Abstract
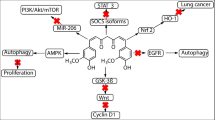
Recent population studies provide clues that the use of curcumin may be associated with reduced incidence and improved prognosis of certain cancers. In the present study, we demonstrated that curcumin acted as a growth inhibitor for lung cancer cells. Our results found that curcumin inhibited cell proliferation, which was associated with upregulation of the cyclin-dependent kinase inhibitors, p27 and p21, and downregulation of cyclin D1. In addition, we showed that curcumin induced the expression of forkhead box protein O1 (FOXO1) through activation of extracellular signal-regulated kinase 1/2 signaling. These findings provide evidence for a mechanism that may contribute to the antineoplastic effects of curcumin and justify further work to explore potential roles for activators of FOXO1 in the prevention and treatment of lung cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lung cancer has become the highest incidence and death rates among human neoplasms worldwide [1]. Clinically, non-small cell lung cancer (NSCLC) accounts for about 80 % of the total number of lung cancer cases, with an overall 5-year survival rate of about 15 % [2, 3]. However, the unclear mechanisms underlying the pathogenic development of NSCLC and therapeutic strategy remain largely unexplored.
Forkhead box protein O1 (FOXO1), a member of the FOXO family of transcription factors, was characterized by a conserved forkhead domain containing a DNA binding domain [4]. FOXO1 has been implicated as a critical regulator of many biological events, including cell proliferation, differentiation, DNA damage repair, and stress responses [5–7]. FOXO1 can lead to cell cycle arrest through upregulation of the cyclin-dependent kinase inhibitors, p27and p21, and downregulation of the cell cycle regulator cyclin D1 [8]. Therefore, FOXO1 was considered as a key tumor suppressor. Growing evidence suggested that FOXO1 could inhibit cell proliferation in many types of tumor cells, including lung cancer cells [9].
Curcumin, a natural compound extracted from Curcuma longa, is known to have anti-inflammatory and antiproliferative properties and affects the expression of cell adhesion molecules [10]. Previous studies have shown that curcumin has chemopreventive potential for several cancers through modulating multiple cell signaling pathways [11, 12]. However, the underlying mechanisms of this anticancer effect are still under investigation, especially for its anti-invasive potential in SCLC. Here, our aim is to determine the role of FOXO1 in curcumin-induced cell cycle arrest.
Materials and methods
Chemicals
Curcumin was purchased from Sigma-Aldrich (St. Louis, MO), dissolved in DMSO at a stock concentration of 20 mM, and diluted to the indicated concentration with Dulbecco's modified Eagle medium (DMEM) medium.
Cell culture and transfection
A549 and H460 (human SCLC) cell lines were cultured in DMEM medium supplemented with 10 % fetal bovine serum (Gibco, CA, USA) as well as 100 U/ml penicillin and 100 μg/ml streptomycin. Small interfering RNA (siRNA) against FOXO1 was purchased from Dharmacon (USA). As a negative control, a siRNA sequence targeting green fluorescent protein (GFP) was used. The siRNA sequences were as follows: 5′-CUGGAUCACAGUUUUCCAAAUG-3′ (FOXO1) and 5′-GCAAGCUGACCCUGAAGUUCAU-3′ (GFP). All transient transfections were performed using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions.
Cell proliferation assays
Proliferation of SCLC cells was determined by analyzing 5′-bromo-2′-deoxyuridine (BrdU) incorporation into newly synthesized DNA using a cell proliferation enzyme-linked immunosorbent assay (Beyotime, Shanghai, China). For the BrdU incorporation assays, cells were counted and seeded into 96-well culture plates (2.5 × 103 cells/well). Colorimetric analysis was determined with an ELISA plate reader (Beckman) at 450 nm.
RNA isolation and real-time PCR
Total RNAs were isolated from SCLC cells using the TRIzol reagent (Invitrogen, Shanghai, China), and reverse transcriptions were performed using the TaKaRa RNA PCR Kit (TaKaRa, China) following the manufacturer's instructions. In order to quantify the transcripts of the interest genes, real-time PCR was performed using the SYBR Green Premix Ex Taq (TaKaRa, Japan) on Light Cycler 480 (Roche, Switzerland). Each sample was normalized on the basis of its β-actin content. Primer sequences were as follows: for p27, 5′-TAATTGGGGCTCCGGCTAACT-3′ (forward) and 5′-TGCAGGTCGCTTCCTTATTCC-3′ (reverse); for p21, 5′-TGTCCGTCAGAACCCATGC (forward) and AAAGTCGAAGTTCCATCGCTC-3′ (reverse); for cyclin D1, 5′-CAATGACCCCGCACGATTTC (forward) and CATGGAGGGCGGATTGGAA-3′ (reverse); for FOXO1, 5′-TCGTCATAATCTGTCCCTACACA (forward) and CGGCTTCGGCTCTTAGCAAA-3′ (reverse); and for β-actin, 5′-CATGTACGTTGCTATCCAGGC (forward) and CTCCTTAATGTCACGCACGAT-3′ (reverse).
Western blot
SCLC cells were harvested and lysed with ice-cold lysis buffer (62.5 mM Tris–HCl, pH 6.8, 100 mM DTT, 2 % w/v sodium dodecyl sulfate (SDS), 10 % glycerol). After centrifugation at 20,000 × g for 10 min at 4 °C, proteins in the supernatants were quantified and separated by 10 or 12 % SDS-PAGE and then transferred to NC membrane. After blocking with 5 % nonfat milk in PBS, membranes were immunoblotted with antibodies as indicated, followed by HRP-linked secondary antibodies. The signals were detected using the SuperSignal West Pico Chemiluminescent Substrate Kit according to the manufacturer's instructions. Antibodies anti-p21, anti-p27, anti-cyclin D1, anti-FOXO1, and anti-GAPDH antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA); anti-total extracellular signal-regulated kinase (ERK) 1/2 and anti-phosphorylated ERK1/2 were purchased from Cell Signaling Technology (Danvers, MA, USA).
Statistical analysis
Data were summarized as mean ± SEM. Student's t tests were used to analyze the significant differences between groups. Results were considered to be significant for p values of <0.05.
Results
Curcumin treatment inhibited cell growth in a dose-dependent manner
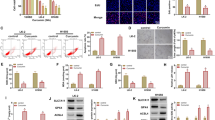
To observe the effects of curcumin on lung cancer cells, we selected two cell lines, A549 cells and H460 cells. Both cell lines were treated with curcumin at several concentrations. After 36 h of treatment, growth was inhibited in a dose-dependent manner in both cell lines (Fig. 1a, b). These results showed that the concentration of curcumin at 10 μM was appropriate in both cell lines. Thus, 10 μM of curcumin was selected for further analysis of genes expression in both cell lines.
Expression of p27, p21, and cyclin D1 protein in curcumin-treated cells
We found that growth inhibition was caused by cell cycle arrest in lung cancer cells following curcumin treatment. To confirm these results, we analyzed the expression of p27, p21, and cyclin D1, which are known as key molecules involved in cell cycle arrest. As shown in Fig. 2, expression levels of p27 and p21 were upregulated in A549 cells (Fig. 2(a, b)). Besides, the contents of cyclin D1 were markedly downregulated in curcumin-treated cells (Fig. 2(c)).
Expression of p27, p21, and cyclin D1 protein in curcumin-treated cells. a, b mRNA (a) and protein (b) levels of p27 and p21 were analyzed in A549 cells treated with curcumin (0, 10 μM) at 12 and 24 h, respectively. c mRNA and protein levels of cyclin D1 were analyzed in A549 cells treated with curcumin (0, 10 μM) at 12 and 24 h, respectively
Roles of FOXO1 in the curcumin-treated lung cancer cells
Previous studies have demonstrated that FOXO1 could regulate a variety of genes relevant to the cell cycle at the transcriptional level, such as p21, p27, and cyclin D1. Therefore, our data suggest that curcumin might modulate the expression of p27, p21, and cyclin D1 by upregulation of FOXO1. As shown in Fig. 3a, b, curcumin treatment increased mRNA and protein abundance of FOXO1 in A549 and H460 cells. Next, we test whether the inhibition of cell proliferation by curcumin is mediated by FOXO1 in lung cancer cells. A549 cells were transfected with siRNA against FOXO1 (Fig. 3c). As a result, knockdown of FOXO1 significantly attenuated the inhibition of cell proliferation by curcumin (Fig. 3d), demonstrating that FOXO1 plays an indispensable role in curcumin-induced cell cycle arrest.
Curcumin increased FOXO1 in human lung cancer cells. (A-B) mRNA and protein levels of FOXO1 were analyzed in A549 cells (A) and H460 cells (B) treated with curcumin (0, 10 μM). c mRNA and protein levels of FOXO1 in A549 cells transfected with siRNA oligos as indicated. Cells were harvest for real-time PCR and Western blot analysis after transfection for 24 or 36 h, respectively. d Cell proliferation ability was determined by BrdU assays in A549 cells after transfection of siRNA oligos as indicated. Cells were treated with curcumin (0, 10 μM) for 24 h
Curcumin induced FOXO1 expression through ERK1/2 signaling
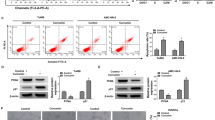
We next sought to determine whether FOXO1 is a molecular target of curcumin. We analyzed the activity of human FOXO1 promoter in A549 cells. In accordance with the regulation of FOXO1 mRNA and protein levels, curcumin activated human FOXO1 promoter activity (Fig. 4a). Through serial deletion of this promoter, we found a minimal responsive region (278–270 bp upstream of the transcriptional start site) that contained a consensus binding motif for activator protein 1 (AP-1) (Fig. 4b). Site mutagenesis experiments further indicated that the AP-1 binding site was required for the induction of promoter activity by curcumin (Fig. 4c). Indeed, we observed the strong activation of ERK1/2, which is upstream of AP1, by curcumin (Fig. 4d). Consistently, inhibition of ERK activation by PD98059 abolished the curcumin-induced promoter activity (Fig. 4e). In addition, PD98059 also blocked the mRNA and protein expression of FOXO1 in curcumin-treated A549 cells (Fig. 4f). Collectively, these findings suggest that curcumin upregulates FOXO1 expression through the ERK1/2–Ap1 signaling axis.
Curcumin regulates FOXO1 expression through ERK1/2 signaling pathway. a Genomic structure showing human FOXO1 promoter (top, the transcription start site was set as +1 bp). Relative activities of luciferase reporters driven by human FOXO1 promoter were determined in A549 cells treated with or without curcumin (10 μM). b Relative luciferase activity of serial deletion of human FOXO1 promoter in A549 cells. c Relative luciferase activity of wild-type FOXO1 promoter (WT) or AP-1 binding site promoter (Mut) in A549 cells. d Western blot analysis of phosphorylated ERK1/2 in A549 cells treated with curcumin for 30 min. Total ERK1/2 was set as a loading control. e Relative luciferase activity of human FOXO1 promoter in A549 cells in the absence or presence of PD98059. Cells were pretreated with PD98059 for 2 h and then exposed to curcumin for another 24 h. f Western blot analysis of FOXO1 protein levels in A549 cells in the absence or presence of PD98059
Discussion
To date, there are no data delineating the effects of curcumin on the FOXO1 expression at the transcriptional level in lung cancer cells. In this study, we demonstrated that curcumin treatment led to a decrease of cell proliferation in lung cancer cell lines. At the molecular level, we found that curcumin promoted p27 and p21 expression while inhibited cyclin D1 expression through induction of FOXO1. Furthermore, our results revealed that curcumin induced FOXO1 mRNA and protein levels by activation of ERK1/2 signaling. Our results will help determine if curcumin has potential roles as a novel therapeutic agent for the treatment of lung cancer.
The members of the FOXO subfamily have been suggested as critical regulators in cancer cell biology. FOXO1 was firstly identified during study of the t(2,13)(q35;q14) and t(1,13)(p36;q14) chromosomal translocations, which are usually found in alveolar rhabdomyosarcoma, a skeletal muscle tumor prevalent in children [13]. In clinical NSCLC samples, the expression and activity of FOXO1 was downregulated, suggesting that FOXO1 expression is a favorable prognostic factor in NSCLC [14]. In addition, the induction or reactivation of FOXO1 might be an efficient strategy to battle with NSCLC.
Curcumin is derived from turmeric (C. longa) and is a natural polyphenol [10]. Curcumin has been used as a food coloring agent and traditional medicine for many years. Especially for its anticarcinogenic property, it still has been the subject of a great deal of interest. Several reports indicated that curcumin has anticancer effects against different types of human tumor cells, including of ovarian cancer cells, colon cancer cells, and glioblastoma cells [15, 16]. The anticancer effect of curcumin was identified through interfering with the cell cycle, inducing apoptosis, and inhibiting the invasive potential of cancers [17, 18].
In summary, for the first time, our study demonstrated the roles of FOXO1 signaling in curcumin-inhibited lung cancer cell proliferation. Future studies are required to identify the molecular mechanisms that are involved in the upregulation of FOXO1 and activation of ERK1/2 signaling by curcumin.
References
Sun S, Schiller JH, Gazdar AF. Lung cancer in never smokers—a different disease. Nat Rev Cancer. 2007;7:778–90.
Spiro SG, Silvestri GA. One hundred years of lung cancer. Am J Respir Crit Care Med. 2005;172:523–9.
Spiro SG, Tanner NT, Silvestri GA, Janes SM, Lim E, Vansteenkiste JF, et al. Lung cancer: progress in diagnosis, staging and therapy. Respirology. 2010;15:44–50.
Huang H, Tindall DJ. Dynamic FoxO transcription factors. J Cell Sci. 2007;120:2479–87.
Kops GJ, Medema RH, Glassford J, Essers MA, Dijkers PF, Coffer PJ, et al. Control of cell cycle exit and entry by protein kinase B-regulated forkhead transcription factors. Mol Cell Biol. 2002;22:2025–36.
Schmidt M, Fernandez de Mattos S, van der Horst A, Klompmaker R, Kops GJ, Lam EW, et al. Cell cycle inhibition by FoxO forkhead transcription factors involves downregulation of cyclin D. Mol Cell Biol. 2002;22:7842–52.
Burgering BM, Medema RH. Decisions on life and death: FOXO forkhead transcription factors are in command when PKB/Akt is off duty. J Leukoc Biol. 2003;73:689–701.
Greer EL, Brunet A. FOXO transcription factors at the interface between longevity and tumor suppression. Oncogene. 2005;24:7410–25.
Sangodkar J, Dhawan NS, Melville H, Singh VJ, Yuan E, Rana H, et al. Targeting the FOXO1/KLF6 axis regulates EGFR signaling and treatment response. J Clin Invest. 2012;122:2637–51.
Manju V, Nalini N. Chemopreventive efficacy of ginger, a naturally occurring anticarcinogen during the initiation, post-initiation stages of 1,2 dimethylhydrazine-induced colon cancer. Clin Chim Acta. 2005;358:60–7.
Shehzad A, Khan S, Sup Lee Y. Curcumin molecular targets in obesity and obesity-related cancers. Future Oncol. 2012;8:179–90.
Saha S, Adhikary A, Bhattacharyya P, Das T, Sa G. Death by design: where curcumin sensitizes drug-resistant tumours. Anticancer Res. 2012;32:2567–84.
Galili N, Davis RJ, Fredericks WJ, Mukhopadhyay S, Rauscher 3rd FJ, Emanuel BS, et al. Fusion of a fork head domain gene to PAX3 in the solid tumour alveolar rhabdomyosarcoma. Nat Genet. 1993;5:230–5.
Maekawa T, Maniwa Y, Doi T, Nishio W, Yoshimura M, Ohbayashi C, et al. Expression and localization of FOXO1 in non-small cell lung cancer. Oncol Rep. 2009;22:57–64.
Dorai T, Aggarwal BB. Role of chemopreventive agents in cancer therapy. Cancer Lett. 2004;215:129–40.
Sinha R, Anderson DE, McDonald SS, Greenwald P. Cancer risk and diet in India. J Postgrad Med. 2003;49:222–8.
Nessa MU, Beale P, Chan C, Yu JQ, Huq F. Studies on combination of platinum drugs cisplatin and oxaliplatin with phytochemicals anethole and curcumin in ovarian tumour models. Anticancer Res. 2012;32:4843–50.
Yogosawa S, Yamada Y, Yasuda S, Sun Q, Takizawa K, Sakai T. Dehydrozingerone, a structural analogue of curcumin, induces cell-cycle arrest at the G2/M phase and accumulates intracellular ROS in HT-29 human colon cancer cells. J Nat Prod. 2012;75:2088–93.
Acknowledgment
This study was supported by the National Natural Science Foundation of China (No. 81071839), Capital Medical Development Foundation of China (2007-2040).
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Additional information
The Publisher and Editor retract this article in accordance with the recommendations of the Committee on Publication Ethics (COPE). After a thorough investigation we have strong reason to believe that the peer review process was compromised.
An erratum to this article can be found online at http://dx.doi.org/10.1007/s13277-017-5487-6.
About this article
Cite this article
Li, ZC., Zhang, LM., Wang, HB. et al. RETRACTED ARTICLE: Curcumin inhibits lung cancer progression and metastasis through induction of FOXO1. Tumor Biol. 35, 111–116 (2014). https://doi.org/10.1007/s13277-013-1013-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-013-1013-7