Abstract
Bioremediation, involving the use of microorganisms to detoxify or remove pollutants, is the most interesting strategy for hydrocarbon remediation. In this aim, four hydrocarbon-degrading bacteria were isolated from oil-contaminated soil in Tunisia. They were identified by the 16S rDNA sequence analysis, as Lysinibacillus bronitolerans RI18 (KF964487), Bacillus thuringiensis RI16 (KM111604), Bacillus weihenstephanensis RI12 (KM094930), and Acinetobacter radioresistens RI7 (KJ829530). Moreover, a lipopeptide biosurfactant produced by Bacillus subtilis SPB1, confirmed to increase diesel solubility, was tested to increase diesel biodegradation along with co-inoculation with two biosurfactant-producing strains. Culture studies revealed the enhancement of diesel biodegradation by the selected consortium with the addition of SPB1 lipopeptide and in the cases of co-inoculation by biosurfactant-producing strain. In fact, an improvement of about 38.42 and 49.65 % of diesel degradation was registered in the presence of 0.1 % lipopeptide biosurfactant and when culturing B. subtilis SPB1 strain with the isolated consortium, respectively. Furthermore, the best improvement, evaluated to about 55.4 %, was recorded when using the consortium cultured with B. subtilis SPB1 and A. radioresistens RI7 strains. Gas chromatography analyses were correlated with the gravimetric evaluation of the residual hydrocarbons. Results suggested the potential applicability of the selected consortium along with the ex situ- and in situ-added biosurfactant for the effective bioremediation of diesel-contaminated water and soil.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The use of enormous amounts of petroleum products contributes highly to soil and ground water contamination. Nowadays, environmental pollution by hydrocarbons receives great concern with respect to environmental and health issues. Bioremediation that involves the use of living microorganisms, bacteria, or fungi, for detoxification of soil and water organic pollutants by biodegradation, biotransformation, and/or mineralization, preferentially in situ, has been proposed as an effective technology for the control of hydrocarbons (Joutey et al. 2013). It has been recognized as powerful alternatives to conventional methods in resolving environmental problems including physical and chemical treatments (Joutey et al. 2013). Gram-negative isolates belonging to the Acinetobacter, Pseudomonas, Alkanivorax, and related genera are well known as the main microorganisms that can consume petroleum hydrocarbons (Mnif et al. 2014). In addition, Gram-positive isolates belonging to the Rhodococci and Bacillus genera are widely recognized by their ability to degrade hydrocarbons (Mnif et al. 2014). In fact, Bacillus sp. has attracted interest in both biotechnological applications and bioremediation strategies (Mnif et al. 2014).
In spite of the greatest variety of hydrocarbon-degrading bacteria, growth of certain microorganisms on hydrocarbon substrates can be limited by numerous factors like the substrate recalcitrance and the low solubility of organic compounds in aqueous systems which limited their bioavailability for biodegradation (Calvo et al. 2009; Joutey et al. 2013). Addition of surface active compounds can promote hydrocarbon solubility making them more available and enhancing therefore their biodegradation (Franzetti et al. 2009; Pacwa-Plociniczak et al. 2011). In this context, many studies have demonstrated the effect of synthetic surfactants in increasing the apparent solubility of certain hydrocarbons (Wong et al. 2004; Swe et al. 2006) and washing contaminated soil (Urum et al. 2006; Akpoveta et al. 2012). Hence, they are widely applied to improve microbial hydrophobic compounds degradation (Van Hamme et al. 2001; Noordman et al. 2002; Helmy et al. 2009; Hadibarata and Tachibana 2010; Chamkha et al. 2011). Nevertheless, the addition of persistent synthetic emulsifiers could be noxious to the environment and living organisms (Dayeh et al. 2004; Pavlic et al. 2005). Furthermore, they can inhibit biodegradation via toxic interactions and sequestration of hydrocarbons into surfactant micelles (Makkar and Rockne 2003). By against, microbial surface active compounds, characterized by their environmental friendly, reduced toxicity and biodegradability, could be considered as best alternatives to chemical surfactants in enhancing hydrocarbons solubility, bioavailability, and biodegradation.
Microbially derived surface active compounds or biosurfactants having an amphiphilic character are classified into diverse groups including glycolipids, lipopeptides, phospholipids, and free fatty acids (Fracchia et al. 2012; Shoeb et al. 2013). They reduce the surface and interface tension at water-oil interface as having both polar and non-polar domains (Fracchia et al. 2012). Owing to their surface activity and biological activities, low toxicity, high biodegradability, and environmentally acceptability, they can be applied in many domains. They are applied in food industry as emulsifying and stabilizing agents, in biomedical sciences and pharmaceutical industries as therapeutic agents to combat many diseases and in agriculture field as biocontrol agents (Fracchia et al. 2012; Shoeb et al. 2013). In addition, they are good candidates for enhanced oil recovery and bioremediation of oil spills (Pacwa-Plociniczak et al. 2011).
Besides the use of surface active compounds as promising strategy to enhance oil biodegradability, inoculation with biosurfactant-producing microorganisms has also been considered as an interesting methodology. This practice would offer the advantage of a continuous supply of a non-toxic and biodegradable surfactant at a low cost. Also, the use of microbes having co-existing capacity to degrade hydrocarbons and produce biosurfactants can effectively be used for speedy bioremediation of oil-contaminated sites (Kumar et al. 2006). In fact, hydrocarbon-degrading bacteria are well known by their ability to produce biosurfactants in situ facilitating therefore their microbial life in environments dominated by hydrophobic compounds (Kumar et al. 2007; Ganesh and Lin 2009; Reddy et al. 2010; Mnif et al. 2011).
In this context, the present work has focused on this approach, aiming to ameliorate diesel biodegradation using a selected consortium by the addition of biological emulsifier and/or co-inoculation with biosurfactant-producing microorganisms. The used consortium was a combination of three newly isolated strains. Two other strains, able to degrade hydrocarbon and produce a bioemulsifier, were used as an in situ biosurfactant-producing strains. An exogenously lipopeptide biosurfactant has been also added. Studies were conducted in order to assess the process feasibility and compare the effectiveness of the different strategies used.
Materials and methods
Strain isolation, characterization, and screening for hydrocarbon degradation
Microbial strains having the ability to degrade hydrocarbon were isolated from hydrocarbon-contaminated soil by the enrichment culture technique. Ten grams of soil were added to 50 ml of minimal-salt medium supplemented with 5 % (v/v) of diesel oil and were incubated at 37 °C at 150 rpm. Three cycles of enrichment were made until complete disappearance of the oil phase. At the end of incubation, serial dilutions (1/10) from the enrichment culture were plated out into mineral salt agar plates coated with diesel oil on their surface and incubated under aerobic conditions at 37 °C until colony formation. Isolated strains were purified by plate streaking method on LB agar medium until having pure isolated colonies. The purity of the isolates was checked microscopically. Strains were stored on LB agar medium at 4 °C until further use. For long-term preservation, the bacterial isolates were stored in 30 % glycerol at −70 °C (Ghribi et al. 2012).
Initially, the newly isolated strains were screened for their growth on liquid mineral salt medium supplemented with diesel oil. Degradation of diesel oil was evaluated by the gravimetric method in the end of cultivation. The strains having the best growth with better diesel biodegradation were subjected to further characterization.
Identification of the newly isolated bacteria and phylogenetic analysis
The bacterial isolates were identified by sequence analysis of nearly complete 16S rRNA gene and phylogenetic analysis. To proceed, the genomic DNA was extracted using the Pure Link Genomic DNA extraction kit, following the manufacturer’s recommended procedure. The 16S rDNA genes were amplified using Rd1 (5′AAGGAGGTGATCCAAGCC3′) and Fd1 (5′AGAGTTTGATCCTGGCTCAG3′) universal primers and a “Gene Amp PCR System 2700” (Applied Biosystems). The 25 μl of PCR mixture contained the following: 0.2 mM of deoxynucleoside triphosphate, 1.32 μM concentration each primer, 0.5 U of DNA polymerase, 5 μl of ×5 buffer, and 1 μl (5 ng) of total DNA template. The PCR reaction conditions included initial denaturation at 94 °C for 5 min followed by 35 cycles including a denaturation step at 94 °C for 1 min, annealing step at 60 °C for 1 min, and extension step at 72 °C for 2 min, and a final extension for 10 min at 72 °C was performed. The purified PCR products (with Invitrogen Gel Extraction Kit, by Life Technologies) were sequenced in both directions using an automated sequencer by Applied Biosystems (USA). Processed sequences were subjected to BLAST (Basic Local Alignment Search Tool) alignment. The MEGA program version 6.06 was used to make multiple alignments between all sequences using default parameters (Thompson et al. 1994).
Preparation of inoculum
The inoculum was prepared in LB medium as described by Mnif et al. (2012). The resultant culture was centrifuged for 10 min at 10,000 rpm, and the cell pellet was washed twice in sterile saline solution (9 ‰) before inoculation (Ganesh and Lin 2009). For the different inoculation conditions, inoculates were mixed at equal proportion and the resultant mixture served for inoculation to start with an optical density of 0.1 (Mnif et al. 2012).
Culture conditions and biodegradation assays
Diesel biodegradation assays were performed in liquid mineral medium as well as in soil amended with hydrocarbons to assess the effect of biosurfactants on diesel solubility and biodegradation. The liquid media composition used for biodegradation study was as described in our previous work with slight modifications (Mnif et al. 2012). It contains (g/l) (NH4)2SO4 (1), K2HPO4 (1), KH2PO4 (0.5), MgSO4 (0.2), NaCl (0.5), MnSO4 (0.001), FeSO4 (0.001), ZnSO4 (0.001), and CaCl2 (0.001). For the treatment of diesel-contaminated water, biodegradation assays were performed in 250-ml Erlenmeyer flasks containing 50 ml of liquid mineral medium supplemented with 5 % diesel (v/v). Un-inoculated control flasks with diesel were incubated in parallel to monitor abiotic losses of the substrate. The culture flasks were incubated for 21 days at 37 °C under shaking at 150 rpm. The growth patterns were obtained by measuring optical density at 600 nm at defined interval times. All experiments were performed in triplicate.
Determination of diesel degradation
The level of diesel degradation was determined using the gravimetric analysis method (Ganesh and Lin 2009), and residual hydrocarbons were analyzed by gas chromatography after 3 weeks of incubation. For liquid medium, the culture broth (50 ml) and abiotic control were collected, centrifuged at 10,000 rpm for 10 min, and the resultant supernatant was extracted with chloroform (v/v) to recover the residual hydrocarbon.
The organic fraction was evaporated by the rotary evaporator system and the remaining hydrocarbon was weighted after desiccation at 60 °C. The percentage of degraded hydrocarbon was calculated as proposed by Ganesh and Lin (2009):
Improvement percentages of oil biodegradation were calculated according to the following formula as proposed by Mnif et al. (2014):
Gas chromatography analyses
Gas chromatography analyses were done according to Mnif et al. (2014). Residual hydrocarbons were extracted using an organic solvent as described in the previous section. The organic solvent extract containing the residual hydrocarbons was subjected to gas chromatography analysis (Shimadzu Gas Chromatograph system, GC-2014). The system was also equipped with a flame ionization detector and a 60-m fused silica capillary column (DB5, Agilent J&W). The injector and detector temperatures were both set at 320 °C with 5 ml/min of hydrogen as the carrier gas. The column temperature was initially set at 100 °C, then raised to 320 °C at the rate of 5 °C/min, and finally set at 320 °C for 10 min (Mnif et al. 2014).
Results and discussions
Isolation and identification of diesel-degrading bacteria
Diesel is a high-diffuse contaminant. Bio-restoration of diesel-contaminated sites requires the involvement of microorganisms having the capability to survive on highly contaminated sites and assimilate diesel. Hence, strain screening was based on the ability of diesel degradation suggesting the utilization of this hydrocarbon as sole carbon and energy source. In fact, after three cycles of enrichment on basal minimal medium containing 5 % (v/v) diesel, the medium became turbid indicating microbial growth. Then, a total of 25 hydrocarbon-utilizing microorganisms were isolated from the contaminated soil after the enrichment process.
The efficiency of bioremediation is often a function of the microbial population or consortium and how it can be enriched and maintained in an environment. Inoculation of bacteria with hydrocarbon biodegradation capabilities shortens the time of the treatment. In fact, the mixed bacterial consortium showed more growth and degradation than did individual strains. Therefore, we selected the most interesting strains, among the isolates, in view of their abilities to grow and degrade diesel oil for further studies. The molecular identification and phylogenetic analysis showed that they correspond to Lysinibacillus bronitolerans RI18 (KF964487), Bacillus thuringiensis RI16 (KM111604), and Bacillus weihenstephanensis strain RI12 (KM094930). A fourth strain, Acinetobacter radioresistens RI7 (KJ829530), having the ability to degrade hydrocarbon and produce biosurfactant was also isolated. The three first strains constituted the consortium, and the fourth strain was used for the co-inoculation by a biosurfactant-producing strain as well as Bacillus subtilis SPB1 (HQ392822) (Ghribi et al. 2012).
According to literature studies, Gram-negative hydrocarbon-tolerant bacteria belong generally to Acinetobacter, Pseudomonas, Alkanivorax, and related genera (Yakimov et al. 2005; Ganesh and Lin 2009). They are generally associated with a fast petroleum degradation phase and are the more efficient (Ganesh and Lin 2009). Gram-positive bacteria such as Bacillus, Rhodococcus, Staphylococcus, and Exiguobacterium have also been found to be hydrocarbon-tolerant bacteria (Lazaroaie 2010).
B. thuringiensis was widely described as a hydrocarbon-degrading bacteria (Maiti et al. 2012; Thamer et al. 2013; Archaya et al. 2014). Lysinibacillus sp. was also published in many previous studies as potent hydrocarbon-degrading microorganisms (Mnif et al. 2011; Ben Hamed et al. 2013). Also, Lysinibacillus sphaericus was reported as potent dibenzothiophene and cyfluthrin-degrading bacteria as described by Bahuguna et al. (2011) and Hu et al. (2014). However, until now, hydrocarbon biodegradation by B. weihenstephanensis strains was not reported by any study. Also, A. radioresistens was widely described as hydrocarbon-degrading (Dehghani et al. 2013) and biosurfactant-producing strain (Barkay et al. 1999; Toren et al. 2001).
Growth kinetics of the selected consortium under different cultural conditions
Regarding literature reports, bacterial consortia were highly used to treat hydrocarbon-contaminated soil and water. In fact, mixed cultures were demonstrated more efficiently than pure culture in hydrocarbon decontamination (Adebusoye et al. 2007; Sathishkumar et al. 2008; Liu and Liu 2011). Since, bacterial degradation could be limited by the low solubility and bioavailability of the target hydrocarbons, diesel biodegradation was studied by the selected consortium in the presence and the absence of the B. subtilis SPB1 biosurfactant, in one hand, and when co-inoculating by biosurfactant-producing strains, B. subtilis SPB1, and a newly isolated strain A. radioresistens RI7, in another hand. In fact, five culture conditions were assayed as follows: consortium alone, consortium + SPB1 biosurfactant, consortium + B. subtilis SPB1 strain, consortium + A. radioresistens RI7, and consortium + A. radioresistens RI7 + B. subtilis SPB1. The used SPB1 biosurfactant was produced and prepared according to the protocol described in our previous work (Mnif et al. 2013a).
In this purpose, kinetics of growth of the microbial consortium on diesel oil was followed by measuring the evolution of optical density at 600 nm (Fig. 1). Growth at the expense of diesel was verified by demonstrating an increase in the optical density at 600 nm, suggesting a bacterial growth. The growth of the consortium composed of the three strains (L. bronitolerans RI18; B. thuringiensis RI16; B. weihenstephanensis RI12) with diesel as a unique carbon source indicates that these microorganisms are potential diesel bio-degraders.
For liquid experiments, after a certain time of incubation, bacterial growth rate becomes stable and the stationary phase was reached (Fig. 1). This is due to the fact that the increasing numbers of bacteria began to compete for the dwindling nutrients and their exponential growth was inhibited. Also, when using hydrocarbons as a unique source of carbon and energy, a short-term stationary phase was observed, suggesting a rapidly diminishing rate of nutrient availability essential to maintain live culture. At the end of the third week of incubation, a gradual decrease of the bacterial growth was observed, suggesting the initiation of the death phase. It was assumed that detrimental environmental changes, such as nutrient deprivation and the buildup of toxic wastes, caused irreparable harm and loss of viability (Mnif et al. 2014). This was verified by the fact that, even when bacterial cells were transferred to a fresh medium, no cellular growth was observed (Mnif et al. 2014).
Distinct amelioration of bacterial consortium growth was recorded when adding biological emulsifiers or when co-cultured with biosurfactant-producing bacteria. The enhancement of bacterial growth could be owed to a better assimilation of diesel oil as a unique source of carbon and energy. Also, biosurfactant addition seemed to accelerate the first growth phase that can be due to the assimilation of emulsifier as an easier carbon source for the initiation of growth stimulating after that growth on immiscible substrate. Furthermore, the enhancement of growth was more pronounced with co-culture with B. subtilis SPB1 or A. radioresistens RI7.
Estimation of diesel biodegradation potency and gas chromatography analyses of residual hydrocarbons
In order to determine the biodegradation rate of diesel, the residual oil content was determined using the gravimetric analysis method (Ganesh and Lin 2009). Results of Table 1 confirmed that the addition of the biological emulsifier supported an enhancement of hydrocarbon degradation. In fact, SPB1 lipopeptide biosurfactant supplement hold up an improvement of about 38.42 %. The comparison of the enhancement of oil biodegradation due to the addition of lipopeptide bioemulsifier with those of co-culture with biosurfactant-producing strain (A. radioresistens RI7 and/or B. subtilis SPB1) was done. Results were better when culturing B. subtilis SPB1 strain with the respective consortium with an improvement of about 49.65 %. Furthermore, the best improvement evaluated to be about 55.4 % was recorded for the consortium cultured with B. subtilis SPB1 and A. radioresistens RI7 strains. The results demonstrated that bacteria with the catabolic potential to degrade hydrophobic contaminants could interact synergistically with surfactant-producing strains to hasten the biodegradation of diesel. In fact, the biosurfactant produced in situ can contribute significantly to the emulsification and solubilization of diesel oil increasing therefore its bioavailability for hydrocarbon-degrading strain. Moreover, the biosurfactant-producing strains can participate in the biodegradation process enhancing diesel digestion.
To confirm the obtained results, gas chromatographic analyses of the residual hydrocarbons were carried out after 21 days of aerobic incubation. In fact, regarding previous literature studies, gas chromatography has been widely applied in order to assess hydrocarbon degradation (Marquez-Rocha et al. 2001; Abalos et al. 2004; Chamkha et al. 2011; Mnif et al. 2014). The obtained chromatograms are presented in Fig. 2. They confirmed the described results and suggested that the present consortium was highly effective on different diesel compounds. Assays were compared with abiotic control, carried out at the same conditions. In fact, gas chromatography profiles showed a decrease of the peak intensity of each compound. The appearance or disappearance of novel peak probably resulted either from the degradation of the compounds or the synthesis of novel metabolites in the fermentation media. Also, the improvement of diesel degradability due to the different combination realized was confirmed by gas chromatography analysis.
Gas chromatography profiles of diesel oil remaining in the mineral salt medium in liquid experiments after incubation without SPB1 biosurfactant (b), with SPB1 biosurfactant (c), with B. subtilis SPB1 (d), with A. radioresistens RI7 (e), and with B. subtilis SPB1 + A. radioresistens RI7 (f). The first spectrum (a) shows a negative control prepared in the same conditions but without inoculation
Biodegradability of hydrocarbons and hence their degree of persistence in natural environments are influenced by various factors, most vital of which are the chemical structures of the hydrocarbons, the presence of viable microbial population able to degrade them, and the environmental conditions optimal for microbial biodegradation activities. Diesel oil derives from the distillation of crude oil, having a complex hydrocarbon combination with carbon numbers that range approximately from C9 to C20, iso-alcanes, cyclo-alcanes, paraffin, olefins, naphtha, and aromatic compounds (Yakimov et al. 2005). This oil mixture represents an excellent substrate in the study of hydrocarbon biodegradation due to the complexity of its composition (Mnif et al. 2014).
The use of bacterial consortia able to degrade hydrocarbons knows a great interest. It is generally considered that mixed cultures have a higher metabolic versatility than pure cultures. Previous studies reported the selection of diverse bacterial consortia for the bioremediation of hydrocarbons; Pseudomonas sp. BPS1-8, Bacillus sp. IOS1-7, Pseudomonas sp. HPS2-5, and Corynebacterium sp. BPS2-6 described by Sathishkumar et al. (2008); Achromobacter, Alcaligenes, and Cupriavidus described by Bacosa et al. (2010); and Acinetobacter faecalis WD2, Staphylococcus sp. DD3, and Neisseria elongate TDA4 described by Mukred et al. (2008). Also, Owsianiak et al. (2009) and Marquez-Rocha et al. (2001) reported the biodegradation of diesel oil by microbial consortia. Our present study reported the use of a mixed culture. Certainly, the reduced solubility of hydrocarbons in aqueous media can limit their bioavailability and biodegradation. Certain strains of B. subtilis were able to produce biosurfactants (Bacosa et al. 2010; Chander et al. 2012; Ghribi et al. 2012; Santos et al. 2014) and degrade hydrocarbon (Lily et al. 2012; Yuliani et al. 2012). Other B. subtilis strains are well known by their ability to produce biosurfactant simultaneously with hydrocarbon degradation for enhancing hydrocarbon bioavaibility and uptake (Wijanarko et al. 2012; Gudina et al. 2013; Yengejeh et al. 2014). In this aim, B. subtilis SPB1, a biosurfactant-producing strain and demonstrated in our previous work to have the ability to degrade hydrocarbons (Mnif et al. 2014), was used as an indigenous biosurfactant-producing strain in order to increase the consortium efficiency. Furthermore, addition of biosurfactants alone can enhance the process feasibility. In fact, biosurfactants can be used as alternatives of synthetic emulsifiers to enhance hydrocarbon biodegradation by the enhancement of mobilization, solubilization, or emulsification of hydrophobic compounds (Pacwa-Plociniczak et al. 2011). Generally, surfactants enhance bioremediation by increasing the surface area of hydrophobic substrates available for biodegradation and increase the bioavailability of these substrates (Pacwa-Plociniczak et al. 2011). Moreover, our recent study confirmed that SPB1 biosurfactant addition enhances remarkably diesel solubilization in water promoting its emulsification (Mnif et al. 2013b). These can predict its potential role on the enhancement of diesel bioavailability for hydrocarbon bacterial-degrading flora, stimulating then its assimilation. So, SPB1 bioemulsifier was added in order to enhance the diesel biodegradation.
The obtained results showed the enhancement of diesel biodegradation in all the studies cases. In fact, either biosurfactant added alone or that produced in situ acts to enhance diesel oil removal. Moreover, SPB1 bioemulsifiers added alone can be of great interest as it ensured alone about 70 % of the whole improvement when compared with co-culture of the two biosurfactant-producing strain. But, for field bioremediation application based on bioaugmentation, addition of the biosurfactant-producing bacteria may be beneficial and more practical than exogenously adding purified biosurfactant (Kumar et al. 2006). This could avoid the high cost of production and preparation of biosurfactant and facilitate the process conduction. However, the effect of in situ biosurfactant production by co-culture of the biosurfactant-producing and hydrocarbon-degrading bacteria on hydrocarbon degradation is relatively less studied than the supplement by biosurfactant alone (Lai et al. 2009). Furthermore, in all experiments, SPB1 biosurfactants addition seemed not to have a deleterious effect on growth patterns, in contrary; it seems to induce consortium growth and diesel degradation probably through its solubilization. Literature reviews discussed the enhancement of hydrocarbon solubility in the presence of microbially derived surfactants (Ji et al. 2009; Abouseoud et al. 2010; Pacwa-Plociniczak et al. 2011).
To date, many literature studies reported the enhancement of hydrocarbon assimilation in liquid media by microbial strains due to the addition of biological emulsifiers. P. aeruginosa-derived rhamnolipid and B. subtilis-derived surfactin were demonstrated by Whang et al. (2008) to increase microbial growth and diesel biodegradation. In fact, they significantly enhanced biomass growth and improved diesel biodegradation from 40 to 94 % when adding 40 mg/L of surfactin and from 40 to 100 % when adding 80 mg/L of rhamnolipid. Moran et al. (2000) reported the augmentation of hydrocarbon digestion by B. subtilis O9 surfactin supplement. Moreover, adding of rhamnolipid produced by P. aeruginosa AT10 improved significantly biodegradation potency of crude oil, by a bacterial consortium, from 32 to 61 % after 10 days of incubation (Maiti et al. 2012). Rhamnolipid biosurfactants were also demonstrated by Owsianiak et al. (2009) as enhancers of diesel, biodiesel, and gasoline degradation. Candida antarctica T-34-derived biosurfactants were shown to improve the biodegradation rate of some n-alkanes, a mixture of n-alkanes and kerosene (Hua et al. 2004). At the same way, it was demonstrated by Saeki et al. (2009) that Gordonia sp. JE-1058 biosurfactant addition stimulated crude petroleum biodegradation by indigene marine flora.
Another hypothesis proposes that the enhancement of bacterial growth and therefore hydrocarbon degradation when adding biosurfactant could be due to the fact that this biosurfactant is biodegradable and could be used as a primary and easier carbon source to initiate microorganisms’ growth on water-immiscible substrates (Bautista et al. 2009).
Otherwise, in addition to the reduced bioavailability and solubility of the pollutants recognized as substrates, biodegradation ability of bacteria is assumed to be associated with the production of different enzymes and their potent activity. Accordingly, we can assume the beneficial effect of the emulsifiers on diesel biodegradation through the increase of bacterial membrane permeability facilitating then substrates diffusion and/or metabolites and enzymes secretion. Many previous reports described the same findings for surfactants (Ji et al. 2009; Gül and Dönmez 2012; Hadibarata et al. 2013) or biosurfactants (Jadhav et al. 2011; Zhou et al. 2011). Moreover, as described in many other researches, surfactants could promote enzymes activities (Kristensen et al. 2007; Champagne et al. 2010; Zhang et al. 2012).
Conclusion
An effective diesel-degrading consortium was isolated from Tunisian contaminated soil. Diesel biodegradation was studied by the selected consortium under different cultural conditions. The consortium was composed with bacterial strains belonged to Bacilus and Acinetobacter genera. Various conditions were studied in order to improve the capacity of diesel oil degradation by this consortium. Among them, we found out the co-inoculation with biosurfacatant-producing bacteria B. subtilis and A. radioresistens increased the degradation for more than 50 %. The direct addition of crude biosurfactant improves also the yield of degradation to about 38.42 %. Gas chromatography analyses are correlated with the gravimetric evaluation of the residual hydrocarbons. Our finding suggested the potential applicability of the selected consortium along with the ex situ- and in situ-added biosurfactant for the bioremediation of diesel-contaminated water.
References
Abalos A, Vinas M, Sabate J, Manresa MA, Solanas AM (2004) Enhanced biodegradation of Casablanca crude oil by a microbial consortium in presence of a rhamnolipid produced by Pseudomonas aeruginosa AT10. Biodegradation 15:249–260
Abouseoud M, Yataghene A, Amrane A, Maachi R (2010) Production of a biosurfactant by Pseudomonas fluorescens—solubilizing and wetting capacity. J Hazard Mater 183:131–136
Adebusoye SA, Ilori MO, Amund OO, Teniola OD, Olatope SO (2007) Microbial degradation of petroleum hydrocarbons in a polluted tropical stream. World J Microbiol Biotechnol 23(8):1149–1159
Akpoveta VO, Osakwe S, Egharevba F, Medjor WO, Asia IO, Ize-Iyamu OK (2012) Surfactant enhanced soil washing technique and its kinetics on the remediation of crude oil contaminated soil. Pac J Sci Technol 13(1):443–456
Archaya S, Gopinath LR, Sangeetha S, Bhuvaneswari R (2014) Molecular characterization of kerosene degrading bacteria isolated from kerosene polluted soil. Int J Adv Res 2(4):1117–1124
Bacosa H, Suto K, Inoue C (2010) Preferential degradation of aromatic hydrocarbons in kerosene by a microbial consortium. Int Biodeter Biodegr 64:702–710
Bahuguna A, Lily MK, Munjal A, Singh RN, Dangwal K (2011) Desulfurization of dibenzothiophene (DBT) by a novel strain Lysinibacillus sphaericus DMT-7 isolated from diesel contaminated soil. J Environ Sci 23(6):975–982
Barkay T, Navon-Venezia S, Ron EZ, Rosenberg E (1999) Enhancement of solubilization and biodegradation of polyaromatic hydrocarbons by the bioemulsifier alasan. Appl Environ Microbiol 65(6):2697–2702
Bautista FL, Sanz R, Molina MC, Gonzalez N, Sanchez D (2009) Effect of different non-ionic surfactants on the biodegradation of PAHs by diverse aerobic bacteria. Int Biodeter Biodegr 63:913–922
Ben Hamed S, Maaroufi A, Ghram A, Zouhaier BAG, Labat M (2013) Isolation of four hydrocarbon effluent-degrading Bacillaceae species and evaluation of their ability to grow under high-temperature or high-salinity conditions. Afr J Biotechnol 12(14):1636–1643
Calvo C, Manzanera M, Silva-Castro GA, Uad I, González-López J (2009) Application of bioemulsifiers in soil oil bioremediation processes. Future Prosp Sci Total Environ 407:3634–3640
Chamkha M, Trabelsi Y, Mnif S, Sayadi S (2011) Isolation and characterization of Klebsiellaoxytoca strain degrading crude oil from a Tunisian off-shore oil field. J Basic Microbiol 51:580–589
Champagne P-P, Nesheim ME, Ramsay JA (2010) Effect of a non-ionic surfactant, Merpol, on dye decolorization of reactive blue 19 by laccase. Enzymol Microb Technol 46:147–152
Chander CRS, Lohitnath T, Mukesh Kumar DJ, Kalaichelvan PT (2012) Production and characterization of biosurfactant from Bacillus subtilis MTCC441 and its evaluation to use as bioemulsifier for food bio-preservative. Adv Appl Sci Res 3(3):1827–1831
Dayeh VR, Chow SL, Schirmer K, Bols NC (2004) Evaluating the toxicity of Triton X-100 to protozoan, fish, and mammalian cells using fluorescent dyes as indicators of cell viability. Ecotoxicol Environ Saf 57:375–382
Dehghani M, Taatizadeh SB, Samaei MR (2013) Biodegradation of n-Hexadecane in Acinetobacter radioresistens liquid culture. Health Scope 2(3):162–167
Fracchia L, Cavallo M, Martinotti MG, Banat IM (2012) Biosurfactants and bioemulsifiers biomedical and related applications—present status and future potentials, biomedical science, engineering and technology. Prof. Dhanjoo N. Ghista (Ed.), (2012) ISBN: 978-953-307-471-9
Franzetti A, Caredda P, Ruggeri C, La Colla P, Tamburini E, Papacchini M, Bestetti G (2009) Potential applications of surface active compounds by Gordonia sp. strain BS29 in soil remediation technologies. Chemosphere 75(6):801–807
Ganesh A, Lin J (2009) Diesel degradation and biosurfactant production by gram-positive isolates. Afr J Biotechnol 8(21):5847–5854
Ghribi D, Abdelkefi-Mesrati L, Mnif I, Kammoun R, Ayadi I, Saadaoui I, Maktouf S, Chaabouni-Ellouze S (2012) Investigation of antimicrobial activity and statistical optimization of Bacillus subtilis SPB1 biosurfactant production in solid-state fermentation. J Biomed Biotechnol. doi:10.1155/2012/373682
Gudina EJ, Pereira JFB, Costa R, Coutinho JAP, Teixeira JA, Rodrigues LR (2013) Biosurfactant-producing and oil-degrading Bacillus subtilis strains enhance oil recovery in laboratory sand-pack columns. J Hazard Mater 261:106–113
Gül UD, Dönmez G (2012) Effects of dodecyl trimethyl ammonium bromide surfactant on decolorization of remazol blue by a living Aspergillus versicolor strain. J Surfactants Deterg 15:797–803
Hadibarata T, Tachibana S (2010) Characterization of phenanthrene degradation by strain Polyporus sp. S133. J Environ Sci 22(1):142–149
Hadibarata T, Adnan LA, Mohd Yusoff AR, Yuniarto A, Meor R, Ahmad Zubir MF, Khudhair AB, Teh ZC, Naser MA (2013) Microbial decolorization of an azo dye reactive black 5 using white-rot fungus Pleurotus eryngii F032. Water Air Soil Pollut 224:1595
Helmy Q, Kardena E, Wisjnuprapto (2009) Performance of petrofilic consortia and effect of surfactant tween 80 addition in the oil sludge removal process. J Appl Sci Environ Sanit 4(3):207–218
Hu GP, Zhao Y, Song FQ, Liu B, Vasseur L, Douglas C, You MS (2014) Isolation, identification and cyfluthrin-degrading potential of a novel Lysinibacillus sphaericus strain FLQ-11-1. Res Microbiol 165(2):110–118
Hua Z, Chen Y, Du C, Chen J (2004) Effects of biosurfactants produced by Candida antarctica on the biodegradation of petroleum compounds. World J Microbiol Biotechnol 20:25–29
Jadhav M, Kalme S, Tamboli D, Govindwar S (2011) Rhamnolipid from Pseudomonas desmolyticum NCIM-2112 and its role in the degradation of Brown 3REL. J Basic Microbiol 51:385–396
Ji G, Zhang H, Huang F, Huang X (2009) Effects of nonionic surfactant Triton X-100 on the laccase-catalyzed conversion of bisphenol A. J Environ Sci 21:1486–1490
Joutey NT, Bahafid W, Sayel H, ElGhachtouli N (2013) Biodegradation: involved microorganisms and genetically engineered microorganisms. Chapter 11; doi: 10.5772/56194
Kristensen JB, Borjesson J, Bruun MH, Tjerneld F, Jørgensen H (2007) Use of surface active additives in enzymatic hydrolysis of wheat straw lignocellulose. Enzymol Microbial Technol 40:888–895
Kumar M, Leon V, De Sisto A, Ilzins OA (2006) Enhancemant of oil degradation by co-culture of hydrocarbon and biosurfactant producing bacteria. Polish J Microbiol 55(2):139–146
Kumar M, Leon V, De Sisto AM, Ilzins OA (2007) A halotolerant and thermotolerant Bacillus sp. degrades hydrocarbons and produces tension-active emulsifying agent. World J Microbiol Biotechnol 23:211–220
Lai C-C, Huang Y-C, Wei Y-H, Chang J-S (2009) Biosurfactant-enhanced removal of total petroleum hydrocarbons from contaminated soil. J Hazard Mater 167(1–3):609–614
Lazaroaie MM (2010) Multiple responses of gram-positive and gram-negative bacteria to mixture of hydrocarbons. Braz J Microbiol 41:649–667
Lily MK, Bahuguna A, Bhatt KK, Garg V, Dangwal K (2012) Strain improvement of a potent benzo-A-pyrene (BAP) degrader Bacillus subtilis BMT4I (MTCC 9447). Int J Adv Biotechnol Res 3(2):570–577
Liu C-W, Liu H-S (2011) Rhodococcus erythropolis strain NTU-1 efficiently degrades and traps diesel and crude oil in batch and fed-batch bioreactors. Process Biochem 46:202–209
Maiti A, Das S, Bhattacharyya N (2012) Bioremediation of high molecular weight polycyclic aromatic hydrocarbons by Bacillus thuringiensis strain NA2. J Sci 1(4):72–75
Makkar RS, Rockne KJ (2003) Comparison of synthetic surfactants and biosurfactants in enhanced biodegradation of polycyclic aromatic hydrocarbons. Environ Toxicol Chem 22(10):2280–2292
Marquez-Rocha FJ, Hernandez-Rodriguez V, Lamela MT (2001) Biodegradation of diesel oil by a microbial consortium. Water Air Soil Pollut 128:313–320
Mnif S, Chamkha M, Labat M, Sayadi S (2011) Simultaneous hydrocarbon biodegradation and biosurfactant production by oilfield-selected bacteria. J Appl Microbiol 111:525–536
Mnif I, Ellouze-Chaabouni S, Ghribi D (2012) Response surface methodological approach to optimize the nutritional parameters for enhanced production of lipopeptide biosurfactant in submerged culture by B. subtilis SPB1. J Adv Sci Res 3(1):87–94
Mnif I, Sahnoun R, Ellouze-Chaabouni S, Ghribi D (2013a) Evaluation of B. subtilis SPB1 biosurfactants’ potency for diesel-contaminated soil washing: optimization of oil desorption using Taguchi design. Environ Sci Pollut Res. doi:10.1007/s11356-013-1894-4
Mnif I, Ellouze-Chaabouni S, Ghribi D (2013b) Economic production of Bacillus subtilis SPB1 biosurfactant using local agro-industrial wastes and its application in enhancing solubility of diesel. J Chem Technol Biotechnol 88:779–787
Mnif I, Ellouze-Chaabouni S, Ayedi Y, Ghribi D (2014) Treatment of diesel- and kerosene-contaminated water by B. subtilis SPB1 biosurfactant-producing strain. Water Environ Res 86(8):707–716
Moran AC, Olivera N, Commendatore M, Esteves JL, Siñeriz F (2000) Enhancement of hydrocarbon waste biodegradation by addition of a biosurfactant from Bacillus subtilis O9. Biodegradation 11:65–71
Mukred AM, Hamid AA, Hamzah A, Yusoff WM (2008) Development of three bacteria consortium for the bioremediation of crude petroleum-oil in contaminated water. Online J Biol Sci 8(4):73–79
Noordman WH, Wahter JH, De Boer GJ, Janssen DB (2002) The enhancement by surfactants of hexadecane degradation by Pseudomonas aeruginosa varies with substrate availability. J Biotechnol 94:195–212
Owsianiak M, Chrzanowski L, Szulc A, Staniewski J, Olszanowski A, Olejnik-Schmidt AK, Heipieper HJ (2009) Biodegradation of diesel/biodiesel blends by a consortium of hydrocarbon degraders: effect of the type of blend and the addition of biosurfactants. Bioresour Technol 100:1497–1500
Pacwa-Plociniczak M, Plaza GA, Piotrowska-Seget Z, Cameotra SS (2011) Environmental applications of biosurfactants: recent advances. Int J Mol Sci 12:633–654
Pavlic Z, Cifrek ZV, Puntaric D (2005) Toxicity of surfactants to green microalgae Pseudokirchneriella subcapitata and Scenedesmus subspicatus and to marine diatoms Phaeodactylum tricornutum and Skeletonema costatum. Chemosphere 61:1061–1068
Reddy MS, Naresh B, Leela T, Prashanthi M, Madhusudhan NC, Dhanasri G, Devi P (2010) Biodegradation of phenanthrene with biosurfactant production by a new strain of Brevibacillus sp. Bioresour Technol 101:7980–7983
Saeki H, Sasaki M, Komatsu K, Miura A, Matsuda H (2009) Oil spill remediation by using the remediation agent JE1058BS that contains a biosurfactant produced by Gordonia sp. strain JE-1058. Bioresour Technol 100:572–577
Santos BF, Ponezi AN, Filetia AMF (2014) Strategy of using waste for biosurfactant production through fermentation by Bacillus subtilis. Chem Eng Trans 37:727–732
Sathishkumar M, Binupriya AR, Baik S-H, Yun S-E (2008) Biodegradation of crude oil by individual bacterial strains and a mixed bacterial consortium isolated from hydrocarbon contaminated areas. Clean 36(1):92–96
Shoeb E, Akhlaq F, Badar U, Akhter J, Imtiaz S (2013) Classification and industrial applications of biosurfactants. Part-I: Nat Appl Sci 4(3):242–252
Swe MM, Yu LE, Hung K-C, Chen B-H (2006) Solubilization of selected polycyclic aromatic compounds by nonionic surfactants. J Surfactants Deterg 9(3):237–244
Thamer M, Al-Kubaisi AR, Zahraw Z, Abdullah HA, Hindy I, Abd Khadium A (2013) Biodegradation of Kirkuk light crude oil by Bacillus thuringiensis. North Iraq Nat Sci 5(7):865–873
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTALW: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–468
Toren A, Navon-Venezia S, Ron EZ, Rosenberg E (2001) Emulsifying activity of purified alasan proteins from Acinetobacter radioresistens. Appl Environ Microbiol 67:1102–1106
Urum K, Grigson S, Pekdemir T, McMenamy S (2006) A comparison of the efficiency of different surfactants for removal of crude oil from contaminated soils. Chemosphere 62:1403–1410
Van Hamme JD, Ward OP (2001) Physical and metabolic interactions of Pseudomonas sp. Strain JA5-B45 and Rhodococcus sp. Strain F9-D79 during growth on crude oil and effect of a chemical surfactant on them. Appl Environ Microbiol 67:4874–4879
Whang L-M, Liu P-WG, Ma C-C, Cheng S-S (2008) Application of biosurfactants, rhamnolipid, and surfactin, for enhanced biodegradation of diesel-contaminated water and soil. J Hazard Mater 151:155–163
Wijanarko A, Yuliani H, Hermansyah H, Sahlan M (2012) Isolation and properties characterization of biosurfactant synthesized by pyrene degrading Bacillus subtilis C19. J Chem Eng 6:889–896
Wong JWC, Fang M, Zhao Z, Xing B (2004) Effect of surfactants on solubilization and degradation of phenanthrene under thermophilic conditions. J Environ Qual 33:2015–2025
Yakimov MM, Denaro R, Genovese M, Cappello S, D’Auria G, Chernikova TN, Timmis KN, Golyshi PN (2005) Natural microbial diversity in superficial sediments of Milazzo Harbor (Sicily) and community successions during microcosm enrichment with various hydrocarbons. Environ Microbiol 7:1426–1441
Yengejeh JR, Sekhavatjou MS, Maktabi P, Arbab Soleimani N, Khadivi S, Pourjafarian V (2014) The biodegradation of crude oil by Bacillus subtilis isolated from contaminated soil in hot weather areas. Int J Environ Res 8(2):509–514
Yuliani H, Sahlan M, Hermansyah H, Wijanarko A (2012) Selection and identification of polyaromatic hydrocarbon degrading bacteria. World Appl Sci J 20(8):1133–1138
Zhang Y, Zeng Z, Zeng G, Liud X, Liu Z, Chen M, Liu L, Lie J, Xie G (2012) Effect of Triton X-100 on the removal of aqueous phenol by laccase analyzed with a combined approach of experiments and molecular docking. Colloids Surf B 97:7–12
Zhou MF, Yuan XZ, Zhong H, Liu ZF, Li H, Jiang LL, Zeng GM (2011) Effect of biosurfactants on laccase production and phenol biodegradation in solid-state fermentation. Appl Biochem Biotechnol 164:103–114
Acknowledgments
This work has been supported by grants from “Tunisian Ministry of Higher Education, Scientific Research and Technology.”
Ethical statement
The experimental protocols were conducted in accordance with the Guide for the Care and Use of Laboratory Animals issued by the University of Sfax, Tunisia, and approved by the Tunisia Committee of Animal Ethics.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Robert Duran
Rights and permissions
About this article
Cite this article
Mnif, I., Mnif, S., Sahnoun, R. et al. Biodegradation of diesel oil by a novel microbial consortium: comparison between co-inoculation with biosurfactant-producing strain and exogenously added biosurfactants. Environ Sci Pollut Res 22, 14852–14861 (2015). https://doi.org/10.1007/s11356-015-4488-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-4488-5


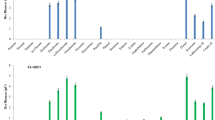



 , with SPB1 biosurfactant
, with SPB1 biosurfactant  , with B. subtilis SPB1
, with B. subtilis SPB1  , with A. radioresistens RI7
, with A. radioresistens RI7  , and with A. radioresistens RI7 and B. subtilis SPB1
, and with A. radioresistens RI7 and B. subtilis SPB1  in liquid medium supplemented with 5 % diesel oil (v/v)
in liquid medium supplemented with 5 % diesel oil (v/v)