Abstract
Costus spicatus (spiked spiralflag ginger) is traditionally utilised for its advantages such as antidiabetic, antihyperlipidemic, diuretic, antimicrobial, and anticancer properties. However, there is no scientific evidence on the nephroprotective potential of this plant. Thus, this study tested the nephroprotective effect of the polyphenol-rich extract of Costus spicatus leaves (PCSL) using preclinical models, including the HeK cell line and Wistar albino rats against cisplatin-induced toxicity. It also determined the polyphenolic compounds using high-performance thin-layer chromatography (HPTLC). PCSL showed significant (p < 0.05) nephroprotective potential against cisplatin-induced nephrotoxicity in HeK cells. Moreover, in vivo studies revealed significant (p < 0.05) amelioration in serum biochemical markers and antioxidant enzymes against cisplatin-induced nephrotoxicity. PCSL significantly inhibited the level of inflammatory cytokines such as TNF-α, IL-6, and IL-1β. Moreover, PCSL restored the damage of the kidney tissues and ameliorated interstitial haemorrhage, congestion in capillaries, inflammatory cell infiltration, vacuolated cytoplasm, and tubular epithelial injury with widened Bowman's space. In addition, HPTLC analysis revealed that PCSL comprised polyphenolic compounds such as caffeic acid, quercetin, and ferulic acid. In conclusion, PCSL exerted nephroprotective potential by modulating the expression of inflammation, oxidative stress, and histological architecture of kidney tissues.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nephrotoxicity is one of the most recurrent kidney problems characterised by kidney malfunction through either direct or indirect exposure to toxins or medications (Pizzorno 2015). The kidney is the principal osmoregulatory organ that perpetuates the physiological functions of the body by regulating blood filtration and removing waste metabolites from the body to maintain proper tonicity, fluid volume, pH balance, and electrolyte homeostasis (Al-Naimi et al. 2019). With time, multiple pathogenic events such as deleterious imbalance of antioxidant, oxidative stress, and inflammatory cytokines, as well as progressive renal dysfunction, lead to chronic kidney disease (CKD) (Daenen et al. 2019). It is one of the most serious public health problems in the world, with a global prevalence of 13.4%. CKD is driven by other diseases such as cardiovascular disease, hypertension, diabetes, dyslipidemia, obesity, and ageing (Lv and Zhang 2019).
Cisplatin is a frequently used medicament to treat numerous solid tumours like ovarian cancer, lung cancer, and stomach cancer. However, it produces a number of negative side effects, including bone marrow suppression, nephrotoxicity, and neural toxicity. Of the total patients, 25–30% of the patients utilising cisplatin develop nephrotoxicity (Dasari and Tchounwou 2014; Miller et al. 2010). Herewith, the development of nephrotoxicity by cisplatin is associated with major aggression factors such as transporter-mediated cisplatin accumulation, transformation into nephrotoxins, DNA adduct formation, mitochondrial dysfunction due to nitrosative and oxidative stress (OS) and inflammation, signal transducers, and apoptosis activation. However, evidence suggests that OS and inflammation play an essential role in toxicity-induced cisplatin (Xavier et al. 2017).
Herbal medicines contain a large number of bioactive compounds that exert several bioactivities such as antiautoimmune, inflammatory, antidiabetic, anticancer, and antioxidant activities (Batiha et al. 2020; Fierascu et al. 2020). Hence, the trend in medicine selection has been switched from synthetic to herbal medicine for disease management and treatment, indicating that people are returning to nature. Herbal medicines have grown exponentially in recent decades and gained popularity in both developed and developing countries, including India. Moreover, herbal medicines have been used for millennia and are widely regarded around the world as a treatment regimen for disease prevention owing to its natural origins, easy accessibility and availability, and fewer side effects. In the present scenario, there is a lack of effective therapy to prevent cisplatin-induced nephrotoxicity, although several efficacious and less toxic plant-based medicines have been currently developed to protect against nephrotoxicity (Fang et al. 2021).
Costus spicatus was frequently used in different parts of the world as nutraceuticals as well as pharmaceuticals. It is one of the most popular medicinal plants frequently utilised in the Ayurvedic system of medicine to medicate various ailments such as chronic headache, gastroenteritis, diarrhoea, hyperlipidemia, diabetes, cancer, ringworm infections, and skin inflammations (Shediwah et al. 2019). C. spicatus is enriched with polyphenols (phenols and flavonoids), alkaloids, glycosides many more, which are mainly responsible for various bioactivities (Moreno et al. 2021). Furthermore, the polyphenolic components in C. spicatus are designed to prevent the progressive degradation of the kidney function, restore the physiological and cellular strength of individuals with renal challenges by improving kidney function parameters, antioxidant, and anti-inflammatory properties. However, there is still lack of evidence-based studies that assess the nephroprotective activity of C. spicatus. Therefore, the present study aims to test the nephroprotective effect of the polyphenol-rich extract of C. spicatus (PCSL) using a rodent model of nephrotoxicity.
Materials and methods
Procurement of sample and preparation of extract
C. spicatus leaves were obtained from local market of Delhi and taxonomic identification were performed and the specimen was deposited at the Department of Pharmacy, Integral University (IU/2021/Costus spicatus-2). The dried leaves of C. spicatus (50 g) were placed in a conical flask containing methanol (500 mL) and macerated for three consecutive days with continuous stirring at 25 °C. After complete maceration, the extract was filtered through filter paper (Whatman No. 1). Finally, the obtained extract was dried using vacuum evaporation and stored in an airtight container for future experiments. The extractive percentage yield was 13.76%.
Total polyphenolic content (phenol and flavonoid)
The Folin–Ciocalteu technique, with some modifications, was used to estimate the total phenol concentration in PCSL (Chlopicka et al. 2012). Briefly, an extract stock solution (10 mg/mL) was produced in high-performance liquid chromatography (HPLC)-grade methanol. 1000 µL of the extract from the stock solution was mixed with the Folin–Ciocalteu reagent (5.0 mL; 1:10, v/v). Thereafter, sodium bicarbonate solution (5.0 mL) was poured and maintained for 30 min with occasional shaking. Finally, the absorbance was recorded at 765 nm.
The total content of flavonoids in PCSL was estimated using the aluminium chloride method with some moderation (Chlopicka et al. 2012). From the aforesaid stock solution, 1000 μL of extract solution was combined with 3.0 mL methanol and incubated for 5 min. Thereafter, aluminium chloride (0.2 mL 10%), sodium acetate (0.2 mL; 1 M), and distilled water (3.6 mL) were added, and the mixture was kept for another 40 min at 25°Cbefore recording absorbance at 415 nm. The standard curves for total phenol and flavonoid content estimation were plotted using standard gallic acid and quercetin, respectively, and the results are represented as mg gallic acid/quercetin equivalent/gm extract (mg gallic acid/quercetin/gm extract).
2,2–Diphenyl–1–picrylhydrazyl (DPPH) inhibition activity
The antioxidant activity of PCSL was measured using a conventional procedure with a few modifications (Chlopicka et al. 2012). Briefly, 20 µL of the test sample (25–500 µg/mL) was mixed with 180 μL of DPPH solution. The resultant mixtures were kept in a dark room at 25 °C for 30 min, and then absorbance was recorded at 517 nm. Ascorbic acid was utilised as the standard. The DPPH scavenging activity is assessed by the following equation:
High-performance thin-layer chromatography (HPTLC) determination of caffeic acid, quercetin, and ferulic acid in PCSL
The solvate (50 mg/mL of the extract and 0.5 mg/mL of standards) was prepared by dissolving the extract in ultra-performance liquid chromatography (UPLC) grade methanol and filtered through a Millipore Milles-GN Nylon syringe filter (0.2 µm). CAMAG-Linomat 5 was used to apply the extract and standard (caffeic acid, quercetin, and ferulic acid) to TLC plates with the following settings. The band length was 6 mm, the track margin was 4 mm, the application rate was 150 nL/s, and the distance between the side edge and the bottom was 1.5 and 2 cm. Then, the TLC plate was transferred in a presaturated TLC chamber having mobile phases such as toluene, ethyl acetate, and formic acid (5.5:4.0:0.5 v/v/v). After complete development, the plate was air-dried, scanned at 254 and 366 nm, and analysed with Wincats1.2.3.
In vitro cytotoxicity and nephroprotective activity of PCSL in human embryonic kidney cell line
The human embryonic kidney 293 (HeK293) cell line was procured from NCSS, Pune, Maharashtra, India, and in vitro cell line studies were performed to investigate the nephroprotective potential of PCSL against cisplatin-induced toxicity on the HeK cells line. The study includes cytotoxicity and nephroprotective activity using MTT assay. The HEK cells were cultured and grown in Dulbecco's Modified Eagle Medium (DMEM) with 10% FBS and 50 μg/mL antibiotics (penicillin–streptomycin solution) at 37 °C in a CO2 humidified incubator (5% CO2; 95% air). Samples of different concentrations (25–1000 µg/mL) were incubated with cells for 24 h. Thereafter, the culture medium was gently replaced with 100 µL of DMEM followed by the addition of the MTT reagent (10 µL; 5 mg/mL) and again incubated for 3 h. Finally, the supernatant was removed and formazan crystals were dissolved in 100 µL of a solubilising agent; then, absorbance was recorded using a microplate ELISA reader at 540 nm.
Furthermore, the nephroprotective assay of PCSL was assessed against cisplatin-induced nephrotoxicity following the described protocol. Briefly, 100 µL of cisplatin (16 µg/mL) and 100 µL of samples (25–1000 µg/mL) were poured in ELISA plates and incubated for 24 h. After 24 h, the medium was gently replaced with fresh DMEM (100 µL) followed by the addition of the MTT reagent (10 µL; 5 mg/mL) and again incubated for 3 h. Absorbance was recorded using a microplate ELISA reader at 540 nm. The nephroprotective effect of PCSL was determined in terms of percentage cell viability.
Ascorbic acid was used as the positive control (Xavier et al. 2017).
Experimental animal models
The study was approved by Ethics Committee (43–101), Taif University, Saudi Arabia. The in vivo nephroprotective activity was performed on Wistar albino rats (200–225 g) as per the described protocol with some modifications (Abd El-Rhman et al. 2020). All the animals were housed in standard laboratory conditions and fed a standard diet throughout experimentation. The experimental design of the animal study is presented in Fig. 1. Ascorbic acid (AA) was used as a standard drug for comparative evaluation. The body weight of all the animals was observed at end of the experiment. On the 14th day, blood samples were collected and allowed to clot for 20 min and centrifuged at 1350 rpm for 10 min to gently separate the serum, which was the stored at − 4 °C for further analysis. Moreover, the animals were euthanised to collect kidney tissue from each animal to determine the antioxidant and histopathological analysis (Akca et al. 2018; Singh et al. 2020).
Estimation of serum biochemical markers
Biochemical markers were evaluated to assess the biochemical changes in the kidney. In the analysis of kidney markers, blood urea (BU), uric acid (UA), creatinine (Cr), total bilirubin (TB), total protein (TP), direct bilirubin (DB), albumin (Alb), globulin (Glb), calcium (Ca), sodium (Na), phosphorus (P), and potassium (K) levels were measured in the blood serum (Sultana et al. 2012; Lin et al. 2015; Kpemissi et al. 2019; Ingale et al. 2013).
Antioxidant status
In sterile phosphate buffer (50 mM, pH 7.4), kidney tissues were homogenised (10% w/v) and centrifuged at 1300 rpm for 10 min at 4 °C. The kidney's antioxidant state was assessed spectrophotometrically by detecting the expression of superoxide dismutase (SOD), catalase (CAT), glutathione (GPx), and malondialdehyde (MDA) in the supernatant (Sultana et al. 2012; Lin et al. 2015; Kpemissi et al. 2019).
Assessment of inflammatory biomarkers
Using an ELISA reader, the expression of inflammatory biomarkers, including TNF-α (E-EL-H0109), IL-6 (E-EL-0156), and IL-1β (E-EL-R0012), were measured sequentially as per the manufacturer's protocol (Elabscience; Texas, USA).
Histopathology
The small piece of the kidney specimen was fixed in formalin solution (10%) overnight and then embedded in paraffin blocks. Furthermore, tissue samples were sliced at 5 m thickness with a rotatory microtome, stained with hematoxylin and eosin, dried, and photomicrographed using a microscope (Motic) (Rezaee-Khorasany et al. 2020).
Statistical analysis
The Tukey test and One Way ANOVA were used to portray the data statistically as mean standard deviation. P values less than 0.05 were considered statistically significant. The control group and treatment groups were compared with the toxic group.
Results
Total phenol and flavonoid contents in PCSL
In the present study, the total contents of phenols and flavonoids were assessed. The results reveal that the total phenol content of PCSL was 64.59 ± 1.25 mg equivalents to GAE/gm extract, whereas the total flavonoid content was 31.45 ± 0.73 mg equivalent to rutin/gm extract. The results reveal that PCSL is enriched with polyphenols.
Determination of antioxidant potential of PCSL
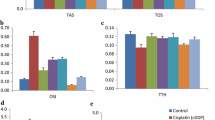
Biochemical assays are widely used to determine the antioxidant activity of single compounds as well as extracts. DPPH, a purple solution, combines with antioxidant chemicals in the test samples (PCSL) to produce a pale yellow colour. Results of the study reveal that PCSL shows significant inhibitory potential in graded dose response on DPPH free radicals at the tested concentration (25–500 µg/mL). The IC50 values of the test sample and standard were 84.34 ± 5.18 and 77.36 ± 3.36 μg/mL, respectively.
Simultaneous determination of caffeic acid, ferulic acid, and quercetin in PCSL
In this study, the standard bioactive markers were determined using HPTLC. For this purpose, we used various solvent systems with different compositions to achieve better separation. Toluene:ethyl acetate:formic acid (5.5:4.0:0.5 v/v/v/) was found to be the best among all mobile phases for all the bioactive markers. This mobile phase offers better results over the earlier reported method (Dixit et al. 2017). Chromatographic separation of the extract showed that all the polyphenolic compounds were well resolved and separated on TLC plates, and the fluorescence bands of all the polyphenolic compounds were visible at 254 nm. Rf values of 0.30, 0.42, and 0.46 for the spots of PCSL were detected as caffeic acid, ferulic acid, and quercetin, respectively (Fig. 2). The percentage of polyphenol content in PCSL was in the order of caffeic acid, ferulic acid, and quercetin. In addition, peaks were symmetrical, and no tailing was observed at 254 nm.
In vitro assays for cytotoxicity and nephroprotective potential of PCSL
Cell line studies were conducted to assess the cytotoxicity and potential of PCSL. The obtained data clearly show that only a higher concentration of PCSL, i.e. 1000 µg/mL, possesses cell cytotoxicity, whereas lesser concentration was found safe (Fig. 3a). In addition, for cisplatin (16 µg/mL) the cell viability was remarkably reduced to 45.5 ± 3.51%. Moreover, data obtained from nephroprotective potential reveal that PCSL significantly (p ≤ 0.05) enhanced the cell viability against cisplatin up to 64.05 ± 4.34% and 70.65 ± 4.15%, respectively, at tested concentrations of 250–500 µg/mL (Fig. 3b). The percentage cell viability was expressed statistically after 24 h of incubation with treatment using one-way ANOVA with the Tukey test.
Measurement of body weight
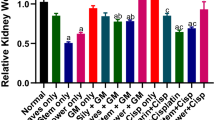
The body weight of all the animals was measured before and after experimentation. The presented data reveal a significant loss in the body in toxic groups as compared to other treated groups. The higher dose of PCSL and standard show a similar body weight pattern and significantly ameliorates to normal as compared to toxic (Fig. 4). Reduced body weight indicates nephrotoxicity by cisplatin.
Estimation of biochemical markers in blood serum
After consecutive administration of cisplatin to experimental rats, the biochemical markers of blood significantly changed. In the toxic group, the levels of biochemical markers such as urea, uric acid, creatinine, total protein, albumin, globulin, and total bilirubin as well as the level of electrolyte sodium increased. By contrast, the levels of calcium, phosphorus, and potassium significantly (p ≤ 0.05) reduced. The above description shows the typical characteristic feature of nephrotoxicity. Interestingly, the treatment groups protect and maintain the biochemical levels near to normal. Higher doses of PCSL show comparable results to those of AA. The overall results are depicted in Figs. 5, 6 and 7.
Effect of PCSL on urea, uric acid, creatinine, total protein, albumin, globulin and total bilirubin in all the groups. The data was expressed statistically as Mean ± SD (n = 6). The comparisons were made between control to toxic (#), and toxic to drug-treated group (*). The significance level was observed at #/*p < 0.05. The ns represents not significant
Effect of PCSL on calcium, phosphorus, sodium and potassium in all the groups. Data was expressed statistically as Mean ± SD (n = 6). The comparisons were made between control to toxic (#), and toxic to drug-treated group (*). The significance level was observed at #/*p < 0.05. The ns represents not significant
Histopathological examination of the control groups showed normal histo-architecture of the kidney tissues while in the case of the toxic group a severe inflammatory cell infiltration (white arrow), irregular dilated lumina (thick), interstitial haemorrhage (curvy arrow), vacuolated cytoplasm and destructured brush border cells (head arrow), atrophy and tubular epithelial injury with widened Bowman's space along with congestion in capillaries of the glomerulus. Treatment groups ameliorated the destruction of kidney tissue damage towards normal
Estimation of antioxidant status of kidney tissue in the experimental groups
OS is a characteristic feature of cisplatin-induced nephrotoxicity. Hence, in this study, the antioxidant status (SOD, CAT, GPx, and MDA) was assessed in kidney tissue homogenates. The obtained results reveal that, in the treatment group, the SOD, CAT, and GPx levels increased markedly, whereas the MDA level reduced. In treatment groups, the levels of the above antioxidant status significantly (p ≤ 0.05) ameliorated to the normal level against cisplatin-induced OS in kidney tissues. Almost similar efficacy of SOD, CAT, and GPx was observed in the higher dose of treatment and standard. Table 1 presents all the antioxidant observations.
Estimation of inflammatory markers in the experimental groups
The expression of TNF-α, IL-6, and IL-1β was examined in all the experimental groups. In the toxic group, a concurrent increment was observed in the expression of all the markers. However, in the treatment groups, the expression of all the inflammatory cytokines was significantly restored near to normal. A high dose of PCSL showed better protective activity as compared to a lower dose. Interestingly, a high dose of PCSL showed a comparable effect as that of standard ascorbic acid (Table 2). Thus, it can be inferred that PCSL protects the nephrotoxicity induced by cisplatin.
Histological observations of the kidney
Histopathological observation of kidney tissues was observed normally in the control group. Cisplatin alone showed impaired renal morphology and caused significant inflammatory cell infiltration, interstitial haemorrhage, irregular dilated lumina, vacuolated cytoplasm, and destructured brush border cells atrophy, as well as expanded Bowman’s space and congestion in glomerular capillaries. In the treatment groups (PCSL and standard), appreciable results were observed, and the cellular architecture of the kidney tissue was observed as normal (Fig. 6). Thus, it can say that PCSL has efficacy to protect the renal damage.
Discussion
Cisplatin-treated rats displayed typical clinical and pathological symptoms like reduction in relative body weight and significant changes in kidney function parameters such as creatinine, urea, uric acid, total protein, albumin, and electrolytes. This observation implies that cisplatin caused nephrotoxicity, as evidenced by a decrease in the glomerular filtration rate. (Sultana et al. 2012). Our findings are comparable to those of previous studies on cisplatin-induced nephrotoxicity. Interestingly, in kidney ailments, the levels of urea, creatinine, and uric acid in serum are considered important markers of nephrotoxicity (Sultana et al. 2012; Lin et al. 2015). Previous reports suggested that creatinine is the most reliable marker in kidney injury. The kidneys maintain creatinine levels by excreting it from the body, and its elevation in the serum shows kidney malfunction (Daenen et al. 2019). In addition, urea is an important marker that can indicate renal cell injuries. Furthermore, cited literature demonstrated that renal physiology is highly responsible for the excretion of urine, water and electrolytes (Na+, K+, Ca2+, and phosphate) (Ingale et al. 2013). PCSL significantly reduces cisplatin-induced nephrotoxicity by returning electrolyte levels in the serum to normal.
OS plays a crucial role in overwhelming renal toxicity induced by cisplatin. Oxidation of cellular lipids and proteins is strongly associated with structural and physiological functions (Kumar et al. 2017). Cited studies reported that free radicals induced by cisplatin counteract the antioxidant enzymes such as SOD, CAT, and GPx. The antioxidants neutralise oxidative free radicals and constitute the primary cellular defence barrier grid in living systems against oxidative stress (Ighodaro and Akinloye 2018). The results of this investigation reveal that cisplatin injection dramatically reduced the levels of SOD, CAT, and GPx in tissue homogenate while significantly increasing the level of MDA. PCSL administration significantly enhanced the levels of SOD, CAT, and GPx and reduced the level of MDA. As compared to a lower dose, a higher dose of PCSL showed more efficacy in antioxidant enzymes. Higher doses of PCSL showed almost similar efficacy as the standard. Thus, the antioxidant efficacy and the nephroprotective activity of PCSL are confirmed.
Furthermore, inflammatory cytokines such as TNF-, IL-6, and IL-1β play a key role in cellular inflammation and are strongly associated with the pathophysiology of nephrotoxicity. Previous studies suggested that cisplatin trigger phosphorylation and subsequently transit the NF-κB transcription factor to the nucleus with the deterioration of the inhibitory IκBα protein (Humanes et al. 2017). The impelling of NF-κB stimulates the transcription of inflammatory mediators and constructs immune, antiapoptotic, proliferative, and inflammatory responses. As a result, the level of TNF-α in kidney cells increases, which is an important inflammatory cytokine in systemic inflammation and acute phase responses caused by cisplatin administration (Peres and da Cunha 2013). IL-6 and IL-1β play a key role in kidney homeostasis and are promptly released in response to renal cell injuries by cisplatin and stimulate acute phase responses (Elsawy et al. 2021; Mohamed et al. 2020). Our results indicate that PCSL ameliorates all the examined inflammatory cytokines near to normal. This result is consistent with several published reports that reveal the anti-inflammatory potential of PCSL in cisplatin-induced nephrotoxicity in rodents. These results signify the anti-inflammatory potential of PCSL. Afsar et al. (2021) reported that polyphenols are excellent drug candidates for preventing cisplatin-induced nephrotoxicity. Consistent with previous reports, this study also revealed high levels of TNF-α, IL-6, and IL-1β.
This study demonstrated that cisplatin administration impairs the histological architecture of the kidney tissues and decreases the body weight. Histopathological examination revealed that cisplatin induces severe inflammatory cell infiltration, irregularly dilated lumina, interstitial haemorrhage, vacuolated cytoplasm and destructured brush border cells, atrophy, and tubular epithelial injury with widened Bowman's space as well as congestion in capillaries of the glomerulus. These disfigurements are clinically confirmed by the elevated serum creatinine level, electrolyte imbalance, and acute kidney failure. Various interstitial congestion and inflammatory infiltration have also been noted (Ewees et al. 2018). Evaluation of the PCSL-treated group indicates that the polyphenol-rich extract counteracts the inimical effects of cisplatin and restores the histological architecture of the kidney near to normal. The histopathological findings are concurrent with biochemical results and agree well with the earlier reports (Wang et al. 2018).
Polyphenolic compounds such as caffeic acid, quercetin, and ferulic acid are effective candidates to hinder the generation of free radicals induced by OS; similarly, these compounds inhibit OS-induced inflammation in the biological systems (Özen et al. 2004; Sanchez-Gonzalez et al. 2011. Interestingly, oral intake of caffeic acid, ferulic acid, and quercetin attenuates the elevated levels of creatinine, urea, uric acid, and MDA. It also ameliorates the status of antioxidants enzymes such as SOD, CAT, and GPx and regain normal histology of the kidney against cisplatin-induced nephrotoxicity (Bami et al. 2017). Of the most prevalent drug development employed in the current decades, mechanisms implicated in the accumulation of oxidative stress, inflammation, and certain biochemical markers related to kidney disorders have been scrutinised in great detail. Polyphenols or other phytochemicals against the multiple targets described above show potential as a class of therapeutics for the management of nephrotoxicity. Moreover, the use of medicinal plants might prevent the occurrence of toxicity caused by synthetic drugs. However, the major drawback is their relatively slower action compared with synthetic medicines. Hence, in emergency cases, the use of medicinal plants is not recommended. Further research is recommended regarding the molecular efficacy, toxicity, and safety of medicinal plants to translate their actual action on human subjects.
Conclusions
The present study revealed based on preclinical models that PCSL exerts a nephroprotective effect against cisplatin-induced toxicity by ameliorating the levels of creatinine, antioxidants, and anti-inflammatory status as well as restoring the histological architecture of the kidney tissues. Hence, we proposed that PCSL could be utilised as a nephroprotective agent in cisplatin chemotherapy. Moreover, further molecular and clinical examinations confirmed these findings.
Abbreviations
- PCSL:
-
Polyphenolic enriched extract of Costus spicatus leaves
- HPTLC:
-
High-performance thin-layer chromatography
- CKD:
-
Chronic kidney disease
- OS:
-
Oxidative stress
- MTT:
-
(3-[4,5-Dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide)
- DMEM:
-
Dulbecco's Modified Eagle Medium
- PBS:
-
Phosphate buffer saline
- TNF-α:
-
Tumour necrosis factor
- IL-6:
-
Interleukin 6
- IL-1β:
-
Interleukin -1β
- SOD:
-
Superoxide dismutase
- CAT:
-
Catalase
- GPx:
-
Glutathione peroxidase
- GAE:
-
Gallic acid equivalent
- MDA:
-
Malondialdehyde
- NCSS:
-
National Centre For Cell Science
References
Abd El-Rhman RH, El-Naga RN, Gad AM et al (2020) Dibenzazepine attenuates against cisplatin-induced nephrotoxicity in rats: involvement of NOTCH pathway. Front Pharmacol 11:567852
Afsar T, Razak S, Aldisi D, Shabbir M et al (2021) Acacia hydaspica R Parker ethyl-acetate extract abrogates cisplatin-induced nephrotoxicity by targeting ROS and inflammatory cytokines. Sci Rep 11:17248
Akca G, Eren H, Tumkaya L, Mercantepe T, Horsanali MO, Deveci E, Dil E, Yilmaz A (2018) The protective effect of astaxanthin against cisplatin-induced nephrotoxicity in rats. Biomed Pharmacother 100:575–582
Al-Naimi MS, Rasheed HA, Hussien NR, Al-Kuraishy HM, Al-Gareeb AI (2019) Nephrotoxicity: role and significance of renal biomarkers in the early detection of acute renal injury. J Adv Pharm Technol Res 10:95–99
Bami E, Ozakpınar OB, Ozdemir-Kumral ZN, Köroglu K, Ercan F, Cirakli Z, Sekerler T, Izzettin FV, Sancar M, Okuyan B (2017) Protective effect of ferulic acid on cisplatin induced nephrotoxicity in rats. Environ Toxicol Pharmacol 54:105–111
Batiha EG, Magdy Beshbishy A, Wasef GL, Elewa YH, Al-Sagan AA, El-Hack A, Mohamed E, Taha AE, Abd-Elhakim MY, Prasad Devkota H (2020) Chemical constituents and pharmacological activities of garlic A review. Nutrients 12(3):872
Chlopicka J, Pasko P, Gorinstein S, Jedryas A, Zagrodzki P (2012) Total phenolic and total flavonoid content, antioxidant activity and sensory evaluation of pseudocereal breads. LWT-Food Science and Technology 46:548–555
Daenen K, Andries A, Mekahli D, Van Schepdael A, Jouret F, Bammens B (2019) Oxidative stress in chronic kidney disease. Pediatr Nephrol 34:975–991
Dasari S, Tchounwou BP (2014) Cisplatin in cancer therapy: Molecular mechanisms of action. European Journal of Pharmacology. Eur J Pharmacol 740:364–378
Dixit V, Irshad S, Singh H, Agnihotri P, Husain T, Khatoon S (2017) High-performance thin-layer chromatographic determination of three therapeutic phenolic components in Leucas species. JPC-J Planar Chromat 30:25–31
Elsawy H, Alzahrani AM, Alfwuaires M, Abdel-Moneim AM, Khalil M (2021) Nephroprotective effect of naringin in methotrexate induced renal toxicity in male rats. Biomed Pharmacother 143:112180
Ewees MG, Messiha BA, Abo-Saif AA, Bayoumi A, Abdel-Bakky MS (2018) Erratum: interference with coagulation cascade as a novel approach to counteract cisplatin-induced acute tubular necrosis; an experimental study in rats. Front Pharmacol 9:1155
Fang CY, Lou DY, Zhou LQ, Wang JC, Yang B, He QJ, Wang JJ, Weng QJ (2021) Natural products: potential treatments for cisplatin-induced nephrotoxicity. Acta Pharmacol Sin 9:1–9
Farooqui Z, Ahmed F, Rizwan S, Shahid F, Khan AA, Khan F (2017) Protective effect of Nigella sativa oil on cisplatin induced nephrotoxicity and oxidative damage in rat kidney. Biomed Pharmacother 85:7–15
Fierascu RC, Fierascu I, Ortan A, Georgiev MI, Sieniawska E (2020) Innovative approaches for recovery of phytoconstituents from medicinal/aromatic plants and biotechnological production. Molecules 25:309
Humanes B, Camaño S, Lara JM, Sabbisetti V, González-Nicolás MÁ, Bonventre JV, Tejedor A, Lázaro A (2017) Cisplatin-induced renal inflammation is ameliorated by cilastatin nephroprotection. Nephrol Dial Transplant 32:1645–1655
Ighodaro OM, Akinloye OA (2018) First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alexandria J Med 54:287–293
Ingale KG, Thakurdesai PA, Vyawahare NS (2013) Protective effect of Hygrophila spinosa against cisplatin induced nephrotoxicity in rats. Indian J Pharmacol 45:232–236
Kpemissi M, Eklu-Gadegbeku K, Veerapur VP, Negru M, Taulescu M, Chandramohan V, Hiriyan J, Banakar SM, Thimmaiah NV, Suhas DS, Puneeth TA (2019) Nephroprotective activity of Combretum micranthum G Don in cisplatin induced nephrotoxicity in rats: In-vitro, in-vivo and in-silico experiments. Biomed Pharmacother 116:108961
Kumar M, Dahiya V, Kasala ER, Bodduluru LN, Lahkar M (2017) The renoprotective activity of hesperetin in cisplatin induced nephrotoxicity in rats: molecular and biochemical evidence. Biomed Pharmacother 89:1207–1215
Lin L, Zheng J, Zhu W, Jia N (2015) Nephroprotective effect of gelsemine against cisplatin-induced toxicity is mediated via attenuation of oxidative stress. Cell Biochem Biophys 71:535–541
Lv JC, Zhang LX (2019) Prevalence and disease burden of chronic kidney disease. Adv Exp Med Biol 1165:3–15
Miller RP, Tadagavadi RK, Ramesh G, Reeves WB (2010) Mechanisms of cisplatin nephrotoxicity. Toxins 2:2490–2518
Mohamed ME, Abduldaium YS, Younis NS (2020) Ameliorative effect of linalool in cisplatin-induced nephrotoxicity: The role of HMGB1/TLR4/NF-κB and NRF2/HO1 pathways. Biomolecules 10:1–19
Moreno KG, Junior AG, Dos Santos AC, Palozi RA, Guarnier LP, Marques AA, Romão PV, Lorençone BR, Cassemiro NS, Silva DB, Tirloni CA (2021) Nephroprotective and antilithiatic activities of Costus spicatus Sw: Ethnopharmacological investigation of a species from the Dourados region, Mato Grosso do Sul State. Brazil J Ethnopharmacol 266:113409
Neelima S, Dwarakanadha Reddy P, Kothapalli Bannoth CS (2020) Nephroprotective activity of Annona Squamosa leaves against paracetamol-induced nephrotoxicity in rats: in vitro and in vivo experiments. Future J Pharm Sci 6:1–8
Özen S, Akyol Ö, Iraz M, Söğüt S, Özuğurlu F, Özyurt H, Odacı E, Yıldırım Z (2004) Role of caffeic acid phenethyl ester, an active component of propolis, against cisplatin-induced nephrotoxicity in rats. J Appl Toxicol 24:27–35
Peres LA, Cunha Júnior AD (2013) Acute nephrotoxicity of cisplatin: molecular mechanisms. J Bras Nefrol 35(4):332–340
Pizzorno J (2015) The kidney dysfunction epidemic, part 1: causes. Integrative Med: Clin J 14:8
Rezaee-Khorasany A, Razavi BM, Taghiabadi E, Yazdi AT, Hosseinzadeh H (2020) Effect of crocin, an active saffron constituent, on ethanol toxicity in the rat: Histopathological and biochemical studies. Iran J Basic Med Sci 23:51–62
Sanchez-Gonzalez PD, Lopez-Hernandez FJ, Perez-Barriocanal F, Morales AI, Lopez-Novoa JM (2011) Quercetin reduces cisplatin nephrotoxicity in rats without compromising its anti-tumour activity. Nephrol Dial Transplant 26:3484–3495
Shediwah FM, Naji KM, Gumaih HS, Alhadi FA, Al-Hammami AL, D’Souza MR (2019) Antioxidant and antihyperlipidemic activity of Costus speciosus against atherogenic diet-induced hyperlipidemia in rabbits. J Integr Med 17:181–191
Singh HP, Singh TG, Singh R (2020) Sinapic acid attenuates cisplatin-induced nephrotoxicity through peroxisome proliferator-activated receptor gamma agonism in rats. J Pharm Bioallied Sci 12:146–154
Sultana S, Verma K, Khan R (2012) Nephroprotective efficacy of chrysin against cisplatin-induced toxicity via attenuation of oxidative stress. J Pharm Pharmacol 64:872–881
Wang TE, Liu HT, Lai YH, Jan TR, Nomura N, Chang HW, Chou CC, Lee YJ, Tsai PS (2018) Honokiol, a polyphenol natural compound, attenuates cisplatin-induced acute cytotoxicity in renal epithelial cells through cellular oxidative stress and cytoskeleton modulations. Front Pharmacol 9:357
Xavier S, Haneefa SM, Anand DR (2017) Antioxidant and nephroprotective activities of the extract and fractions of Homonoia riparia Lour. Pharmacog Mag 13:25–30
Acknowledgements
Authors are thankful to the Taif University Researchers Supporting Project (Number TURSP-2020/124), Taif University, Taif, Saudi Arabia for supporting this work.
Funding
This research was funded by Taif University Research Supporting Project number “TURSP-2020/124”.
Author information
Authors and Affiliations
Contributions
The authors confirm their contribution as follows: Study conception and design: AA, ABA (Abuzer Ali), and WA; data collection and analysis: WA, MA, and KA; interpretation of results: WA, PA, A AH (Adil Ahamad) and AA; draft manuscript preparation: MA, ABA (Abuze Ali), KA, SW, AT, PA and A AH (Adil Ahamad). All authors reviewed the results and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
The study was approved by Ethics Committee (43–101), Taif University, Saudi Arabia.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ali, A., Ali, A., Ahmad, W. et al. Nephroprotective effect of polyphenol-rich extract of Costus spicatus in cisplatin-induced nephrotoxicity in Wistar albino rats. 3 Biotech 12, 189 (2022). https://doi.org/10.1007/s13205-022-03233-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-022-03233-z