Abstract
Crude oil contamination has been widely recognized as a major environmental issue due to its various adverse effects. The use of inhabitant microorganisms (native to the contaminated sites) to detoxify/remove pollutants owing to their diverse metabolic capabilities is an evolving method for the removal/degradation of petroleum industry contaminants. The present study deals with the exploitation of native resident bacteria from crude oil contaminated site (oil exploration field) for bioremediation procedures. Fifteen (out of forty-four) bioremediation-relevant aerobic bacterial strains, belonging to the genera of Bacillus, Stenotrophomonas, Pseudomonas, Paenibacillus, Rhizobium, Burkholderia, and Franconibacter, isolated from crude oil containing sludge, have been selected for the present bioremediation study. Crude oil bioremediation performance of the selected bacterial consortium was assessed using microcosm-based studies. Stimulation of the microbial consortium with nitrogen or phosphorous led to the degradation of 60–70% of total petroleum hydrocarbon (TPH) in 0.25% and 0.5% crude oil experimental sets. CO2 evolution, indicative of crude oil mineralization, was evident with the highest evolution being 28.6 mg mL−1. Ecotoxicity of treated crude oil-containing media was assessed using plant seed germination assay, in which most of the 0.25% and 0.5% treated crude oil sets gave positive results thereby suggesting a reduction in crude oil toxicity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Exploration of crude oil is an age-old process that has been leading to severe contamination of land masses, water bodies and groundwater reserves through accidental spillage, storing, refining, production and distribution and sometimes drilling site abandonment (Holliger et al. 1997; Ulrich et al. 2009; Roy et al. 2014). Oily sludge is a waste product that is generated in various stages of petroleum industries, including extraction, storage, transportation, and refining of petroleum crude oil. The practical and effective disposal of this oily sludge has become a global issue due to its toxic nature and large quantity produced every year. Indian refineries produce more than 28,220 tons of sludge every year (Singh and Kumar 2020). Crude oil, extracted from this oily sludge, remains in the environment for a long time due to its persistent nature which rigorously affects the quality of soil by altering physical, physiological, biochemical properties and intrinsic heterogeneous microbial diversity (Margesin et al. 2003; Head et al. 2006). Oil exposure being phytotoxic in nature, has affected plant growth by limiting the nutrient availability for plants (de Jong 1980; Udo and Fayemi 1975; Odukoya et al. 2019). Although oil provides a rich source of carbon and energy, it contains no significant amounts of biologically available nitrogen or phosphorus essential for microbial growth (Prince et al. 2013). These inherent complications led to the development of ecofriendly strategies which gained considerable importance as it relies on the metabolic potential of inhabitant microorganisms for reclamation of crude oil contaminated sites (Megharaj et al. 2011). Bioaugmentation and biostimulation-based bioremediation strategies have been undertaken by many investigators to remediate crude oil or other petroleum hydrocarbon-associated environments (Tahhan et al. 2011; Sun et al. 2012; Suja et al. 2014; Jasmine and Mukherji 2015; Wu et al. 2016; Mukjang et al. 2022; Muthukumar et al. 2023; Rondon-Afanador et al. 2023; Nong et al. 2023; Omenna et al.2024). Biostimulation (addition of appropriate nutrients like N and/or P) have been observed to improve metabolic activity of indigenous microorganisms thereby accelerating the hydrocarbon degradation process (Suja et al. 2014; Smith et al. 2015; Wu et al. 2019). Nitrogen and phosphorous-containing water-soluble salts can be supplemented to compensate for the required amount nitrogen and phosphorous in the initial phases of microbial cell growth during hydrocarbon metabolism (Thavasi et al. 2011). The efficacy of this process may get reduced due to the scarcity of efficient microorganisms in highly contaminated areas (Almeida et al. 2013). The availability of suitable microorganisms, especially those possessing the capacity of hydrocarbon degradation influences the process of degrading hazardous petroleum waste (Jasmine and Mukherji 2015; Poorsoleiman et al. 2020). Bioaugmentation strategy that uses specialist microbial isolates in contaminated sites has faced strong competition and predation by autochthonous microorganisms. This has led to the preferred use of native microbes (for bioremediation procedures), a concept known as ‘‘autochthonous bioaugmentation’’ (ABA), proposed by Ueno et al. (2007), for their easy adaptation and acclimatization in the familiar environment thereby increasing the bioremediation efficiency (that is mainly governed by nature and climatic condition of the contaminated sites, Gogoi et al. 2003; Suja et al. 2014; Jasmine and Mukherji 2015; Ambust et al. 2021). Study reports suggest that there are a limited number of microbial strains that are individually capable of biodegrading all the constituents of crude oil. Therefore, it is important to use a combination of strains to achieve proper bioremediation thereby utilizing their broad enzymatic capacities and their synergistic actions (Shetaia et al. 2016; Talukdar et al. 2023; Tripathi et al. 2024). Various studies have thus explored the effectiveness of using combined biostimulation and bioaugmentation as means of restoring petroleum-contaminated habitats (Sun et al. 2012; Roy et al. 2014, 2018; Wu et al. 2017). Microorganisms capable of hydrocarbon degradation as well as beneficial for plant growth have potentially been used in recovering the contaminated sites and improving plant health (Glick 2010; Gkorezis et al. 2016). Rhizosphere associated or free-living rhizobacteria show both plant growth-promoting (PGP) activity as well as degradation of persistent petroleum hydrocarbon (Yenn et al. 2014). Several reports are available on crude oil-degrading bacteria from diverse environments belonging to the genera of Pseudomonas, Bacillus, Marinobacter, Alcanivorax, Rhodococcus, Mycobacterium, Cycloclasticus, Enterobacter, Dietzia, Alcaligenes, etc. (Kasai et al. 2002; Wang et al. 2011; Yan et al. 2013; Fathepure 2014; Das et al. 2015; Kim et al. 2015; Zhang et al. 2015; Chen et al. 2017; Parthipan et al. 2017; Pi et al. 2017; Mohammed et al. 2023; Talukdar et al. 2023; Tripathi et al. 2024). These bacteria have been extensively used in preparing consortium because of their inherent potential of hydrocarbon degradation and their ability to produce biosurfactant. However, the use of oil-degrading consortium has not yielded satisfactory results all the time and this calls for the need of employing bacterial consortia from a petro-chemically important or extensively crude oil contaminated regions, which is unfortunately very limitedly explored (Zhao et al. 2011; Patowary et al. 2016).
Crude oil being a complex mixture has been the major source of petroleum hydrocarbon contamination of different habitats. Majority of the reports have focused on the fate of oil degradation by isolated microbial strains related to marine environments and contaminated soil in the aerobic shake flask technique (Nikolopoulou et al. 2013; Roy et al. 2014; Suja et al. 2014; Kristensen et al. 2015; Varjani et al. 2015; Ma et al. 2021). This study was undertaken with an attempt to investigate the crude oil biodegradation potential of a microbial consortium consisting of fifteen strains isolated from oil-containing sludge of Duliajan oil field, Assam, India in the presence/absence of exogenous nutrient availability where the TPH reduction from different concentrations of crude oil was monitored. Seed germination assay was also performed to assess the relative lowering of TPH toxicity during the course of the biodegradation study.
Materials and methods
Study area and sample collection
Samples were collected from Duliajan oil field, Assam, India (27.3667° N, 95.3167° E). Duliajan is an industrial town of Dibrugarh district in the Indian state of Assam. It is particularly known for its oil-related industry, Oil India Limited, one of the country's largest oil and gas companies. Crude oil-contaminated waste sludge was collected in pre-sterilized DEPC-treated screw-capped bottles (1 L capacity) from oil exploration sites, brought and stored in the lab under aseptic conditions until further processing.
Formulation of hydrocarbon utilizing bacteria
The consortium used in this study was formulated by mixing fifteen bacterial isolates (DJ5, DJ25, DJ26, DJ27, DJ29, DJ30, DJ31, DJ32, DJ33, DJ34, DJ-E1, DJ-E2, DJ-E4, DJ-E8 and DJ-E9) obtained after screening of forty-four isolates obtained from oil containing sludge of Duliajan oil fields following the method of Das and Kazy (2014). Selection of these particular isolates was done based on their physiological, metabolic and phylogenetic characterization to ensure the microcosm study represented a considerable proportion of the culturable bacterial diversity present in the oily sludge. The potential of the isolates to utilize various hydrocarbons as the sole carbon source was evaluated in MSM. The cultures were incubated at 30 °C for 3 days in the presence of Benzene (B), Toluene (T), Ethyl Benzene (E) or Xylene (X) at a concentration of 50 mg L−1 and BTEX mixture at 200 mg L−1. Utilization of alkanes as a sole source of carbon by the bacterium was carried out during growth in 100 mL Erlenmeyer flasks containing 20 mL MSM supplemented with pentadecane (C15) or hexadecane (C16) at a concentration of 250 mg L−1. Cell numbers were determined after every 7 days and the residual n-alkane was analysed by gas chromatography. The residual n-alkane from the medium was extracted by adding an equal volume of n-hexane and deep freezing the lower water layer to collect the upper organic phase. Remaining water was absorbed by adding Na2SO4. The residual concentrations of the alkanes were analyzed using a GC (Agilent 7820A) equipped with a split/splitless injector, FID detector and an HP-5 column (30 m × 0.32 mm and i.d. 0.25 µm thickness). Nitrogen was used as carrier gas (flow rate 25 mL min−1). The oven program was set initially at 80 °C for 2 min, followed by increasing to 210 °C at 10 °C rise per minute. Utilization of crude oil containing sludge (1%) was analyzed gravimetrically (Mishra et al. 2001) whereas utilization of crude oil was estimated by CFU count. The strains were also tested for their ability to grow in the presence of various temperatures (4–50 °C), at different NaCl concentrations (0–10%) and at different pH range (1.0–11.0), tolerate various heavy metals (Pb, Ni and Cd) at different concentrations (0.1–5 mM). Surfactant production ability of the isolates were examined following the method of Pal et al. (2017). Extraction of genomic DNA and molecular identification by amplification of 16S rRNA gene was performed by following the method as described by Das and Kazy (2014). As amplification and identification of partial 16S rRNA gene was only performed and no polyphasic approached were employed, so the isolates could not be identified to the species level.
Preparation of crude oil degrading microcosms
Estimation of the total organic carbon (TOC) of crude oil was done following the method of Walkley and Black (1934). Briefly, 0.125 ml of crude oil was gently mixed with 5 ml of 1N K2Cr2O7 till maximum dispersion of crude oil has occurred. To this mixture, concentrated H2SO4 (7.5 ml) was added which was kept in boiling water bath for 30 min. The extract was collected and measured spectrophotometrically at 600 nm. Nitrate was estimated by following the method of Cataldo et al. (1975). Briefly, aliquots of 1 ml of extracted samples were pipette out into 50 ml of Erlenmeyer flasks, and mixed thoroughly with 0.8 ml of 5% (w/v) salicylic acid in concentrated sulphuric acid (SA- H2SO4). The flasks were kept at room temperature and after 20 min, 19 ml of 2N NaOH were added slowly to raise the pH above 12. The samples were then incubated for 10 min at room temperature and absorbance was measured at 410 nm. Phosphate in crude oil was determined according to the method of Murphy and Riley (1962). The concentration of phosphate was determined spectrophotometrically at 880 nm. Microcosms were prepared in butyl rubber capped serum vials (27 ml; Sigma-Aldrich, USA) with 4 ml normal saline (0.85%) and different concentrations of crude oil (0.25%, 0.5%, 1% and 2%). N and P amendments were done to maintain a final C: N: P ratio of 100:10:1 within each set (Roy et al. 2018).
Fifteen bacterial strains were selected for assessing their crude oil biodegradation potential by forming a consortium (MC setup). Microcosm with MC setup was supplemented with nitrate (MC + N), phosphate (MC + P) and a combination of nitrate and phosphate (MC + NP) for assessing the possible differences in bioremediation potential when the microbial strains were amended with nutrients. However, GC–MS analysis of the final formulation was not carried out. Details of various microcosm setups have been illustrated in Table 1. Nitrate (N) amendment was done with the addition of NaNO3 and phosphate (P) by adding K2HPO4. Initial inoculum was used in between 108 and 1010 cells mL−1 for each isolate. An uninoculated microcosm setup (Abiotic control) was prepared to evaluate the possible abiotic losses. Microcosm setups were prepared in triplicates for each time point for different treatments and incubated at 30 °C in static conditions. The time points were set at 0, 15, 30, 60, 90 and 120 days for each sacrificial vial. The amendments of nutrients made in various microcosm setups have been illustrated in Table 2.
Assessment of heterotrophic cell viability and crude oil TPH degradation within microcosms
Aerobic heterotrophic cell count was enumerated by serial dilution plating technique. 100 µl of the diluted sample (10–9–10–10) from each microcosm setup (Abiotic control, MC, MC + N, MC + P and MC + NP of 0.25%, 0.5%, 1% and 2% crude oil) was plated on Nutrient agar (HIMEDIA, India), incubated overnight at 30 °C to determine the cell numbers. This was performed to delineate any relationship between the reduction in TPH and cell viability. Residual TPH of crude oil at different time points was extracted by mixing an equal volume of n-hexane with a microcosm mixture followed by vigorous vortexing for 1 h. Particulates present were separated by collecting the supernatant in a fresh tube after centrifuging the whole mixture for 10 min at 10,000 rpm. The extracted TPH content and reduction percentage were determined by gas chromatographic (GC-FID) analysis as described by Roy et al. (2018).
Analysis of residual nitrate and phosphate in microcosms
After the extracted TPH (in organic phase) being separated, the aqueous phase is subjected to analysis of residual nitrate and phosphate as mentioned above, to determine its utilization by microorganisms as essential nutrients for their metabolism. MC + NP, MC + N and Abiotic control setups were tested for residual nitrate where 1 ml of extracted samples in test tubes were mixed thoroughly with 0.8 ml of 5% (w/v) salicylic acid in concentrated sulphuric acid (SA- H2SO4). The flasks were kept at room temperature and after 20 min, 19 ml of 2N NaOH was added slowly to raise the pH above 12. The samples were then incubated for 10 min at room temperature and absorbance was measured at 410 nm (Cataldo et al. 1975). Residual phosphate was estimated for MC + NP, MC + P and Abiotic control setups by following the method of Murphy and Riley (1962).
Assessment of CO2 evolution in microcosms
Evolved carbon dioxide (CO2) was periodically collected every 30 days in alkali (NaOH), which has been back titrated with HCl (Tahhan et al. 2011; de Quadros et al. 2016). Briefly, a CO2 trapping system was setup for each vial by wrapping rubber balloon containing 2 ml of 1 M NaOH around the neck of the vial and kept for 24 h. This provided a closed expandable system for the containment of CO2 and necessary contact time for its maximum absorption by NaOH. Vials were sacrificed at different time points for measuring the entrapped CO2. The resulting mixture of excess NaOH and Na2CO3 was titrated with standard HCl (1 M). Excess of NaOH was neutralized when the titration reached the first colorless phenolphthalein endpoint and all the Na2CO3 was converted to NaHCO3. Continuing the titration till the second methyl orange endpoint converted NaHCO3 to H2O and CO2. The volume difference between the first and second endpoints was used to calculate the CO2 evolved by microbial activity in microcosms by following the equation as described by Crossno et al. (1996).
Ecotoxicity bioassay by seed germination and plant growth
Plant seed germination assay was performed to assess the bioremediation efficacy of crude oil degrading microcosms with (MC + NP, MC + N, MC + P) or without (MC) various amendments. At the end of 120th day, treated and untreated samples were mixed with garden soil at equal (1:1) and twofold (1:2) proportion (sample:soil, w/v) in small earthen pots, where seeds of white mustard (Sinapis alba) were sown in triplicates. White mustard is a widely and easily grown crop in the Indian scenario. It is a fast-growing plant. The difference in growth of the plant under various conditions (control and treated) was easier to identify. Therefore, white mustard seeds were chosen for the assay. Various ecotoxicity studies have also reported the use of seeds of white mustard (Salanitro et al. 1997; Jiang et al. 2016; Hawrot-Paw et al. 2020; Ambust et al. 2021). Moisture content was maintained by keeping the pot partially immersed in water. Growth of seedlings was monitored till 15 days and growth parameters (germination, shoot height and root length) of the seedlings was recorded at the end of 15 days following the method of Tang et al. (2011).
Results and discussion
Crude oil characteristics; taxonomic assignment, formulation, physiological and metabolic potential of hydrocarbon utilizing bacterial consortium
Total organic carbon (TOC) of crude oil (1%) was estimated to be 3.09 × 105 ppm. Total nitrogen (N) and phosphorus (P) in crude oil was estimated to be 59.34 ppm and 38.03 ppm respectively.
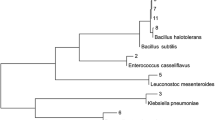
A microbial consortium was formulated to assess the crude oil bioremediation performance of the native microorganisms isolated from crude oil-contaminated sludge. Fifteen bacterial isolates were screened from 44 isolates obtained from the sludge based on their physiological, metabolic and phylogenetic characterization. Seven Bacillus, three Stenotrophomonas and one each of Pseudomonas, Paenibacillus, Franconibacter, Rhizobium and Burkholderia strains were selected for constructing the hydrocarbon utilizing bacterial consortium. Phylogenetic affiliation (the assigned taxonomy) and various physiological (growth under different pH, temperature, salinity and heavy metal concentration) and metabolic potential (degradation of various hydrocarbons, biosurfactant production, etc.) of the strains have been briefed in Table 3. The observation suggested their potential of degrading hydrocarbons and tolerating various physicochemical conditions. Most of the strains could utilize crude oil, petroleum sludge and BTEX compounds except DJ-E1, E4, E8, E9. Pentadecane and hexadecane were utilized at different proportions by the strains with the maximum utilization shown by DJ25, DJ30, DJ31, DJ32 and DJ34. All the strains could produce biosurfactant and displayed dominant growth in the presence of 1 mm of Pb and Ni, but Cd proved to be lethal for few strains.
A group of microorganisms were selected because low microbial population and insufficient microbial diversity can affect bioremediation efficacy (Lin et al. 2010). The use of such exogenous/endogenous microorganisms in hydrocarbon-associated habitats and their efficiency in hydrocarbon degradation have been demonstrated in various studies (Cerqueira et al. 2011; Teng et al. 2011; Yang et al. 2016; Yuan et al. 2017; Roy et al. 2018; Muthukumar et al. 2023; Omenna et al. 2024). Biosurfactant production has been an essential property for promoting hydrocarbon uptake and degradation by lowering its interfacial surface tension thereby enhancing its solubility (Pal et al. 2017). Heavy metals are ubiquitous in petroleum contaminated areas. Bacteria possess various transporters for exporting heavy metals out of the cells in order to survive in contaminated habitats. These properties justified their selection for constructing the bacterial consortium to be used in microcosms and evaluate the best possible way for utilization of crude oil.
Cell viability and crude oil TPH degradation within microcosms
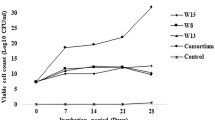
The potential of the isolates to bioremediate TPH in microcosms were assessed for its possible adoption as an approach for reclaiming contaminated sites. Almost 102 fold increase in cell count was observed in all the setups for different concentrations of crude oil. However, the maximum cell growth occurred at different time points for different concentrations of crude oil used. For 0.25% crude oil concentration, the highest cell number was reached within 15 days after which it gradually dropped. For the rest of the concentrations, the maximum cell yield was obtained within 30–45th day (Fig. 1). No viable cells were observed for abiotic control sets. The increase in cell counts and viability hinted towards the capability of the organisms to degrade the crude oil components and utilize the simpler products, which in turn supported their growth. Chaineau et al. (2005) reported that a sharp increase in viable cell count mostly occurs within the first 15 days of adding nutrients. However, the efficiency of the microorganisms gradually decreased with increasing TPH content. Reports from similar kind of experiments showed the reduction of TPH biodegradation with increasing crude oil concentrations, although the cell viability remained almost same (Rahman et al. 2003; Behera et al. 2021).
TPH degradation of 0.25% crude oil was nearly 67% for the MC + N and MC + P set, which were also recorded for the maximum cell yield. Considerable degradation of more than 60% was also observed in the setup with only microbial consortium (MC setup). Crude oil concentration of 0.5% was also reduced following a similar pattern as MC + N and MC + P resulted in maximum TPH reduction followed by MC and MC + NP setup. However, when 1% and 2% crude oil concentrations were used, MC setup resulted in the maximum degradation although the maximum microbial count was less than that of nutrient-amended setups. The results clearly suggested that the microorganisms used to construct the microcosms (which represented a considerable fraction of the petroleum sludge community) have their inherent capacity for synergistic petroleum hydrocarbon utilization (Fig. 2).
Using a microbial consortium instead of the pure culture of microorganisms could be more advantageous from the application perspective of bioremediation and satisfactory degradation results can be obtained if a mixed bacterial culture is used. Before field application, depending on the requirement, consortium could be defined for providing the necessary metabolic diversity and robustness. Bioremediation process in nature depends on cooperative metabolic activities of mixed microbial populations where the degrading bacteria get benefits from synergistic interactions, thereby increasing the bioremediation efficiency (Gallego et al. 2007; Jacques et al. 2008; Mukred et al. 2008; Cao et al. 2009; Cerqueira et al. 2011; Janbandhu and Fulekar 2011; Tyagi et al. 2011; Morris et al. 2013; Wu et al. 2013; Yenn et al. 2014; Patowary et al. 2016; Dhote et al. 2016; Koolivand et al. 2017; Kumari et al. 2018; Suganthi et al. 2018).
Various earlier studies have reported the successful utilization of microbial consortium (using microorganisms with crude oil biodegradation potential) for bioremediation of hydrocarbons, that produced better degradation outcomes than their individual counterparts. The use of consortium led to an effective reduction of TPH, thereby decreasing the toxicity of the contaminated environments. Varjani et al. (2015) illustrated the use of a microbial consortium formulated by six indigenous bacterial strains consisting of Ochrobactrum sp. (01), Stenotrophomonas maltophilia (02) and Pseudomonas aeruginosa (03) in removing more than 80% of crude oil TPH. Bacterial consortium consisting of five pure bacterial cultures of Stenotrophomonas acidaminiphila, Bacillus megaterium, Bacillus cibi, Pseudomonas aeruginosa and Bacillus cereus obtained from petrochemical oily sludge and soil contaminated by petrochemical waste was reported to biodegrade aliphatic and aromatic hydrocarbons of petrochemical oily sludge in liquid medium (Cerqueira et al. 2012). The consortium was capable of reducing 90.7% of the aliphatic fraction and 51.8% of the aromatic fraction during 40 days of the experiment. Consortium consisting of four bacterial strains namely, Achromobacter sp. BAB239, Pseudomonas sp. DV-AL2, Enterobacter sp. BAB240 and Pseudomonas sp. BAB241, showed efficient naphthalene degradation even in the presence of other pollutants as compared to individual bacterial strain (Patel et al. 2012). Tao et al. (2017) investigated the reduction in crude oil TPH by 85% within 7 days when Bacillus subtilis strain was exogenously added to an existing bacterial consortium composed of Betaproteobacteria and Gammaproteobacteria members as compared to 71% when only the indigenous microorganisms were used. Bacillus sp. IOS1-7, Corynebacterium sp. BPS2-6, Pseudomonas sp. HPS2-5 and Pseudomonas sp. BPS1-8 were isolated from oil-contaminated soil samples and were considered for constructing an efficient consortium capable of utilizing crude oil (Sathishkumar et al. 2008). While utilizing 1% crude oil, this consortium could reduce 77% of crude oil TPH which was the highest when these strains were used individually. Koolivand et al. (2017), reported a two-stage composting treatment of tank bottom sludge with effective removal of TPH by combining different strains of Pseudomonas sp., Bacillus sp., Klebsiella sp., Staphylococcus sp., and Proteus sp. TPH removal of 96% was also achieved when tank bottom sludge was treated by a bacterial consortium constituting Shewanalla chilikensis, Bacillus firmus, and Halomonas hamiltonii (Suganthi et al. 2018). Patowary et al. (2016) applied different sets of consortia constituting crude oil degrading strains among which some were biosurfactant producer and some were not. Maximum reduction of crude oil TPH was exhibited by the consortium which had biosurfactant-producing strains. Mnif et al. (2015) also highlighted the fact that co-inoculation of biosurfactant-producing strain could enhance the performance of a consortium in the biodegradation of hydrocarbons. They isolated four strains namely Lysinibacillus bronitolerans RI18, Bacillus thuringiensis RI16, Bacillus weihenstephanensis strain RI12 and a biosurfactant-producing Acinetobacter radioresistens RI7 from oil-contaminated soil. Consortium of L. bronitolerans RI18, B. thuringiensis RI16 and B. weihenstephanensis strain RI12 when co-inoculated with A. radioresistens RI7 and an exogenous Bacillus subtilis SPB1 showed maximum diesel degradation of about 55.4%. Molaei et al. (2022) conducted a pioneering study reporting enhanced biodegradation of TPH and COD removal by a bacterial consortium (removal efficiencies of above 99% and 96%, respectively), that was also capable of in situ generation of biosurfactant. Gojgic-Cvijovic et al. (2012) characterized strains belonging to the genera of Pseudomonas, Achromobacter, Bacillus and Micromonospora from oil refinery storage fuel tank and polluted soil from its vicinity. Consortium constructed from these strains were used for bioaugmentation of the polluted sample in laboratory condition which was further biostimulated with the addition of nitrogen, phosphorous and surfactant. Over the course of the experiment for twelve weeks, a reduction of 80–90% of TPH was achieved for petroleum sludge and polluted soil. Zhao et al. (2011), formulated a consortium with Pseudomonas sp., Rhodococcus sp., Bacillus sp., Microbacterium sp., Roseomonas sp., Brucella sp. and Rhizobiales sp. isolated from oil field contaminated soil which exhibited nearly 52% crude oil removal. Bioaugmentation of contaminated soil with this particular consortium in microcosm test confirmed its effectiveness by reducing more than 50% crude oil over a period of 60 days. Microbial consortium consisting of two strains each of Bacillus sp. and Pseudomonas sp. were also considered for the degradation study of 2% (w/v) oil sludge (Dhote et al. 2016). The consortium yielded 75% reduction of TPH as compared to the individual strains which were almost 20% lower than the consortium. The result also emphasized the use of biosurfactant-producing strains while constructing the consortium.
However, it is important to stimulate those microorganisms with nutrients that are lacking in the environment to obtain maximum degradation as insufficient N and/or P concentration could retard hydrocarbon biodegradation (Smith et al. 2015). Majority of the studies suggested the use of combined bioaugmentation and biostimulation (especially with N and P) in enhancing microbial growth and hydrocarbon degradation (Sun et al. 2012; Almeida et al. 2013; Ghaly et al. 2013; Suja et al. 2014; Roy et al. 2018). In our study, the combined use of N and P could not help in attaining substantial degradation. Therefore, this study contradicted the use of both N and P in combination for biodegradation.
Estimation of residual nitrate and phosphate in microcosms
To confirm the utilization of N and P during the degradation of crude oil, residual nitrate and phosphate in the medium was determined. Decline in nitrate and phosphate concentration in test samples suggested their consumption during the treatment period. Changes in the concentration of nitrate (plotted on y-axis) in microcosms have been presented in Fig. 3. There was a gradual reduction of nitrate concentration in all the treated setups till 120 days of incubation. It was interesting to observe that the consumption of nitrate in MC + NP and MC + N sets were similar for all the crude oil concentrations used. Nitrate serves as an important nitrogenous nutrient as well as a terminal electron acceptor for many microorganisms. Thus, in nitrogen-limited condition, input of nitrate could influence nitrogen cycling and subsequent biogeochemical processes which are mostly driven by microorganisms (Galloway et al. 2008). Due to its thermodynamic favorability as an electron acceptor, large number of microorganisms during anaerobic respiration reduce nitrate in the process of oxidizing organic matter or other reduced substrates. Nitrate amendments in contaminated sediments for in situ bioremediation have been demonstrated in quite a number of studies which suggested its role in promoting degradation of organic carbon (Cunningham et al. 2001; Kutvonen et al. 2015; Xu et al. 2015; Romantschuk et al. 2023).
Concentration of residual phosphate in microcosms showed a gradual decrease over the entire bioremediation treatment process when compared to the abiotic control. Reports suggest that microbial metabolic traits shift with phosphate availability and the amount of phosphate present acts as a critical controller of hydrocarbon degradation (Oliverio et al. 2020). Siciliano et al. (2016) established the essential role of phosphate in enhancing the catabolic potential of microorganisms. Phosphate was consumed more for the MC + P set than MC + NP for all the crude oil concentrations used. Changes in the concentration of phosphate (plotted on y-axis) in microcosms have been presented in Fig. 4.
This finding could suggest that in the presence of both N and P as nutrient, microorganisms would prefer using N over P. This could probably happen due to the complex formation between phosphorus and different metals present in crude oil making the phosphate less available for utilization (Mattingly 1975; USEPA 1985, 2013; Fragkou et al. 2021).
Assessment of crude oil mineralization via CO2 evolution
All the microcosm setups containing different concentrations of crude oil showed higher mineralization activities, compared to abiotic controls during the 120 days of the experiment indicating the utilization of crude oil TPH as the sole carbon source by the amended microbial community (Fig. 5). The highest mineralization occurred in the treatments of 0.25% crude oil by MC + P setup (28.6 ± 0 mg/mL of evolved CO2) followed up by MC + N (24.2 ± 0 mg/mL), which also showed the maximum cell yield and TPH degradation. For 0.5% and 1% crude oil, MC setup showed the highest mineralization of 24.2 ± 0 mg/mL of CO2 evolution, whereas the MC + P set of 2% crude oil yielded 19.8 ± 0 mg/mL of evolved CO2 as the highest during the 120 days. Quantification of microbial CO2 evolution and the results obtained from crude oil TPH degradation through gas chromatography highlighted the important role of autochthonous microorganisms in TPH removal.
Previous investigators have also demonstrated that addition of hydrocarbon-degrading bacteria to the petroleum-contaminated soil could result in a substantial increase in TPH mineralization and removal, which strongly and positively correlated with the overall outcome of the hydrocarbon removal process (Tahhan et al. 2011; Abena et al. 2019). The degrading bacteria can follow various pathways of assimilation, metabolism, and cellular decomposition to degrade individual crude oil components following the formation for CO2. Alkanes are either converted to alcohol which gets oxidized to an alkanal and dehydrogenated by aldehyde dehydrogenase to the corresponding fatty acid, or, the aldehyde is further oxidized to generate alcohol and acetic acid that undergoes terminal oxidation to generate fatty acid. The fatty acid formed undergoes beta-oxidation with the generation of fatty acyl-CoA and acetyl-CoA. The fatty acyl-CoA continues to be oxidized to generate carbon dioxide and water (Varjani and Upasani 2017). Cycloalkanes are successively oxidized to cycloalkanol, cycloalkanone and then finally to adipic acid. Adipic acid goes directly for β-oxidation, where it is oxidatively decomposed into water and carbon dioxide (Abbasian et al. 2015; Hazaimeh and Ahmed 2021; Xue et al. 2015). Monocyclic aromatic compounds are catalyzed by oxygenases and dehydrogenase to produce catechol which undergoes further cleavage. Intermediate products undergo oxidation and the TCA cycle, which eventually mineralize compounds into carbon dioxide and water (Chunyan et al. 2023).
The mineralization rate was more in the initial 60 days of experimental setup probably because the more labile fraction in the crude oil could have been readily used as a source of carbon and energy for the microorganisms, which also corroborated with the maximum microbial population that was observed in this particular period. Marin et al. (2005) showed that the CO2 emissions gradually decreased while the most labile hydrocarbon fractions disappeared, leaving behind the unutilized recalcitrant fraction. Furthermore, it was observed that the setups with lower concentrations of crude oil showed higher mineralization rate than the treatments with higher nutrient amounts. Similar behavior was observed for the rate of mineralization in the work realized by Tahhan and Abu-Ateih (2009), wherein the inhibitory effect of the added nutrients (NH4NO3 and KH2PO4) was observed during the mineralization of TPH from the oily sludge. Since nutrients were added as a ratio of the added carbon, inhibition was the greatest with the highest TPH treatment. This could be attributed to the possibility of biodegradation inhibition by high concentrations of nutrients. Earlier reports also suggest similar attributes where there was a permanent inhibition of hydrocarbons assimilation with a high input of nutrients thereby revealing that different nutrient supplies should be added in an optimum for the unhindered continuation of the degradation process (Chaineau et al. 2005).
Ecotoxicity bioassay by seed germination and plant growth
Data for the seed germination bioassay of treated and untreated crude oil has been summarized in Table 4. Germination studies have been considered as a primary assay for determining the reduction in toxicity of pollutants (Roy et al. 2014). The seed germination results corresponded well with the findings on TPH degradation. No seed germination took place for untreated crude oil setups (abiotic controls) whereas all seeds germinated in uncontaminated garden soil. Maximum seed germination occurred for the treated 0.25% and 0.5% crude oil sets suggesting that these crude oil concentrations were the most suitable for bacterial degradation and utilization for 120-day period in static conditions. This ecotoxicity study provided two highlights (i) the reduction in toxicity of the treated sludge following the effective TPH removal and (ii) renewed activities of introduced bioaugmented bacteria (Roy et al. 2014). Along with the decrease in TPH content, the reduction of heavy metal toxicity due to the introduction of microorganisms could also be a reason for the germination of seeds. Improved root and shoot lengths were observed at a lower concentration ratio (1:2) than in the case of 1:1 ratio for the lowest (0.25%) concentrations of crude oil (Fig. 6).
Root shoot length for MC setup degrading 0.5% crude oil (in both 1:1 and 1:2 ratio) was similar to that of seeds growing in uncontaminated garden soil. Growth of seedlings was also significant for MC setup while degrading 1% crude oil. Amendments with N or P also showed significant germination and growth of seedlings. It has been reported that petroleum hydrocarbon content in the soil could negatively affect plant germination and growth (Tang et al. 2011). In the present experiment, the higher concentrations of crude oil inhibited the seed germination as their degradation was also lesser compared to lower crude oil concentrations. These findings have been consistent with previous reports, which documented an improved germination and plant growth on applying bioremediation (Wang and Bartha 1990; Salanitro et al. 1997; Saterbak et al. 2000; Roy et al. 2014).
Conclusion
The present study deals with the exploitation of native resident bacteria from crude oil-contaminated site for bioremediation procedures. Microbial consortium formulated from fifteen isolated bacterial strains (out of forty-four, selected based on their metabolic potential) showed their inherent potential of crude oil biodegradation and mineralization in microcosms with or without nutrient amendment. Throughout the 120-day degradation study, 60–65% of TPH was removed for 0.25% and 0.5% crude oil. However, for 1% and 2% crude oil, 30–40% of TPH could be reduced by the microbial consortium. Germination of Sinapis alba seeds suggested the reduction in toxicity of crude oil components. Overall, the study concluded that the consortium developed from diverse indigenous microorganisms of crude oil-impacted regions could be a potential tool for in-situ bioremediation of crude oil-contaminated sites. With the emergence of high-throughput omics techniques (e.g., genomics and metagenomics), the individual bio-degraders, hydrocarbon-degrading microbial communities, metabolic pathways and interactions can be described at a contaminated site. Single microorganisms or microbial communities can be examined at the system level and the metabolic networks, interspecies interactions during hydrocarbon mineralization can be elucidated. Genomic studies of the microbial isolates to identify the active genes and proteins responsible for the inherent capability of hydrocarbon degradation could help us in amplifying the biodegradation potential of the formulated consortium. Moreover, in-depth analysis of the crude oil sample may help unravel novel unculturable hydrocarbon-degrading strains and enzymes which could be fitted into the metabolic networks of the community, which in turn would support the scaling up of these microcosm studies for ex-situ applications and field trials.
References
Abbasian F, Lockington R, Mallavarapu M, Naidu R (2015) A comprehensive review of aliphatic hydrocarbon biodegradation by bacteria. Appl Biochem Biotechnol 176:670–699
Abena MTB, Li T, Shah MN, Zhong W (2019) Biodegradation of total petroleum hydrocarbons (TPH) in highly contaminated soils by natural attenuation and bioaugmentation. Chemosphere 234:864–874
Almeida CMR, Reis I, Couto MN, Bordalo AA, Mucha AP (2013) Potential of the microbial community present in an unimpacted beach sediment to remediate petroleum hydrocarbons. Environ Sci Pollut Res 20:3176–3184. https://doi.org/10.1007/s11356-012-1240-2
Ambust S, Das AJ, Kumar R (2021) Bioremediation of petroleum contaminated soil through biosurfactant and Pseudomonas sp. SA3 amended design treatments. Curr Res Microb Sci. 2:100031. https://doi.org/10.1016/j.crmicr.2021.100031
Behera ID, Nayak M, Biswas S, Meikap BC, Sen R (2021) Enhanced biodegradation of total petroleum hydrocarbons by implementing a novel two-step bioaugmentation strategy using indigenous bacterial consortium. J Environ Manag 292:112746
Cao B, Nagarajan K, Loh C-K (2009) Biodegradation of aromatic compounds: current status and opportunities for biomolecular approaches. Appl Microbiol Biotechnol 85:207–228
Cataldo DA, Haroon M, Schrader LE, Youngs VL (1975) Rapid colorimetric determination of nitrate in plant tissue by nitration of salicylic acid. Commun Soil Sci Plant Anal 6(1):71–80
Cerqueira VS, Hollenbach EB, Maboni F, Vainstein MH, Camargo FAO, Peralba MCR, Bento FM (2011) Biodegradation potential of oily sludge by pure and mixed bacterial cultures. Bioresour Technol 102:11003–11010
Cerqueira VS, Hollenbach EB, Maboni F, Camargo FAO, Peralba do MCR, Bento FM (2012) Bioprospection and selection of bacteria isolated from environments contaminated with petrochemical residues for application in bioremediation. World J Microbiol Technol 28:1203–1222
Chaineau CH, Rougeux G, Yepremian C, Oudot J (2005a) Effects of nutrient concentration on the biodegradation of crude oil and associated microbial populations in the soil. Soil Biol Biochem 37(8):1490–1497
Chen W, Li J, Sun X, Min J, Hu X (2017) High efficiency degradation of alkanes and crude oil by a salt-tolerant bacterium Dietzia species CN-3. Int Biodeterior Biodegrad 118:110–118
Chunyan X, Qaria MA, Qi X, Daochen Z (2023) The role of microorganisms in petroleum degradation: current development and prospects. Sci Total Environ 865:161112
Crossno SK, Kalbus LH, Kalbus GE (1996) Determinations of carbon di oxide by titration. J Chem Educ 73(2):175–176
Cunningham JA, Rahme H, Hopkins GD, Lebron C, Reinhard M (2001) Enhanced in situ bioremediation of BTEX-contaminated groundwater by combined injection of nitrate and sulfate. Environ Sci Technol 35(8):1663–1670
Das R, Kazy SK (2014) Microbial diversity, community composition and metabolic potential in hydrocarbon contaminated oily sludge: prospects for in situ bioremediation. Environ Sci Pollut Res 21:7369–7389
Das D, Baruah R, Roy AS, Singh AK, Boruah HPD, Kalita J, Bora TC (2015) Complete genome sequence analysis of Pseudomonas aeruginosa N002 reveals its genetic adaptation for crude oil degradation. Genomics 105:182–190
de Jong E (1980) The effect of crude oil spill on cereals. Environ Pollut 22:187–196
de Quadros PD, Cerqueira VS, Cazarolli JC, Peralba MCR, Camargo FAO, Giongo A et al (2016) Oily sludge stimulates microbial activity and changes microbial structure in a landfarming soil. Int Biodeterior Biodegrad 115:90–101
Dhote M, Kumar A, Juwarkar A (2016) Petroleum contaminated oil sludge degradation by defined consortium: influence of biosurfactant production. Proc Natl Acad Sci 88(2):517–523
Fathepure BZ (2014) Recent studies in microbial degradation of petroleum hydrocarbons in hypersaline environments. Front Microbiol 14:173. https://doi.org/10.3389/fmicb.2014.00173
Fragkou E, Antoniou E, Daliakopoulos I, Manios T, Theodorakopoulou M, Kalogerakis N (2021) In situ aerobic bioremediation of sediments polluted with petroleum hydrocarbons: a critical review. J Mar Sci Eng 9(9):1003
Gallego JLR, Garcia-Martinez MJ, Llamas JF, Belloch C, Pelaez AI, Sanchez J (2007) Biodegradation of oil tank bottom sludge using microbial consortia. Biodegradation 18:269–281
Galloway JN, Townsend AR, Erisman JW, Bekunda M, Cai Z, Freney JR et al (2008) Transformation of the nitrogen cycle: recent trends, questions, and potential solutions. Science 320:889–892
Ghaly AE, Yusran A, Dave D (2013) Effects of biostimulation and bioaugmentation on the degradation of pyrene in soil. J Bioremed Biodegrad S7:005. https://doi.org/10.4172/2155-6199.S7-005
Gkorezis P, Daghio M, Franzetti A, Van Hamme JD, Sillen W, Vangronsveld J (2016) The interaction between plants and bacteria in the remediation of petroleum hydrocarbons: an environmental perspective. Front Microbiol 7:1836
Glick BR (2010) Using soil bacteria to facilitate phytoremediation. Biotechnol Adv 28:367–374
Gogoi BK, Dutta NN, Goswami P, Krishna Mohan TR (2003) A case study of bioremediation of petroleum-hydrocarbon contaminated soil at a crude oil spill site. Adv Environ Res 7(4):767–782
Gojgic-Cvijovic GD, Milic JS, Solevic TM, Beskoski VP, Ilic MV, Djokic LS et al (2012) Biodegradation of petroleum sludge and petroleum polluted soil by a bacterial consortium: a laboratory study. Biodegradation 23:1–14
Hawrot-Paw M, Koniuszy A, Zając G, Szyszlak-Bargłowicz J (2020) Ecotoxicity of soil contaminated with diesel fuel and biodiesel. Sci Rep 10(1):16436
Hazaimeh MD, Ahmed ES (2021) Bioremediation perspectives and progress in petroleum pollution in the marine environment: a review. Environ Sci Pollut Res 28:54238–54259
Head IM, Jones MD, Roling WFM (2006) Marine microorganisms make a meal of oil. Nat Rev Microbiol 4:173–182
Holliger C, Gaspard S, Glod G, Heijman C, Schumacher W, Schwarzenbach RP, Vazquez F (1997) Contaminated environments in the subsurface and bioremediation: organic contaminants. FEMS Microbiol Rev 20:517–523. https://doi.org/10.1111/j.1574-6976.1997.tb00334.x
Jacques RJS, Okeke BC, Bento FM, Teixeira AS, Peralba MCR, Camargo FAO (2008) Microbial consortium bioaugmentation of a polycyclic aromatic hydrocarbons contaminated soil. Bioresour Technol 99:2637–2643
Janbandhu A, Fulekar MH (2011) Biodegradation of phenanthrene using adapted microbial consortium isolated from petrochemical contaminated environment. J Hazard Mater 187(1–3):333–340. https://doi.org/10.1016/j.jhazmat.2011.01.034
Jasmine J, Mukherji S (2015) Characterization of oily sludge from a refinery and biodegradability assessment using various hydrocarbon degrading strains and reconstituted consortia. J Environ Manag 149:118–125. https://doi.org/10.1016/j.jenvman.2014.10.007
Jiang Y, Brassington KJ, Prpich G, Paton GI, Semple KT, Pollard SJ, Coulon F (2016) Insights into the biodegradation of weathered hydrocarbons in contaminated soils by bioaugmentation and nutrient stimulation. Chemosphere 161:300–307
Kasai Y, Kishira H, Harayama S (2002) Bacteria belonging to the genus cycloclasticus play a primary role in the degradation of aromatic hydrocarbons released in a marine environment. Appl Environ Microbiol 68(11):5625–5633
Kim S-J, Kweon O, Sutherland JB, Kim H-L, Jones RC, Burback BL et al (2015) Dynamic response of Mycobacterium vanbaalenii PYR-1 to BP deepwater horizon crude oil. Appl Environ Microbiol 81(13):4263–4276
Koolivand A, Rajaei MS, Ghanadzadeh MJ, Saeedi R, Abtahi H, Godini K (2017) Bioremediation of Storage Tank Bottom Sludge by Using a Two-Stage Composting System: Effect of Mixing Ratio and Nutrients Addition 235:240–249
Kristensen M, Johnsen AR, Christensen JH (2015) Marine biodegradation of crude oil in temperate and Arctic water samples. J Hazard Mater 300:75–83
Kumari S, Regar RK, Manickam N (2018) Improved polycyclic aromatic hydrocarbon degradation in a crude oil by individual and a consortium of bacteria. Bioresour Technol 254:174–179
Kutvonen H, Rajala P, Carpén L, Bomberg M (2015) Nitrate and ammonia as nitrogen sources for deep subsurface microorganisms. Front Microbiol 6:1079
Lin TC, Pan PT, Cheng S-S (2010) Ex situ bioremediation of oil-contaminated soil. J Hazard Mater 176:27–34
Ma M, Zheng L, Yin X, Gao W, Han B, Li Q, Zhu A, Chen H, Yang H (2021) Reconstruction and evaluation of oil-degrading consortia isolated from sediments of hydrothermal vents in the South Mid-Atlantic Ridge. Sci Rep 11(1):1456
Margesin R, Labbe D, Schinner F, Greer CW, Whyte LG (2003) Characterization of hydrocarbon-degrading microbial populations in contaminated and pristine alpine soils. Appl Environ Microbiol 69(6):3085–3092
Marin JA, Hernandez T, Garcia C (2005) Bioremediation of oil refinery sludge by landfarming in semiarid conditions: influence on soil microbial activity. Environ Res 98:185–195
Mattingly GEG (1975) Labile phosphates in soils. Soil Sci 119:369–375
Megharaj M, Ramakrishnan B, Venkateswarlu K, Sethunathan N, Naidu R (2011) Bioremediation approaches for organic pollutants: a critical perspective. Environ Int 37:1362–1375
Mishra S, Jyot J, Kuhad RC, Lal B (2001) In situ bioremediation potential of oily-sludge degrading bacterial consortium. Curr Microbiol 43(5):328–335
Mnif I, Mnif S, Sahnoun R, Maktouf S, Ayedi Y, Ellouze-Chaabouni S et al (2015) Biodegradation of diesel oil by a novel microbial consortium: comparison between co-inoculation with biosurfactant-producing strain and exogenously added biosurfactants. Environ Sci Pollut Res 22(19):14852–14861
Mohammed SA, Omar Zrary TJ, Hasan AH (2023) Degradation of crude oil and the pure hydrocarbon fractions by indigenous soil microorganisms. Biologia 78(12):3637–3651
Molaei S, Moussavi G, Talebbeydokhti N, Shekoohiyan S (2022) Biodegradation of the petroleum hydrocarbons using an anoxic packed-bed biofilm reactor with in-situ biosurfactant-producing bacteria. J Hazard Mater 421:126699
Morris BE, Henneberger R, Huber H, Moissl-Eichinger C (2013) Microbial syntrophy: interaction for the common good. FEMS Microbiol Rev 37(3):384–406. https://doi.org/10.1111/1574-6976.12019
Mukjang N, Chitov T, Mhuantong W, Champreda V, Pathom-Aree W, Sattayawat P, Bovonsombut S (2022) Bacterial communities associated with crude oil bioremediation through composting approaches with indigenous bacterial isolate. Life (Basel). 12(11):1712. https://doi.org/10.3390/life12111712
Mukred AM, Hamid AA, Hamzah A, Yusoff WMW (2008) Development of three bacterial consortium for the bioremediation of crude petroleum–oil in contaminated water. Int J Biol Sci 8:73–79
Murphy J, Riley JP (1962) A modified single solution method for the determination of phosphate in natural waters. Anal Chim Acta 27:31–36
Muthukumar B, Surya S, Sivakumar K, AlSalhi MS, Rao TN, Devanesan S, Arunkumar P, Rajasekar A (2023) Influence of bioaugmentation in crude oil contaminated soil by Pseudomonas species on the removal of total petroleum hydrocarbon. Chemosphere 310:136826
Nikolopoulou M, Pasadakis N, Kalogerakis N (2013) Evaluation of autochthonous bioaugmentation and biostimulation during microcosm-simulated oil spills. Mar Pollut Bull 72(1):165–173
Nong J, Peng P, Pan J, Shen T, Xie Q (2023) Effect of bioaugmentation and biostimulation on hydrocarbon degradation and bacterial community composition in different petroleum-contaminated soil layers. Water Air Soil Pollut 234(3):189
Odukoya J, Lambert R, Sakrabani R (2019) Understanding the impacts of crude oil and its induced abiotic stresses on agrifood production: a review. Horticulturae 5(2):47
Oliverio AM, Bissett A, McGuire K, Saltonstall K, Turner BL, Fierer N (2020) The role of phosphorus limitation in shaping soil bacterial communities and their metabolic capabilities. MBio 11(5):10–1128
Omenna EC, Omage K, Ezaka E, Azeke MA (2024) Bio-augmentation and bio-stimulation with kenaf core enhanced bacterial enzyme activities during bio-degradation of petroleum hydrocarbon in polluted soil. Sci Rep 14(1):8
Pal S, Kundu A, Banerjee TD, Mohapatra B, Roy A, Manna R, Sar P, Kazy SK (2017) Genome analysis of crude oil degrading Franconibacter pulveris strain DJ34 revealed its genetic basis for hydrocarbon degradation and survival in oil contaminated environment. Genomics 109:374–382
Parthipan P, Preetham E, Machuca LL, Rahman PKSM, Murugan K, Rajasekar A (2017) Biosurfactant and degradative enzymes mediated crude oil degradation by bacterium Bacillus subtilis A1. Front Microbiol. https://doi.org/10.3389/fmicb.2017.00193
Patel V, Jain S, Madamwar D (2012) Naphthalene degradation by bacterial consortium (DV-AL) developed from Alang-Sosiya ship breaking yard, Gujarat, India. Bioresour Technol 107:122–130
Patowary K, Patowary R, Kalita MC, Deka S (2016) Development of an efficient bacterial consortium for the potential remediation of hydrocarbons from contaminated sites. Front Microbiol 7:1092. https://doi.org/10.3389/fmicb.2016.01092
Pi Y, Chen B, Bao M, Fan F, Cai Q, Ze L et al (2017) Microbial degradation of four crude oil by biosurfactant producing strain Rhodococcus sp. Bioresour Technol 232:263–269
Poorsoleiman MS, Hosseini SA, Etminan A, Abtahi H, Koolivand A (2020) Effect of two-step bioaugmentation of an indigenous bacterial strain isolated from oily waste sludge on petroleum hydrocarbons biodegradation: scaling-up from a liquid mineral medium to a two-stage composting process. Environ Technol Innov 17:100558
Prince RC, McFarlin KM, Butler JD, Febbo EJ, Wang FC, Nedwed TJ (2013) The primary biodegradation of dispersed crude oil in the sea. Chemosphere 90(2):521–526
Rahman KSM, Rahman TJ, Kourkoutas Y, Petsas I, Marchant R, Banat IM (2003) Enhanced bioremediation of n-alkane in petroleum sludge using bacterial consortium amended with rhamnolipid and micronutrients. Bioresour Technol 90:159–168
Romantschuk M, Lahti-Leikas K, Kontro M, Galitskaya P, Talvenmäki H, Simpanen S, Allen JA, Sinkkonen A (2023) Bioremediation of contaminated soil and groundwater by in situ biostimulation. Front Microbiol 14:1258148. https://doi.org/10.3389/fmicb.2023.1258148
Rondon-Afanador C, Pinilla-Meza G, Casallas-Cuervo FC, Diaz-Vanegas C, Barreto-Gomez D, Benavides C, Buitrago N, Calvo M, Forero-Forero C, Galvis-Ibarra V, Moscoso-Urdaneta V (2023) Bioremediation of heavy oily sludge: a microcosms study. Biodegradation 34(1):1–20
Roy AS, Baruah R, Borah M, Yenn, Singh AK, Deka Boruah HP, Saikia N, Deka M, Dutta N, Bora TC (2014) Bioremediation potential of native hydrocarbon degrading bacterial strains in crude oil contaminated soil under microcosm study. Int Biodeterior Biodegrad 94:79–89
Roy A, Dutta A, Pal S, Gupta A, Sarkar J, Chatterjee A et al (2018) Biostimulation and bioaugmentation of native microbial community accelerated bioremediation of oil refinery sludge. Bioresour Technol 253:22–32
Salanitro JP, Dorn PB, Huesemann MH, Moore KO, Rhodes IA, Jackson LMR et al (1997) Crude oil hydrocarbon bioremediation and soil ecotoxicity assessment. Environ Sci Technol 31:1769–1776
Saterbak A, Toy RJ, McMain BJ, Williams MP, Dorn PB (2000) Ecotoxicological and analytical assessment of effects of bioremediation on hydrocarbon containing soils. Environ Toxicol Chem 19:2643–2652
Sathishkumar M, Binupriya AR, Bail S-H, Yun S-E (2008) Biodegradation of crude oil by individual bacterial strains and a mixed bacterial consortium isolated from hydrocarbon contaminated areas. Clean 36(1):92–96
Shetaia YMH, El Khalik WAA, Mohamed TM, Farahat LA, ElMekawy A (2016) Potential biodegradation of crude petroleum oil by newly isolated halotolerant microbial strains from polluted Red Sea area. Mar Pollut Bull 111:435–442
Siciliano SD, Chen T, Phillips C, Hamilton J, Hilger D, Chartrand B et al (2016) Total phosphate influences the rate of hydrocarbon degradation but phosphate mineralogy shapes microbial community composition in cold-region calcareous soils. Environ Sci Technol 50:5197–5206. https://doi.org/10.1021/acs.est.5b05911
Singh B, Kumar P (2020) Physicochemical characteristics of hazardous sludge from effluent treatment plant of petroleum refinery as feedstock for thermochemical processes. J Environ Chem Eng 8(4):103817
Smith E, Thavamani P, Ramadass K, Naidu R, Srivastava P, Megharaj M (2015) Remediation trials for hydrocarbon-contaminated soils in arid environments: evaluation of bioslurry and biopiling techniques. Int Biodeterior Biodegrad 101:56–65. https://doi.org/10.1016/j.ibiod.2015.03.029
Suganthi SH, Murshid S, Sriram S, Ramani K (2018) Enhanced biodegradation of hydrocarbons in petroleum tank bottom oil sludge and characterization of biocatalysts and biosurfactants. J Environ Manag 220:87–95
Suja F, Rahim F, Taha MR, Hambali N, Razali MR, Khalid A, Hamzah A (2014) Effects of local microbial bioaugmentation and biostimulation on the bioremediation of total petroleum hydrocarbons TPH in crude oil contaminated soil based on laboratory and field observations. Int Biodeterior Biodegrad 90:115–122
Sun GD, Xu Y, Jin JH, Zhong ZP, Liu Y, Luo M, Liu ZP (2012) Pilot scale ex-situ bioremediation of heavily PAHs-contaminated soil by indigenous microorganisms and bioaugmentation by a PAHs-degrading and bioemulsifier-producing strain. J Hazard Mater 233–234:72–78
Tahhan RA, Abu-Ateih RY (2009) Biodegradaation of petroleum industry oily-sludge using Jordanian oil refinery contaminated soil. Int Biodeterior Biodegrad 63:1054–1060
Tahhan RA, Ammari TG, Goussous SJ, Al-Shdaifat HI (2011) Enhancing the biodegradation of total petroleum hydrocarbons in oily sludge by a modified bioaugmentation strategy. Int Biodeterior Biodegrad 65:130–134
Talukdar P, Bordoloi P, Bora PP, Yadav A, Saikia R, Geed SR (2023) Assessment of oily sludge biodegradation in lab scale composting and slurry bioreactor by bacterial consortium. J Environ Manag 342:118360
Tang J, Wang M, Wang F, Sun Q, Zhou Q (2011) Eco-toxicity of petroleum hydrocarbon contaminated soil. J Environ Sci 235:845–851
Tao K, Liu X, Chen X, Hu X, Cao L, Yuan X (2017) Biodegradation of crude oil by a defined co-culture of indigenous bacterial consortium and exogenous Bacillus subtilis. Bioresour Technol 224:327–332. https://doi.org/10.1016/j.biortech.2016.10.073
Teng Y, Shen Y, Luo Y, Sun X, Sun M, Fu D, Li Z, Christie P (2011) Influence of Rhizobium meliloti on phytoremediation of polycyclic aromatic hydrocarbons by alfalfa in an aged contaminated soil. J Hazard Mater 186:1271–1276
Thavasi R, Jayalakshmi S, Banat IM (2011) Effect of biosurfactant and fertilizer on biodegradation of crude oil by marine isolates of Bacillus megaterium, Corynebacterium kutscheri and Pseudomonas aeruginosa. Bioresour Technol 102:772–778
Tripathi V, Gaur VK, Kaur I, Srivastava PK, Manickam N (2024) Unlocking bioremediation potential for site restoration: a comprehensive approach for crude oil degradation in agricultural soil and phytotoxicity assessment. J Environ Manag 355:120508
Tyagi M, da Fonseca MMR, de Carvalho CCCR (2011) Bioaugmentation and biostimulation strategies to improve the effectiveness of bioremediation processes. Biodegradation 22:231–241
Udo EJ, Fayemi AA (1975) Effects of oil pollution of soil on germination, growth and nutrient Uptake of corn. J Environ Qual 4:537–540
Ueno A, Ito Y, Yumoto I, Okuyama H (2007) Isolation and characterization of bacteria from soil contaminated with diesel oil and the possible use of these in autochthonous bioaugmentation. World J Microbiol Biotechnol 23:1739–1745
Ulrich AC, Guigard SE, Foght JM, Semple KM, Pooley K, Armstrong JE, Biggar KW (2009) Effect of salt on aerobic biodegradation of petroleum hydrocarbons in contaminated groundwater. Biodegradation 20:27–38
US EPA (1985) Handbook for Remedial Action at Waste Disposal Sites. Revised. EPA/625/6-85/006
US EPA (2013) Introduction to In Situ Bioremediation of Groundwater (pdf) 542-R-13-008
Varjani SJ, Upasani VN (2017) A new look on factors affecting microbial degradation of petroleum hydrocarbon pollutants. Int Biodeterior Biodegradation 120:71–83
Varjani SJ, Rana DP, Jain AK, Bateja S, Upasani VN (2015) Synergistic ex-situ biodegradation of crude oil by halotolerant bacterial consortium of indigenous strains isolated from on shore sites of Gujarat, India. Int Biodeterior Biodegrad 103:116–124
Walkley A, Black IA (1934) An examination of degtjareffmethod for determining soil organic matter and a proposed modification of the chromic acid titration method. Soil Sci 37:29–37
Wang X, Bartha R (1990) Effects of bioremediation on residues, activity and toxicity in soil contaminated by fuel spills. Soil Biol Biochem 22(4):501–505
Wang XB, Chi CQ, Nie Y, Tang YQ, Tan Y, Wu G, Wu XL (2011) Degradation of petroleum hydrocarbons (C6–C40) and crude oil by a novel Dietzia strain. Bioresour Technol 102(17):7755–7761. https://doi.org/10.1016/j.biortech.2011.06.009
Wu M, Chen L, Tian Y, Ding Y, Dick WA (2013) Degradation of polycyclic aromatic hydrocarboms by microbial consortia enriched from three soils using two different culture media. Environ Pollut 178:152–158
Wu M, Dick WA, Li W, Wang X, Yang Q, Wang T et al (2016) Bioaugmentation and biostimulation of hydrocarbon degradation and the microbial community in a petroleum-contaminated soil. Int Biodeterior Biodegrad 107:158–164
Wu M, Ye X, Chen K, Li W, Yuan J, Jiang X (2017) Bacterial community shift and hydrocarbon transformation during bioremediation of short-term petroleum-contaminated soil. Environ Pollut 223:657–664. https://doi.org/10.1016/j.envpol.2017.01.079
Wu M, Wu J, Zhang X, Ye X (2019) Effect of bioaugmentation and biostimulation on hydrocarbon degradation and microbial community composition in petroleum-contaminated loessal soil. Chemosphere 237:124456
Xu M, Zhang Q, Xia C, Zhong Y, Sun G, Guo J, Yuan T, Zhou J, He Z (2015) Elevated nitrate enriches microbial functional genes for potential bioremediation of complexly contaminated sediments. ISME J 9(2):532. https://doi.org/10.1038/ismej.2014.229. (Epub 2015 Jan 20. Erratum for: 10.1038/ismej.2014.42)
Xue J, Yu Y, Bai Y, Wang L, Wu Y (2015) Marine oil-degrading microorganisms and biodegradation process of petroleum hydrocarbon in marine environments: a review. Curr Microbiol 71:220–228
Yan S, Wang Q, Qu L, Li C (2013) Characterization of oil-degrading bacteria from oil- contaminated soil and activity of their enzymes. Biotechnol Biotechnol Equip 27(4):3932–3938
Yang S, Wen X, Shi Y, Liebner S, Jin H, Perfumo A (2016) Hydrocarbon degraders establish at the costs of microbial richness, abundance and keystone taxa after crude oil contamination in permafrost environments. Sci Rep 6:37473. https://doi.org/10.1038/srep3747
Yenn R, Borah M, Deka Boruah HP, Sarma Roy A, Baruah R, Saikia N, Sahu OP, Tamuli AK (2014) Phytoremediation of abandoned crude oil contaminated drill sites of Assam with the aid of a hydrocarbon-degrading bacterial formulation. Int J Phytoremed 16:909–925
Yuan K, Chen B, Qing Q, Zou S, Wang X (2017) Polycyclic aromatic hydrocarbons (PAHs) enrich their degrading genera and genes in human-impacted aquatic environments. Environ Pollut 230:936–944
Zhang F, Su S, Yu G, Zheng B, Shu F, Wang Z, Xiang T, Dong H, Zhang Z, Hou D, She Y (2015) High quality genome sequence and description of Enterobacter mori strain 5–4, isolated from a mixture of formation water and crude-oil. Stand Genomic Sci 10:9
Zhao D, Liu C, Liu L, Zhang Y, Liu Q, Wu W-M (2011) Selection of functional consortium for crude oil-contaminated soil remediation. Int Biodeterior Biodegrad 65:1244–1248
Acknowledgements
This work was supported by NER TWINNING Project (grant no. BT/226/NE/TBP/2011) from the Department of Biotechnology, Government of India. We gratefully acknowledge the support of Oil India Limited, Duliajan, Assam, in sample collection.
Author information
Authors and Affiliations
Contributions
Not Applicable.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest in the publication.
Accession numbers
Not applicable.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pal, S., Hait, A., Mandal, S. et al. Crude oil degrading efficiency of formulated consortium of bacterial strains isolated from petroleum-contaminated sludge. 3 Biotech 14, 220 (2024). https://doi.org/10.1007/s13205-024-04066-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-024-04066-8