IL16 gene polymorphisms were involve in the pathogenesis of NPC.
In summary, we found that the IL16 rs8034928 T/C polymorphism and the levels of serum IL-16 were significantly associated with increased risk of NPC. These results suggest that the IL16 rs8034928 T/C polymorphism may contribute to an inherited predisposition to NPC through increased expression of IL-16 levels. Additional studies with larger sample sizes will be necessary to confirm our findings. Because genetic polymorphisms often vary among different ethnic groups, further studies are needed to clarify the association of the IL16 polymorphism with the risk of NPC in diverse ethnic populations.
References
Wei WI, Sham JS. Nasopharyngeal carcinoma. Lancet. 2005;365:2041–54.
Chang ET, Adami HO. The enigmatic epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol Biomarkers Prev. 2006;15:1765–77.
Center DM, Cruikshank W. Modulation of lymphocyte migration by human lymphokines. I. Identification and characterization of chemoattractant activity for lymphocytes from mitogen-stimulated mononuclear cells. J Immunol. 1982;128:2563–8.
Kim HS: Assignment of human interleukin 16 (il16) to chromosome 15q26.3 by radiation hybrid mapping. Cytogenet Cell Genet 1999;84:93
Baier M, Bannert N, Werner A, Lang K, Kurth R. Molecular cloning, sequence, expression, and processing of the interleukin 16 precursor. Proc Natl Acad Sci U S A. 1997;94:5273–7.
Zhang Y, Center DM, Wu DM, Cruikshank WW, Yuan J, Andrews DW, et al. Processing and activation of pro-interleukin-16 by caspase-3. J Biol Chem. 1998;273:1144–9.
Laberge S, Cruikshank WW, Kornfeld H, Center DM. Histamine-induced secretion of lymphocyte chemoattractant factor from CD8+ T cells is independent of transcription and translation. Evidence for constitutive protein synthesis and storage. J Immunol. 1995;155:2902–10.
Rumsaeng V, Cruikshank WW, Foster B, Prussin C, Kirshenbaum AS, Davis TA, et al. Human mast cells produce the CD4+ T lymphocyte chemoattractant factor, il-16. J Immunol. 1997;159:2904–10.
Sharma V, Sparks JL, Vail JD. Human B cell lines constitutively express and secrete interleukin-16. Immunology. 2000;99:266–71.
Cruikshank WW, Center DM, Nisar N, Wu M, Natke B, Theodore AC, et al. Molecular and functional analysis of a lymphocyte chemoattractant factor: association of biologic function with CD4 expression. Proc Natl Acad Sci U S A. 1994;91:5109–13.
Center DM, Kornfeld H, Cruikshank WW. Interleukin 16 and its function as a cd4 ligand. Immunol Today. 1996;17:476–81.
Mathy NL, Scheuer W, Lanzendorfer M, Honold K, Ambrosius D, Norley S, et al. Interleukin-16 stimulates the expression and production of pro-inflammatory cytokines by human monocytes. Immunology. 2000;100:63–9.
Mochizuki Y, Nakanishi H, Kodera Y, Ito S, Yamamura Y, Kato T, et al. TNF-alpha promotes progression of peritoneal metastasis as demonstrated using a green fluorescence protein (gfp)-tagged human gastric cancer cell line. Clin Exp Metastasis. 2004;21:39–47.
Kai H, Kitadai Y, Kodama M, Cho S, Kuroda T, Ito M, et al. Involvement of proinflammatory cytokines il-1beta and il-6 in progression of human gastric carcinoma. Anticancer Res. 2005;25:709–13.
Chung YC, Chang YF. Serum interleukin-6 levels reflect the disease status of colorectal cancer. J Surg Oncol. 2003;83:222–6.
Shanmugham LN, Petrarca C, Frydas S, Donelan J, Castellani ML, Boucher W, et al. Il-15 an immunoregulatory and anti-cancer cytokine. Recent advances J Exp Clin Cancer Res. 2006;25:529–36.
Kovacs E. The serum levels of IL-12 and IL-16 in cancer patients. Relation to the tumor stage and previous therapy. Biomed Pharmacother. 2001;55:111–6.
Liebrich M, Guo LH, Schluesener HJ, Schwab JM, Dietz K, Will BE, et al. Expression of interleukin-16 by tumor-associated macrophages/activated microglia in high-grade astrocytic brain tumors. Arch Immunol Ther Exp (Warsz). 2007;55:41–7.
Koike M, Sekigawa I, Okada M, Matsumoto M, Iida N, Hashimoto H, et al. Relationship between cd4(+)/cd8(+) T cell ratio and T cell activation in multiple myeloma: reference to il-16. Leuk Res. 2002;26:705–11.
Alexandrakis MG, Passam FH, Kyriakou DS, Christophoridou AV, Perisinakis K, Hatzivasili A, et al. Serum level of interleukin-16 in multiple myeloma patients and its relationship to disease activity. Am J Hematol. 2004;75:101–6.
Passam FH, Sfiridaki A, Pappa C, Kyriakou D, Petreli E, Roussou PA, et al. Angiogenesis-related growth factors and cytokines in the serum of patients with b non-Hodgkin's lymphoma: relation to clinical features and response to treatment. Int J Lab Hematol. 2008;30:17–25.
Blaschke V, Reich K, Middel P, Letschert M, Sachse F, Harwix S, et al. Expression of the cd4+ cell-specific chemoattractant interleukin-16 in mycosis fungoides. J Investig Dermatol. 1999;113:658–63.
Gao LB, Rao L, Wang YY, Liang WB, Li C, Xue H, et al. The association of interleukin-16 polymorphisms with il-16 serum levels and risk of colorectal and gastric cancer. Carcinogenesis. 2009;30:295–9.
Thomas G, Jacobs KB, Yeager M, Kraft P, Wacholder S, Orr N, et al. Multiple loci identified in a genome-wide association study of prostate cancer. Nat Genet. 2008;40:310–5.
Azimzadeh P, Romani S, Mohebbi SR, Kazemian S, Vahedi M, Almasi S, et al. Interleukin-16 (il-16) gene polymorphisms in Iranian patients with colorectal cancer. J Gastrointest Liver Dis. 2011;20:371–6.
Li S, Deng Y, Chen ZP, Huang S, Liao XC, Lin LW, et al. Genetic polymorphism of interleukin-16 influences susceptibility to HBV-related hepatocellular carcinoma in a Chinese population. Infect Genet Evol. 2011;11:2083–8.
Nakagomi H, Dolcetti R, Bejarano MT, Pisa P, Kiessling R, Masucci MG. The Epstein-Barr virus latent membrane protein-1 (lmp1) induces interleukin-10 production in Burkitt lymphoma lines. Int J Cancer. 1994;57:240–4.
Eliopoulos AG, Stack M, Dawson CW, Kaye KM, Hodgkin L, Sihota S, et al. Epstein-Barr virus-encoded lmp1 and cd40 mediate il-6 production in epithelial cells via an NF-kappa b pathway involving TNF receptor-associated factors. Oncogene. 1997;14:2899–916.
Lu H, Ouyang W, Huang C. Inflammation, a key event in cancer development. Mol Cancer Res. 2006;4:221–33.
Moss SF, Blaser MJ. Mechanisms of disease: inflammation and the origins of cancer. Nat Clin Pract Oncol. 2005;2:90–7. quiz 91 p following 113.
Bani MR, Garofalo A, Scanziani E, Giavazzi R. Effect of interleukin-1-beta on metastasis formation in different tumor systems. J Natl Cancer Inst. 1991;83:119–23.
Yamashita U, Shirakawa F, Nakamura H. Production of interleukin 1 by adult t cell leukemia (atl) cell lines. J Immunol. 1987;138:3284–9.
Ito R, Kitadai Y, Kyo E, Yokozaki H, Yasui W, Yamashita U, et al. Interleukin 1 alpha acts as an autocrine growth stimulator for human gastric carcinoma cells. Cancer Res. 1993;53:4102–6.
Li BY, Mohanraj D, Olson MC, Moradi M, Twiggs L, Carson LF, et al. Human ovarian epithelial cancer cells cultures in vitro express both interleukin 1 alpha and beta genes. Cancer Res. 1992;52:2248–52.
Quirk JT, Kupinski JM. Chronic infection, inflammation, and epithelial ovarian cancer. Med Hypotheses. 2001;57:426–8.
Lin WW, Karin M. A cytokine-mediated link between innate immunity, inflammation, and cancer. J Clin Invest. 2007;117:1175–83.
Rollins BJ. Inflammatory chemokines in cancer growth and progression. Eur J Cancer. 2006;42:760–7.
Muc-Wierzgon M, Nowakowska-Zajdel E, Kokot T, Kozowicz A, Wiczkowski A, Grochowska-Niedworok E, et al. Genetic dysregulation of TNF alpha and TNF alpha type ii receptors in colon cancer at the II and III stage of disease. J Biol Regul Homeost Agents. 2006;20:10–4.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (no. 81260302).
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Xue Qin, Qiliu Peng, and Xiaoxia Lao contributed equally to this work and should be considered as co-first authors.
Rights and permissions
About this article
Cite this article
Qin, X., Peng, Q., Lao, X. et al. The association of interleukin-16 gene polymorphisms with IL-16 serum levels and risk of nasopharyngeal carcinoma in a Chinese population. Tumor Biol. 35, 1917–1924 (2014). https://doi.org/10.1007/s13277-013-1257-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-013-1257-2