Abstract
Cytotoxic T lymphocyte antigen 4 (CTLA-4) gene +49G>A polymorphism was implicated to be associated with risk of malignant bone tumors, but the finding was inconclusive owing to the limited sample of a single study. The objective of the current study was to conduct a pooled analysis of four previously published studies to investigate the association between CTLA-4 +49G>A polymorphism and the risk of malignant bone tumors. Data were extracted, and the pooled odds ratio (OR) with the corresponding 95 % confidence interval (95 % CI) was calculated to assess the association. Those four published studies included a total of 2,165 subjects. The pooled results indicated that CTLA-4 +49G>A polymorphism was significantly associated with risk of malignant bone tumors (AA versus GG: OR = 2.24, 95 % CI 1.67–2.99, P < 0.001; AA/GA versus GG: OR = 1.35, 95 % CI 1.14–1.61, P = 0.001; AA versus GG/GA: OR = 2.00, 95 % CI 1.53–2.62, P < 0.001). Stratified analyses by tumor type showed that CTLA-4 +49G>A polymorphism was associated with risks of both osteosarcoma (AA versus GG: OR = 2.23, 95 % CI 1.45–3.43, P < 0.001; AA/GA versus GG: OR = 1.35, 95 % CI 1.04–1.75, P = 0.024; AA versus GG/GA: OR = 2.00, 95 % CI 1.34–2.98, P = 0.001) and Ewing's sarcoma (AA versus GG: OR = 2.24, 95 % CI 1.51–3.31, P < 0.001; AA/GA versus GG: OR = 1.36, 95 % CI 1.07–1.72, P = 0.011; AA versus GG/GA: OR = 2.01, 95 % CI 1.39–2.89, P < 0.001). Therefore, results from the current pooled analysis suggest that CTLA-4 +49G>A polymorphism is associated with risk of malignant bone tumors, including osteosarcoma and Ewing's sarcoma.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Malignant bone tumors are usually aggressive tumors that require aggressive treatments [1]. Osteosarcoma and Ewing's sarcoma are the two most common primary malignant bone tumors, and both of these tumors cause serious damage to human health [1, 2]. Although many advances have been established in the pathogenesis of bone tumors, the molecular pathogenesis of osteosarcoma and Ewing's sarcoma is unclear [1, 2]. It is no doubt that a better understanding of the pathogenesis of osteosarcoma and Ewing's sarcoma can help us develop new treatments [2, 3]. Previous studies have suggested that both of these tumors develop as multifactorial diseases that result from complex interactions between genetic and environmental factors [4, 5]. Currently, tumor immunity is increasing as a hot spot in cancer research, and the antitumor response involves T lymphocytes and natural killer (NK) cells [5, 6]. Previous studies have suggested that genetic variants in the genes that regulate the activation and proliferation of T lymphocytes and NK cells can affect the development of common cancers [7]. Cytotoxic T lymphocyte antigen 4 (CTLA-4) is a member of the immunoglobulin superfamily and plays a critical role in the negative regulation of T cell proliferation and activation [8, 9]. Previous studies showed that CTLA-4 could elevate the T cell activation threshold, attenuate the antitumor response, and increase the host's susceptibility to common cancers [8, 9]. There are many single-nucleotide polymorphisms identified in the CTLA-4 gene, and CTLA-4 +49G>A polymorphism is the most commonly studied one [10, 11]. Many studies have indicated that CTLA-4 +49G>A polymorphism is involved in the etiology of various cancers, such as pancreatic cancer and breast cancer [12, 13]. There were also several studies assessing the association between CTLA-4 +49G>A polymorphism and risk of malignant bone tumors, but the finding was inconclusive owing to the limited sample of a single study [14–17]. The objective of the current study was to conduct a pooled analysis of four previously published studies to investigate the association between CTLA-4 +49G>A polymorphism and the risk of malignant bone tumors.
Methods
Identification of eligible studies
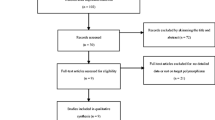
A literature search of PubMed and Wanfang databases (updated to March 19, 2013) was conducted using combinations of the following terms: “polymorphism or variant or mutation” and “bone cancer or bone carcinoma or osteosarcoma or Ewing's sarcoma” and “CTLA-4 or cytotoxic T-lymphocyte antigen-4.” There was no language restriction in the literature search. All studies that evaluated the association between CTLA-4 +49G>A polymorphism and the risk of malignant bone tumors were retrieved. Eligible studies in the meta-analysis must meet all of the following criteria: (1) Assessment of the association between CTLA-4 +49G>A polymorphism and the risk of malignant bone tumors, (2) case–control design, and (3) sufficient genotype numbers for estimating an odds ratio (OR) with a 95 % confidence interval (95 % CI). Abstracts, reviews, or studies that did not report genotype frequency were all excluded.
Data extraction
Two authors extracted all data independently and reached a consensus on all items. The following information was collected: first author's name, year of publication, country of origin, ethnicity, source of the control group (controls), matching factors, genotyping methods, type of bone tumors, total number of cases and controls, and genotype distributions in cases and controls.
Statistical analysis
The strength of the association between CTLA-4 +49G>A polymorphism and the risk of malignant bone tumors were assessed using OR with corresponding 95 % CI. The pooled ORs were calculated with the following models (AA versus GG, AA/GA versus GG, and AA versus GG/GA). The OR was calculated by using a fixed-effects model (the Mantel–Haenszel method) or a random-effects model (the DerSimonian and Laird method) according to heterogeneity. The statistical heterogeneity among studies was assessed with the I 2 test which was used to quantify inconsistency. An I 2 value ≥50 % was considered to represent significant statistical heterogeneity, and the random effect model was used to calculate the pooled OR. An I 2 value <50 % was considered to represent less heterogeneity, and the fixed-effects model was used to calculate the pooled OR. Subgroup analysis by tumor types was also performed. All statistical tests were performed using STATA statistical software package (version 11.0; StataCorp, College Station, TX).
Results
Characteristics of included studies
After literature search and detailed evaluation, four individual studies were included into this pooled analysis [14–17]. The summary of eligible studies included in the meta-analysis was shown in Table 1. Those four published studies included a total of 2,165 subjects, and all studies were performed on Asians [14–17]. Among those four studies, two were on the association between CTLA-4 +49G>A polymorphism and risk of osteosarcoma [14, 15], and the other two were on the association between CTLA-4 +49G>A polymorphism and risk of Ewing's sarcoma [16, 17]. Different genotyping methods were used, and polymerase chain reaction–restriction fragment length polymorphism was the main method. All studies matched the cases and controls by age, sex, and residence area (Table 1).
Quantitative synthesis
The pooled results were shown in Table 2. There was no between-study heterogeneity among those four studies. The pooled results indicated that CTLA-4 +49G>A polymorphism was significantly associated with risk of malignant bone tumors (AA versus GG: OR = 2.24, 95 % CI 1.67–2.99, P < 0.001; AA/GA versus GG: OR = 1.35, 95 % CI 1.14–1.61, P = 0.001; AA versus GG/GA: OR = 2.00, 95 % CI 1.53–2.62, P < 0.001) (Table 2, Figs. 1, 2, and 3).
Stratified analyses by tumor type showed that CTLA-4 +49G>A polymorphism was associated with risks of both osteosarcoma (AA versus GG: OR = 2.23, 95 % CI 1.45–3.43, P < 0.001; AA/GA versus GG: OR = 1.35, 95 % CI 1.04–1.75, P = 0.024; AA versus GG/GA: OR = 2.00, 95 % CI 1.34–2.98, P = 0.001) and Ewing's sarcoma (AA versus GG: OR = 2.24, 95 % CI 1.51–3.31, P < 0.001; AA/GA versus GG: OR = 1.36, 95 % CI 1.07–1.72, P = 0.011; AA versus GG/GA: OR = 2.01, 95 % CI 1.39–2.89, P < 0.001) (Table 2).
Discussion
Osteosarcoma and Ewing's sarcoma are the two most common primary malignant bone tumors, and both of these tumors cause serious damage to human health [1, 2]. It is no doubt that a better understanding of the pathogenesis of osteosarcoma and Ewing's sarcoma can help us develop new treatments, but the molecular pathogenesis of osteosarcoma and Ewing's sarcoma is unclear [2, 3]. Previous studies have suggested that genetic factors also play important roles in the development of common cancers including osteosarcoma and Ewing's sarcoma [4, 5]. Previous studies have suggested that genetic variants in the genes that regulate the activation and proliferation of T lymphocytes and NK cells can affect the development of common cancers, and there are possible roles of T lymphocytes in the development of osteosarcoma and Ewing's sarcoma [7].
Host genetic factors, especially variants of genes involved in the tumorigenesis of osteosarcoma and Ewing's sarcoma, can affect the host's differences in the susceptibility to cancers [18, 19]. Thus, interest in the genetic susceptibility to osteosarcoma and Ewing's sarcoma has led to an increase in the studies on the association between genetic polymorphisms and risks of osteosarcoma and Ewing's sarcoma [20, 21]. CTLA-4 is a member of the immunoglobulin superfamily and plays a critical role in the negative regulation of T cell proliferation and activation [8, 9]. Previous studies showed that CTLA-4 could elevate the T cell activation threshold, attenuate the antitumor response, and increase the host's susceptibility to common cancers including osteosarcoma and Ewing's sarcoma [8, 9]. CTLA-4 +49G>A polymorphism is the most commonly studied single-nucleotide polymorphism in the CTLA-4 gene [10, 11]. Many studies have indicated that CTLA-4 +49G>A polymorphism is involved in the etiology of various cancers, such as pancreatic cancer and breast cancer [12, 13].
Currently, there were also several studies assessing the association between CTLA-4 +49G>A polymorphism and risk of malignant bone tumors, but the finding was inconclusive owing to the limited sample of a single study [14–17]. So, we conducted a pooled analysis of four previously published studies to further investigate the association between CTLA-4 +49G>A polymorphism and the risk of malignant bone tumors. Those four published studies included a total of 2,165 subjects. The pooled results indicated that CTLA-4 +49G>A polymorphism was significantly associated with risk of malignant bone tumors (AA versus GG: OR = 2.24, 95 % CI 1.67–2.99, P < 0.001; AA/GA versus GG: OR = 1.35, 95 % CI 1.14–1.61, P = 0.001; AA versus GG/GA: OR = 2.00, 95 % CI 1.53–2.62, P < 0.001). Stratified analyses by tumor type showed that CTLA-4 +49G>A polymorphism was associated with risks of both osteosarcoma (AA versus GG: OR = 2.23, 95 % CI 1.45–3.43, P < 0.001; AA/GA versus GG: OR = 1.35, 95 % CI 1.04–1.75, P = 0.024; AA versus GG/GA: OR = 2.00, 95 % CI 1.34–2.98, P = 0.001) and Ewing's sarcoma (AA versus GG: OR = 2.24, 95 % CI 1.51–3.31, P < 0.001; AA/GA versus GG: OR = 1.36, 95 % CI 1.07–1.72, P = 0.011; AA versus GG/GA: OR = 2.01, 95 % CI 1.39–2.89, P < 0.001). Therefore, results from the current pooled analysis suggest that CTLA-4 +49G>A polymorphism is associated with risk of malignant bone tumors, including osteosarcoma and Ewing's sarcoma.
However, some limitations should be considered in interpreting the results from the pooled analysis. Firstly, all case–control studies were on Asians; thus, our results may be applicable only to Asian groups. The association between CTLA-4 +49G>A polymorphism and the risk of malignant bone tumors in other populations need further studies. Secondly, our pooled analysis only included published studies which were indexed by the selected databases, but some possible unpublished studies with null results may be missed, which might bias the pooled results in our study. Thirdly, the gene–gene and gene–environment interactions between CTLA-4 polymorphisms and risk of malignant bone tumors were not analyzed owing to the lack of relevant data. Future large-scale studies are needed to clarify the gene–gene and gene–environment interactions between CTLA-4 polymorphisms and risk of malignant bone tumors [22].
In conclusion, the results from this pooled analysis suggest that CTLA-4 +49G>A polymorphism is associated with risk of malignant bone tumors, including osteosarcoma and Ewing's sarcoma. Future large-scale studies are needed to clarify the gene–gene and gene–environment interactions between CTLA-4 polymorphisms and risk of malignant bone tumors.
References
Balamuth NJ, Womer RB. Ewing's sarcoma. Lancet Oncol. 2010;11:184–92.
Ritter J, Bielack SS. Osteosarcoma. Ann Oncol. 2010;21 Suppl 7:vii320–5.
Yarber JL, Agulnik M. Targeted therapies in bone sarcomas: current approach and future directions. Expert Opin Investig Drugs. 2011;20:973–9.
Toomey EC, Schiffman JD, Lessnick SL. Recent advances in the molecular pathogenesis of Ewing's sarcoma. Oncogene. 2010;29:4504–16.
Schwab JH, Springfield DS, Raskin KA, Mankin HJ, Hornicek FJ. What's new in primary bone tumors. J Bone Joint Surg Am. 2012;94:1913–9.
Chen G, Emens LA. Chemoimmunotherapy: reengineering tumor immunity. Cancer Immunol Immunother. 2013;62:203–16.
Dong LM, Potter JD, White E, Ulrich CM, Cardon LR, Peters U. Genetic susceptibility to cancer: the role of polymorphisms in candidate genes. JAMA. 2008;299:2423–36.
Scalapino KJ, Daikh DI. CTLA-4: a key regulatory point in the control of autoimmune disease. Immunol Rev. 2008;223:143–55.
Yuan J, Ginsberg B, Page D, Li Y, Rasalan T, Gallardo HF, et al. CTLA-4 blockade increases antigen-specific CD8(+) T cells in prevaccinated patients with melanoma: three cases. Cancer Immunol Immunother. 2011;60:1137–46.
Sun T, Hu Z, Shen H, Lin D. Genetic polymorphisms in cytotoxic T-lymphocyte antigen 4 and cancer: the dialectical nature of subtle human immune dysregulation. Cancer Res. 2009;69:6011–4.
Benhatchi K, Jochmanova I, Habalova V, Wagnerova H, Lazurova I. CTLA4 exon1 A49G polymorphism in Slovak patients with rheumatoid arthritis and Hashimoto thyroiditis—results and the review of the literature. Clin Rheumatol. 2011;30:1319–24.
Ghaderi A. CTLA4 gene variants in autoimmunity and cancer: a comparative review. Iran J Immunol. 2011;8:127–49.
Yang M, Sun T, Zhou Y, Wang L, Liu L, Zhang X, et al. The functional cytotoxic T lymphocyte-associated protein 4 49G-to-a genetic variant and risk of pancreatic cancer. Cancer. 2012;118:4681–6.
Liu Y, He Z, Feng D, Shi G, Gao R, Wu X, et al. Cytotoxic T-lymphocyte antigen-4 polymorphisms and susceptibility to osteosarcoma. DNA Cell Biol. 2011;30:1051–5.
Wang W, Wang J, Song H, Liu J, Song B, Cao X. Cytotoxic T-lymphocyte antigen-4 +49G/A polymorphism is associated with increased risk of osteosarcoma. Genet Test Mol Biomarkers. 2011;15:503–6.
Yang S, Wang C, Zhou Y, Sun G, Zhu D, Gao S. Cytotoxic T-lymphocyte antigen-4 polymorphisms and susceptibility to Ewing's sarcoma. Genet Test Mol Biomarkers. 2012;16:1236–40.
Feng D, Yang X, Li S, Liu T, Wu Z, Song Y, et al. Cytotoxic T-lymphocyte antigen-4 genetic variants and risk of Ewing's sarcoma. Genet Test Mol Biomarkers. 2013;17:458–63.
Huang HJ, Angelo LS, Rodon J, Sun M, Kuenkele KP, Parsons HA, et al. R1507, an anti-insulin-like growth factor-1 receptor (IGF-1R) antibody, and EWS/FLI-1 siRNA in Ewing's sarcoma: convergence at the IGF/IGFR/AKt axis. PLoS One. 2011;6:e26060.
Postel-Vinay S, Veron AS, Tirode F, Pierron G, Reynaud S, Kovar H, et al. Common variants near TARDBP and EGR2 are associated with susceptibility to Ewing sarcoma. Nat Genet. 2012;44:323–7.
Mackintosh C, Ordonez JL, Garcia-Dominguez DJ, Sevillano V, Llombart-Bosch A, Szuhai K, et al. 1q gain and CDT2 overexpression underlie an aggressive and highly proliferative form of Ewing sarcoma. Oncogene. 2012;31:1287–98.
Silva DS, Sawitzki FR, De Toni EC, Graebin P, Picanco JB, Abujamra AL, et al. Ewing's sarcoma: analysis of single nucleotide polymorphism in the EWS gene. Gene. 2012;509:263–6.
Attia J, Thakkinstian A, D’Este C. Meta-analyses of molecular association studies: methodologic lessons for genetic epidemiology. J Clin Epidemiol. 2003;56:297–303.
Conflicts of interests
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yu, F., Miao, J. Significant association between cytotoxic T lymphocyte antigen 4 +49G>A polymorphism and risk of malignant bone tumors. Tumor Biol. 34, 3371–3375 (2013). https://doi.org/10.1007/s13277-013-0908-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-013-0908-7