Abstract
Sequential pretreatments for sugarcane bagasse (scb) by NaOH followed by organosolv under mild conditions were evaluated for cellulose recovery and dilignification. The best-optimized sequential pretreatment of scb was obtained at 10% (w/v) of raw scb loading at 1% (w/v) NaOH (50 °C, 2 h) followed by treatment with organosolv (85%, v/v phosphoric acid, 50 °C, 1 h) with chilled acetone. This sequentially pretreated scb showed cellulose recovery, 66.1% (w/w) and delignification, 83.2% (w/w). NaOH or organosolv pretreated scb showed lower cellulose recovery 47.4% (w/w) or 54.5% (w/w) with lower delignification, 61% (w/w) or 56% (w/w), respectively. Pretreated solid residue of sequentially pretreated scb was enzymatically saccharified by chimera (β-glucosidase and endoglucanase, CtGH1-L1-CtGH5-F194A) and cellobiohydrolase (CtCBH5A) cloned from Clostridium thermocellum. Enzymatic hydrolysate of best sequentially pretreated scb gave total reducing sugar (TRS) yield, 230 mg/g and glucose yield, 137 mg/g pretreated scb. Only organosolv pretreated scb gave TRS yield, 112.5 mg/g and glucose yield, 72 mg/g of pretreated scb. Thus, sequentially pretreated scb resulted in 37% higher enzymatic digestibility than only orgnaosolv pretreated scb. Higher enzymatic digestibility was supported by higher crystallinity index CrI (45%) than those obtained with only organosolv pretreated (38%) or raw scb (25%). Field Emission Scanning Electron Microscope (FESEM) and Fourier-transform infrared (FT-IR) analyses showed enhanced cellulose exposure in sequentially pretreated scb. Preliminary investigation of bioethanol production at small scale by separate hydrolysis and fermentation (SHF) of enzymatic hydrolysate from best sequentially pretreated scb by Saccharomyces cerevisiae gave maximum ethanol yield of 0.42 g/g of glucose.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Extensive utilization of non-renewable energy sources has led the focus toward the research and technology development in the field of sustainable energy resources (Pathak and Das 2020). Lignocellulosic biomass is one of the sustainable energy resources for the production of bioethanol. The cell wall of lignocellulosic biomass contains cellulose and hemicellulose (Liu et al. 2019). The cellulose is the most abundant carbohydrate which can serve as a potential feedstock for bioethanol production. Sugarcane bagasse (scb) is one of the largest, renewable, and low-cost cellulosic agro-industrial waste used as feedstock in bioethanol production (Pandey et al. 2000). Scb contains approximately 50% (w/w) cellulose, 25% (w/w) each of hemicellulose and lignin (Pandey et al. 2000). Lignin present in the lignocellulosic biomass is responsible for the recalcitrant nature of the biomass. It is known to act as a barrier against the action of various chemicals and microbial degradation processes (Zhao et al. 2009; Rezende et al. 2011). The pretreatment of scb biomass by NaOH removes lignin and increases the accessibility of cellulose and hemicellulose for enzymatic saccharification (Kim et al. 2016). However, to improve the removal of lignin, the increase of NaOH dosage leads to the production of a large number of alkali black liquors, which causes wastewater discharge (Yan et al. 2020). This also causes some of the cellulose solubilization along with the lignin and hemicellulose removal. The acid pretreatments on biomass are employed for the hydrolysis of hemicellulose for increasing the accessibility of cellulose (Sritrakul et al. 2017). However, this could produce inhibitory products, which can negatively affect the downstream process (Sritrakul et al. 2017; Mesa et al. 2011; Zhang et al. 2016). Hence, for efficient delignification and higher cellulose recovery, alternative sequential pretreatment strategies could be designed (Mesa et al. 2011; Zhang et al. 2016; Sindhu et al. 2012). In this regard, the effect of organosolvent, i.e., acetone on acid-pretreated rice straw biomass was explored in a previous study (Sindhu et al. 2012). The rice straw pretreated with 60% (v/v) acetone in the presence of dilute sulphuric acid as catalyst was found to be an effective pretreatment for fermentable sugar yield for the production of bioethanol (Sindhu et al. 2012). However, the use of organosolvent for pretreatment of lignocellulosic biomass is least explored in bioethanol production (Zhang et al. 2016; Sindhu et al. 2012). The organosolvent in the presence of acid or alkali resulted in high cellulose recovery and delignification, therefore is the most promising pretreatment strategy for biomass (Mesa et al. 2011; Zhang et al. 2016; Sindhu et al. 2012). However, the high cost of solvents is the major limiting step in the organosolv pretreatment process which can be overcome using efficient solvent recovery strategies (Zhang et al. 2016). The phosphoric acid-acetone pretreatment is known to be an effective pretreatment strategy for higher removal of hemicellulose and lignin for increasing cellulose exposure (Satari et al. 2019). In this pretreatment, the cellulose obtained after phosphoric acid-acetone pretreatment demonstrated extremely high reactivity for enzymatic digestibility (Marques et al. 2018).
The hydrolysis of cellulosic fraction into glucose for bioethanol production requires cellulases (McKee et al. 2012). The production of these cellulases is cost-intensive process (McKee et al. 2012). The efficiency of the enzymatic hydrolysis process depends on the catalytic efficiency of cellulases and synergistic actions among them against cellulosic substrates (McKee et al. 2012; Lee et al. 2011; Nath et al. 2019). The development of cellulases, with multifunctional activities, reduces the process cost (Lee et al. 2011; Nath et al. 2019). These engineered enzymes were used for hydrolysis of pretreated lignocellulosic biomass into glucose for bioethanol production (Lee et al. 2011; Nath et al. 2019). In an earlier study, the chimera (CtGH1-L1-CtGH5-F194A) was constructed by the fusion of β-glucosidase (CtGH1) and endoglucanase (CtGH5-F194A) from Clostridium thermocellum showed both the activities (Nath et al. 2019). It was thermostable and acted efficiently on Sorghum biomass (Nath et al. 2019). In the present study, the sequential pretreatment strategy for scb was explored using dilute NaOH followed by phosphoric acid–acetone at mild temperature conditions for efficient delignification, cellulose recovery, and improved enzymatic digestibility. The sequentially pretreated scb was enzymatically hydrolyzed using a cocktail of cellulases consisting of a bifunctional chimera (CtGH1-L1-CtGH5-F194A) having β-glucosidase and endoglucanase activities (Nath et al. 2019) and cellobiohydrolase (CtCBH5A) (Nedumaran et al. 2020) cloned earlier from Clostridium thermocellum. The biomass sample after best sequential pretreatment by dilute NaOH for 2 h followed by phosphoric acid–acetone was analyzed by different analytical methods for determining the efficiency of the pretreatment process and was further compared with only organsolv pretreated, only NaOH pretreated, and raw scb. Moreover, the enzymatic hydrolysate obtained from the sequential pretreated scb was subjected to small-scale fermentation through separate hydrolysis fermentation (SHF) by Saccharomyces cerevisiae for preliminary investigation of bioethanol production.
Materials and methods
Scb feedstock preparation
The lignocellulosic feedstock, scb, was collected from a local juice center located in the Sonitpur region of Assam, India. It was washed thoroughly with tap water and dried in the hot air oven at 70 °C for 48 h. The dried substrate was chopped, ground, and sieved through a standard test sieve of 1 mm pore size.
Pretreatment of scb
Stage 1: alkali pretreatment
In stage 1 pretreatment, the raw scb at 7.5% (w/v) biomass loading was chemically pretreated by 1% (w/v) NaOH as reported earlier (Talha et al. 2016). To 1 g of raw scb, 13.3 mL of 1% (w/v) NaOH was added in a 100 mL Erlenmeyer flask and incubated in an incubator shaker (Scigenics Biotech, Model: Orbitek) at 50 °C, 80 rpm. Stage 1 pretreatment was carried out for different time intervals of 1, 2, 3 and 4 h to optimize the reaction time. The alkali pretreated scb pulp was washed with distilled water until the neutral pH 7.0 was achieved. The neutralized samples were then filtered through muslin cloth and dried in a hot air oven at 60 °C for 12 h. The pretreated hydrolysate obtained from this stage 1 pretreatment was analyzed for the estimation of total reducing sugar (TRS) by the method described earlier by Nelson (1944) and Somogyi (1945).
The neutralized dried solid residue obtained after stage 1 pretreatment and raw scb biomass was further subjected to organosolv pretreatment using phosphoric acid-acetone method described below in stage 2 pretreatment.
Stage 2: organosolv pretreatment of alkali pretreated scb
The dried alkali pretreated scb obtained from the stage 1 pretreatment was further pretreated by organosolv using phosphoric acid–acetone method at 10% (w/v) biomass loading as reported earlier (Gupta et al. 2017). To 1 g of raw scb or dried alkali pretreated biomass of each time interval from stage 1, 10 mL of 85% (v/v) phosphoric acid was added and incubated in an incubator shaker (Scigenics Biotech, Model: Orbitek) at 50 °C, 120 rpm for 1 h. After 1 h, 24 mL of chilled acetone was added and centrifuged at 6000g for 10 min, and the pellet was separated. This process of washing with chilled acetone was repeated twice followed by washing with distilled water and the pH of the slurry obtained in the final wash was adjusted to 6.4 by 1.5 M NaOH solution as mentioned earlier (Gupta et al. 2017). This slurry was then separated using a muslin cloth and dried at 25 °C for 12 h. Similarly, the raw scb was also subjected to single-stage organosolv pretreatment. The hydrolysate obtained after stage 2 pretreatments were also analyzed for the total reducing sugar by the method described earlier (Nelson 1944; Somogyi 1945). The monosaccharide estimation (hexoses and pentoses) in the pretreated hydrolysates from all the pretreatments, viz., only NaOH, only organosolv, and sequential pretreatment were analyzed by high-performance liquid chromatography (HPLC coupled with RI detector, Shimadzu, Japan) using monosaccharide analysis HPLC column and the details are mentioned in the analytical methods.
Production of recombinant enzymes: chimera and cellobiohydrolase
The recombinant enzymes, chimera (CtGH1-L1-CtGH5-F194A), and cellobiohydrolase (CtCBH5A) were used for saccharification of pretreated scb. The chimera (Nath et al. 2019) was earlier cloned in Carbohydrate Enzyme Biotechnology Laboratory, Indian Institute of Technology Guwahati, India. Cellobiohydrolase (CtCBH5A) cloned from Clostridium thermocellum was a gift from Professor Carlos M.G.A. Fontes, NZYTech Ltd., Lisbon, Portugal. Both chimera and cellobiohydrolase were expressed in Escherichia coli BL-21 (DE3) cells in LB medium at 24 °C and purified by the method, mentioned earlier (Nedumaran et al. 2020). The 100 mL cultures were centrifuged at 6000g for 10 min at 4 °C and resuspended in 4 mL of 50 mM sodium phosphate buffer, pH 7.0. This cell suspension was sonicated at 33% amplitude at pulse 5 s on and 10 s off (Sonics, Vibra cell) for 10 min. The supernatant containing intracellular recombinant proteins chimera and cellobiohydrolase were purified using immobilized metal-ion affinity chromatography (IMAC) using 5 mL Sepharose column (HiTrap chelating, GE Healthcare). The purified proteins were extensive, dialyzed using 1.0 L of 50 mM sodium phosphate buffer pH 7.0 with 5 times buffer change, giving a total volume of 5.0 L of buffer used. The protein concentration of purified chimera and cellobiohydrolase was determined by Bradford method (Bradford 1976) using BSA as standard. The reaction mixture contained 10 µL of protein in 90 µL of 50 mM sodium phosphate buffer, pH 7.0, and 1 mL Bradford reagent. The reaction mixture was incubated at 25 °C for 15 min and the optical density at 595 nm was measured by UV–visible spectrophotometer (Perkin Elmer, Lambda-45) using 50 mM sodium phosphate buffer, pH 7.0 as blank.
Enzyme activity
The endoglucanase activity of chimera was determined under optimized conditions. The 100 µL reaction mixture containing 1% (w/v) carboxymethylcellulose (CMC) in 20 mM citrate phosphate buffer, pH 5.0, and 10 µL of 0.05 mg/mL purified chimera was incubated at 60 °C, for optimized time period of 4 min, as described earlier (Nath et al. 2019). The β-glucosidase activity of chimera was determined under optimized conditions previously described (Nath et al. 2019) using GOD–POD method (Raabo and Terkildsen 1960). The reaction mixture, 100 µL containing 1% (w/v) cellobiose and 10 μL of the enzyme (0.05 mg/mL) in 20 mM citrate phosphate buffer, pH 5.5 was incubated at 70 °C for 10 min, and then, the reaction was stopped after boiling for 15 min. Subsequently, after cooling, 2.0 μL of reaction mixture was added to a well of 96-well microtitre plate followed by addition of 200 μL of glucose reagent L1 (from a commercial kit) and incubated at 37 °C in an incubator for 15 min. The β-glucosidase activity was determined by measuring the absorbance at 505 nm, on a spectrophotometer (Varian, Cary 100 Bio) using glucose as standard. The cellobiohydrolase, CtCBH5A activity was measured by incubating 100 µL reaction mixture containing 1% (w/v) CMC in 50 mM sodium phosphate buffer, pH 6.4 and 10 µL of 0.05 mg/mL purified enzyme at 65 °C for 2 min as mentioned earlier (Nedumaran et al. 2020). The enzyme activity of chimera (endoglucanase) and cellobiohydrolase was calculated by estimating the reducing sugar released method (Nelson 1944; Somogyi 1945). The reducing sugar was estimated by measuring the absorbance at 500 nm, on a spectrophotometer (Varian, Cary 100 Bio) using glucose as standard.
Optimization of enzymatic saccharification
Optimization of enzyme loading
The saccharification of the pretreated scb biomass from stage 1 and stage 2 pretreatments, viz., only organosolv and all sequentially pretreated scb was carried out using cocktail of chimera and cellobiohydrolase in a previously optimized ratio of 2:3 (Nedumaran et al. 2020). The common 50 mM sodium phosphate buffer, pH 6.4 was used for enzymatic saccharification of pretreated scb, since cellobiohydrolase is stable in 50 mM sodium phosphate buffer, within the pH range, 6.2–6.6 (Nedumaran et al. 2020), and chimera also showed its stability in 50 mM sodium phosphate buffer, pH 6.4, retaining 70–80% of its activity for both β-glucosidase and endoglucanase (Nath et al. 2019). The total enzyme loading concentration of chimera and cellobiohydrolase in optimized 2:3 ratio was varied between 200 and 800 U/g for enzymatic saccharification of each pretreated scb samples.
The saccharification reaction was performed in 1 mL reaction volume using initially 2% (w/v) of pretreated scb in 50 mM sodium phosphate buffer, pH 6.4, and incubated in a shaking incubator at 30 °C at 150 rpm for 48 h in duplicate sets. 0.005% (w/v) sodium azide was added to prevent the reaction mixture from contamination. The above saccharification reactions were stopped after 48 h, by boiling the reaction mixtures in boiling water bath for 10 min. The optimum enzyme loading for saccharification was determined by comparing the TRS yield estimated by the method of Nelson (1944) and Somogyi (1945).
The temperature for saccharification reaction was considered on basis of the previous thermostability analysis described on the same enzyme cocktail comprising chimera and cellobiohydrolase (Nedumaran et al. 2020). Moreover, in the current study, the thermostability study for chimera at 30 °C and 50 °C was also performed. For the thermostability analysis of chimera, 1 mL of 0.05 mg/mL of chimera in 50 mM citrate phosphate buffer (pH 5.0) was incubated at 30 °C and 50 °C for 96 h. The aliquots of 30 µL of chimera were withdrawn periodically and the endoglucanase activity was determined under optimized conditions as described earlier (Nath et al. 2019).
Optimization of pretreated biomass loading
The pretreated biomass loading for the saccharification reaction was optimized by varying the concentration of only organosolv and each sequentially pretreated scb sample from 1 to 4% (w/v). The 1 mL reaction volume under the optimized conditions of 50 mM sodium phosphate buffer, pH 6.4 was incubated at 30 °C in the shaking incubator at 150 rpm for 48 h in duplicate sets. The total enzyme loading of 600 U/g was used in these reactions and 0.005% (w/v) sodium azide was added to prevent contamination. The saccharification reaction was stopped, by boiling the reaction mixtures in boiling water bath for 10 min. The optimum substrate loading for saccharification was determined by comparing the TRS yields estimated by the method of Nelson (1944) and Somogyi (1945).
Optimization of time of incubation for saccharification
Enzymatic saccharification for only organosolv and each sequentially pretreated scb samples was carried out at a biomass loading of 2% (w/v) and an enzyme loading of 600 U/g in 1 mL reaction volume under the optimized reaction conditions for 48 h, 72 h, and 96 h. The reactions were stopped at each time interval by boiling the reaction mixture in boiling water bath for 10 min. The optimum time of incubation for saccharification was determined by comparing the TRS yields, estimated by the method of Nelson (1944) and Somogyi (1945).
Enzymatic saccharification of best sequentially pretreated scb under optimized conditions
The best sequentially pretreated scb (1%, w/v NaOH at 50 °C for 2 h followed by organosolv at 2% (w/v) pretreated biomass loading was saccharified using total enzyme concentration of 600 U/g of chimera and cellobiohydrolase at optimized ratio, 2:3 for 96 h. The saccharification reaction was performed in a 40 mL reaction volume with 50 mM sodium phosphate buffer, pH 6.4 at 30ºC, 150 rpm in the presence of 0.005% (w/v) sodium azide. After 96 h, 1.0 mL aliquot was collected and the TRS yield was estimated by the method described by Nelson (Nelson 1944) and Somogyi (Somogyi 1945). The glucose quantification was performed by HPLC using monosaccharide analysis HPLC column. The enzymatic hydrolysate (34 mL) obtained was then used for small-scale fermentation at shake flask level using S. cerevisiae as mentioned in the subsequent section.
Small-scale fermentation of enzymatic hydrolysate
The fungal strain, S. cerevisiae MTCC170 was procured from the Institute of Microbial Technology (IMTECH), Microbial Type Culture Collection (MTCC), Chandigarh, India. The cultures were maintained in YEPD agar plate containing: yeast extract, 10.0 g/L; peptone, 20 g/L and dextrose, 20 g/L and incubated at 30 °C for 24 h as reported earlier (Bharadwaja et al. 2015). The enzymatic hydrolysate from best sequentially pretreated scb was subjected to small-scale fermentation by S. cerevisiae at shake flask level to investigate preliminary bioethanol production. The glucose present in the enzymatic hydrolysate (34 mL containing 2.78 g/L of concentration was filter sterilized using 0.2 µm membrane) was used as a carbon source for bioethanol production. The fermentation medium (40 mL) containing enzymatic hydrolysate was supplemented with yeast extract (3 g/L), KH2PO4 (1 g/L), (NH4)2SO4 (5 g/L), and MgSO4·7H2O (0.5 g/L), and medium pH was adjusted to 5.0. The inoculum, 15% (v/v) of 12 h grown S. cerevisiae culture (cell OD600 of 3.5) was added and incubated in an incubator shaker (Scigenics Biotech, Model: Orbitek) at 30 °C and 150 rpm following the protocol described earlier (Bharadwaja et al. 2015). After every 2 h of fermentation, 0.5 mL aliquot was withdrawn and the cell growth by the optical density (600nm) of broth, the substrate (glucose), and ethanol concentration were monitored. After the complete consumption of glucose, the samples were withdrawn after every 4 h till 24 h. Ethanol yield and conversion efficiency were calculated using the following formula given earlier (Hatzis et al. 1996):
Mass balance
The mass balance was carried out for 100 g of raw scb for the best sequentially pretreated scb under optimized enzymatic saccharification and fermentation conditions. The cellulose conversion (%) was calculated using the following equation (Hatzis et al. 1996):
Here, ES is the enzymatic saccharification.
Analytical methods
Total reducing sugar, monosaccharide, and ethanol quantification
The hydrolysates obtained after stage 1 and stage 2 pretreatments and enzymatic saccharification of pretreated scb only organosolv and each sequentially pretreated scb were analyzed for total reducing sugar (TRS) yield by the method described by Nelson (1944) and Somogyi (1945). The concentration of the fermenting sugars (hexoses and pentoses) in the pretreated hydrolysates and the concentration of glucose in enzymatic hydrolysates were quantified by HPLC (Coupled with RI detector, Shimadzu, Japan). The 500 µL samples were filtered through the PVDF membrane (pore size 0.2 µm, MF-Millipore, Merck, Massachusetts USA) using a sterile syringe filter. Twenty microliters of filtered sample were injected into the HPLC column Phenomenex Rezex ROA (300 × 7.8 mm) with guard column (50 × 7.8 mm) for monosaccharide sugar analysis. The column temperature was kept constant at 37 °C throughout the analysis. 5 mM H2SO4 was used as the mobile phase at a flow rate of 0.5 mL/min with retention time of 40 min. The standards, glucose, and xylose (Sigma-Aldrich Co. LLC., USA) were used within 0.1 to 1 mg/mL concentrations. The ethanol concentration in 500 µL sample during fermentation was determined by HPLC using Metacarb 67C analyt column. The column temperature was maintained constant at 85 °C. MilliQ water was used as the mobile phase at a flow rate of 0.5 mL/min.
Carbohydrate composition analysis of raw and pretreated scb
The holocellulose, cellulose, hemicellulose, and acid-insoluble lignin (ADL) content of raw scb, scb pretreated by NaOH for 2 h, only organosolv and the best sequentially pretreated scb was determined by TAPPI method (TAPPI 1992). The solid recovery, cellulose, hemicellulose, and lignin removal were calculated by following the method reported earlier (Hatzis et al. 1996):
Structure characterization of raw and pretreated scb biomass
XRD analysis of raw and pretreated scb
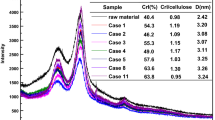
The crystallinity for raw scb, the scb pretreated by NaOH for 2 h, only organosolv, and each sequentially pretreated scb was investigated using X-ray diffractometer (D8 Advance, Bruker, Germany). The diffractometer was set at 40 kV, 40 mA, and radiation source Cu Kα (λ = 1.54 Å). Samples were scanned with 2θ = over range of 5–30 °C with a step of 0.05 °C. The crystallinity index (CrI) of all the samples was calculated by the formula described earlier (Segal et al. 1959):
The CrI (%) of each sample was calculated by:
where Icrystalline is the intensity of the crystalline peak at 2θ = 22° and Iamorphous is the intensity of the amorphous peak at 2θ = 18°.
FESEM analysis of raw and pretreated scb
The FESEM analyses of raw scb, the scb pretreated by NaOH for 2 h, only organosolv, and the best sequentially pretreated scb were carried out to determine and compare the variations in the structural topology of the pretreated biomass. The samples for FESEM analysis were prepared by drying the raw or pretreated biomass in a hot air oven at 60 °C for 24 h. The dried biomass was placed over a carbon tape and a double coating of gold film over samples was performed for 90 s using Polaron Sputter Coater, SC7620 “Mini” (Quorum Technologies, England). Each sample was then examined under FESEM (carl Zeiss, SIGMA VP instrument, Germany).
FT-IR analysis of raw and pretreated scb
The FT-IR-spectral analyses of raw scb, the scb pretreated by NaOH for 2 h, only organosolv, and the best sequentially pretreated scb samples dried under hot air oven were carried out. The pretreated scb samples were grinded with KBr in the ratio 1:100 (w/w) and the mixtures were made into a pellet using hydraulic press. The pellet samples were subjected to spectral analysis by FT-IR spectrometer (Perkin Elmer, USA) and scanned between wavenumber 400 and 4000 cm−1. The peaks corresponding to lignin, hemicelluloses, and cellulose were analyzed.
Results and discussion
Preliminary analysis of pretreatment based on reducing sugar released in pretreated hydrolysates from scb
The stage 1, alkali 1% (w/v) NaOH pretreated scb at 50 °C for 1, 2, 3, and 4 h resulted the TRS yields of 20.3, 13.5, 9.4, and 5.9 mg/g of raw biomass, respectively, as estimated by reducing sugar estimation method of Nelson (1944) and Somogyi (1945) in the hydrolysate (Table 1). The monosaccharide analysis by HPLC showed that the glucose yield from the stage 1 alkali pretreatment of scb for 1, 2, 3, and 4 h in the hydrolysate was very low, i.e., 3.7, 2.6, 1.3, and 0.5 mg/g of raw scb biomass, respectively (Table 1). Similarly, the xylose yield from the stage 1 pretreatment for 1, 2, 3, and 4 h in the pretreated hydrolysate was also very low, i.e., 3.2, 2.5, 1.3, and 0.6 mg/g of raw scb biomass, respectively (Table 1). These low values indicated insignificant hydrolysis of carbohydrate content of scb after alkali pretreatment. Similarly, in a previous study, the low TRS yield of 41.1 mg/g of raw biomass was released in the pretreated hydrolysate after pretreatment of scb with 8% (w/v) NaOH at 180 °C for 30 min, suggesting lower hydrolysis of carbohydrate content after alkali pretreatment (Zhang et al. 2020).
The only organosolv pretreated scb generated TRS yield of only 1.5 mg/g of raw biomass; therefore, glucose and xylose were not detected in the HPLC analysis (Table 1). The stage 2, sequential pretreatments, i.e., NaOH pretreated scb from different time intervals 1, 2, 3, and 4 h followed by organosolv pretreatment generated total sugar (TS), in the pretreated hydrolysates from two successive pretreatments, yield of 20.9, 14.7, 10.4, and 6.7 mg/g, of raw biomass, respectively, (Table 1). The TRS obtained in the hydrolysate after the stage 2 pretreatments was low (0.6–1.2 mg/g of raw biomass), and therefore, glucose and xylose were not detected in the HPLC analysis (Table 1) indicating lower carbohydrate loss or solubilization. An earlier report showed that organosolv pretreated rice straw gives higher delignification and reduced hydrolysis of carbohydrates as compared to raw rice straw biomass (Asadi and Zilouei 2017). In a previous study, two-stage pretreatment of poplar wood chips with steam and organosolv comprising aqueous ethanol resulted in 66% (w/w) delignification with maximum cellulose and hemicellulose recovery of 90% (w/w), suggesting that the organosolv pretreatment caused less carbohydrate degradation (Panagiotopoulos et al. 2013). These results showed that only organosolv and sequentially pretreated scb give much lower TRS in the pretreated hydrolysate. Thus, these pretreated scb were taken further for saccharification by chimera and cellobiohydrolase.
Optimization of saccharification of pretreated scb by cellulases’ enzymes
Optimization of enzyme and pretreated scb loading
The chimera (CtGH1-L1-CtGH5-F194A) showed 4316 U/µmol of endoglucanase activity against CMC and 4984 U/µmol of β-glucosidase activity against cellobiose under optimized reaction conditions. Cellobiohydrolase (CtCBH5A) gave 20 U/mg or 1340 U/µmol against CMC under optimized conditions. Based on the enzyme activities calculated for chimera and cellobiohydrolase, the cocktail of chimera and cellobiohydrolase in the ratio 2:3 was prepared by following the method previously mentioned (Nedumaran et al. 2020). This enzyme cocktail was used for optimizing the enzymatic saccharification reaction for pretreated scb (only organosolv and NaOH pretreated scb for 1, 2, 3, and 4 h followed by organosolv pretreatment). The temperature for saccharification considered was 30 °C, because chimera retained 100% activity at 30 °C (Fig. S1). Moreover, an earlier reported optimization also gave maximum TRS yield at 30 °C as compared to higher temperatures after saccharification of pretreated Sorghum biomass using the same enzyme cocktail (Nedumaran et al. 2020). The enzyme loading and pretreated biomass loading concentration were optimized for maximum TRS yield from above-pretreated scb. The optimum enzyme loading concentration comprising chimera and cellobiohydrolase in a ratio of 2:3 was 600 U/g (240 U/g:360 U/g) at a constant pretreated biomass of 2% (w/v) (Fig. S2). The optimum pretreated biomass loading concentration under the above-mentioned conditions of enzyme loading of 600 U/g was found to be 2%, w/v (Fig. S3).
Optimization of time of incubation for saccharification
The saccharification of each pretreated scb (only organosolv and NaOH pretreated scb for 1, 2, 3, and 4 h followed by organosolv pretreatment) performed using optimized enzyme loading of 600 U/g and optimized substrate concentration of 2% (w/v) of pretreated scb monitored for 48, 72, and 96 h at 30 °C. The best sequential pretreatment for scb was identified on the basis of TRS yield, glucose yield, and CrI determined, as listed in Table 2. The sequentially pretreated (NaOH pretreated for 2 h + organosolv) scb gave maximum TRS yield, 230 mg/g of pretreated scb that was obtained at 30 °C after 96 h of saccharification (Table 2). In a previous report, the enzymatic hydrolysis of microwave-assisted acid-pretreated scb gave maximum TRS yield, 91 mg/g of pretreated scb (Binod et al. 2012). While, dilute nitric acid-pretreated scb (1%, w/v autoclaving at 121 °C and 1 atm pressure for 30 min) gave comparable TRS yield, 280 mg/g of pretreated scb (Ascencio et al. 2019). Although, acid pretreatments on biomass are mostly used for hydrolysis of hemicellulose for increasing the accessibility of cellulose, but it produces inhibitory products that can negatively affect the downstream process (Binod et al. 2012). Hence, the current optimized sequential pretreatment can be used as an alternate possible pretreatment strategy instead of harsh pretreatments for efficient enzymatic digestion of cellulose part of scb by employing further optimization in the enzymatic hydrolysis process.
The enzymatic hydrolysate of all 5 pretreated scb samples obtained after the saccharification was also analyzed for glucose yield by HPLC. The sequentially pretreated (NaOH pretreated for 2 h + organosolv) scb resulted in maximum yield of 137 mg/g of glucose after enzymatic saccharification which was ~ twofold higher than 72 mg/g of pretreated scb obtained with only organosolv (Table 2). These results showed that the enzymatic digestibility or cellulose-to-glucose conversion (%) of this sequentially pretreated scb after enzymatic saccharification was 19% (using Eq. 3), which was 37% higher than 12% obtained with the only organosolv pretreated scb. This could be because in sequential (NaOH pretreated for 2 h + organosolv) pretreatment of scb, the combined effect of alkali and organosolv resulted in enhanced delignification and higher cellulose exposure in the pretreated scb. The exposed cellulose after sequential pretreatment was readily acted upon by cellulase cocktail for higher cellulose-to-glucose conversion as compared to other pretreatments. From previous studies, the cellulose conversion obtained from chemically pretreated scb by NaOH and H2SO4 was in the range, 20–70% (Rezende et al. 2011), while with NH4OH, it was 35% (Cao and Aita 2013). The previous studies also reported that the higher cellulose exposure and cellulose conversion to glucose increases with increase in delignification (Cao and Aita 2013; Liu et al. 2015; Wang et al. 2019).
Composition analysis of raw and pretreated scb
The composition analysis of of raw scb, the scb pretreated by NaOH for 2 h, only organosolv, and the best sequentially pretreated scb were performed and described in Table 3. The raw scb showed 68.3% (w/w) holocellulose, which contained 42.5% (w/w) of cellulose, 25.8% (w/w) of hemicellulose, and 22.1% (w/w) acid-insoluble lignin (ADL) (Table 3). The higher cellulose content makes scb a potential lignocellulosic resource for bioethanol production. In only alkali (1%, w/v NaOH) pretreatment at 50 °C for 2 h showed 70.2% (w/w) solid recovery, giving higher holocellulose content of 70.1% (w/w) consisting of 47.4% (w/w) of cellulose, slightly lower hemicellulose content of 22.8% (w/w), and lower ADL of 12.3% (w/w) as compared to the raw scb biomass. These results indicated maximum 61% (w/w) of lignin removal and 38.1% (w/w) of hemicellulose removal; however, there was relatively lower cellulose removal of 21.4% (w/w) in the pretreated scb hydrolysate (Table 3). The cellulose, hemicellulose, and lignin removal (%) were calculated using Eqs. (4–6), respectively. Therefore, this pretreatment by the use of mild NaOH gave higher lignin removal of scb biomass with less solubilization of cellulose and hemicellulose than raw scb, thereby leading to high carbohydrate recovery from scb biomass.
The only organosolv pretreatment of scb showed 63% (w/w) solid recovery, giving higher holocellulose (69.5%, w/w), cellulose (54.5%, w/w), and ADL (15.5%, w/w) content, but lower hemicellulose content (15%, w/w) than the raw scb (Table 3). These results gave 63.4% (w/w) of hemicellulose removal along with 56% (w/w) of lignin removal and lower cellulose removal of 19.3% (w/w) in the pretreated scb hydrolysate (Table 3). This might be because in the organosolv pretreatment, where the phosphoric acid present could dissolve crystalline cellulose and hemicellulose portion along with the delignification as reported earlier (Zhang et al. 2016).
The best sequentially pretreated scb (NaOH pretreated for 2 h + organosolv) showed solid recovery of 46.1% (w/w) giving increased holocellulose content, 74.5% (w/w) and cellulose content, 66.1% (w/w) with decreased hemicellulose (8%, w/w), and ADL contents (8.1%, w/w) (Table 3). These results showed that there are low cellulose (28.4%, w/w) removal and maximum hemicellulose (85.8%, w/w) and lignin (83.2%, w/w) removal in the pretreated scb hydrolysate (Table 3). Similarly, in the previous studies on scb, the efficient pretreatments were designed for higher cellulose recovery up to 63% (w/w) (Wang et al. 2019), 57.6% (w/w) (Zhang et al. 2006), and 40.3% (w/w) (Gupta et al. 2020). Therefore, the current sequential pretreatment strategy for scb could be considered as an effective approach for higher delignification and cellulose recovery with enhanced cellulose exposure for efficient enzymatic hydrolysis.
XRD analysis of raw and pretreated scb
The CrI analysis of raw scb, the scb pretreated by NaOH for 2 h, only organosolv, and each sequentially pretreated scb were carried out by XRD and compared. The raw scb showed CrI of 25%, while the only NaOH pretreated for 2 h and only organosolv pretreated scb showed similar CrI of 38%. The sequentially pretreated scb showed the highest CrI of 45% among all sequentially and only organosolv pretreated scb (Table 2). The CrI of sequentially pretreated (NaOH pretreated for 2 h + organosolv) scb was 44% higher than the raw scb and 16% higher than both only organosolv and NaOH pretreated for 2 h pretreated scb. In a previous study, the analysis of CrI after acid pretreatment of sugarcane bagasse showed 48% increase in CrI as compared with raw scb (Zhang et al. 2020). This suggested the effective removal of hemicellulose content from scb leaving the crystalline cellulose fraction intact in the pretreated solid residue as mentioned earlier (Rocha et al. 2020). Similarly, in the present study, the enhanced CrI was contributed by the combined action of alkali and organosolv pretreatment. These results demonstrated that the removal of hemicellulose and lignin was higher in sequentially pretreated (NaOH pretreated for 2 h + organosolv) scb than the other pretreatments. It has been also reported earlier that if the pretreatment of biomass is effective, the amorphous nature of cellulose fibers increases that makes it more available for enzymatic hydrolysis (Moura et al. 2018; Qiu et al. 2012).
FESEM analysis of raw and pretreated scb
The surface topology structure analysis of raw scb, the scb pretreated by NaOH for 2 h, only organosolv, and the best sequentially pretreated scb (NaOH pretreated for 2 h + organosolv) scb samples were carried out, as shown in Fig. 1. The rigid and non-porous and rough surface of raw biomass was due to the intact structural integrity of cellulose, hemicelluloses, and lignin (Fig. 1a). The only NaOH pretreated scb showed visible voids (Fig. 1b). These voids generated in the surface morphology of the biomass could be attributed to the delignification. This was also earlier reported that alkali pretreatment of scb forms pores on the surface of biomass, which is mainly due to the lignin removal and that makes the biomass more accessible to the saccharifying enzyme(s) (Rabelo et al. 2011; Sant’Anna and Souza 2012). The only organosolv pretreated scb showed non-porous, with a smooth surface structure more similar to the raw scb biomass (Fig. 1c). The FESEM images of sequentially pretreated (NaOH pretreated for 2 h + organosolv) scb displayed fibrous structures with relatively smooth surface unlike the raw biomass (Fig. 1d). These smooth fibrous structures might be the exposed cellulose fibers. This indicated that the sequential pretreatment removed external fibers (lignin and hemicellulose), which in turn increased the surface area of pretreated biomass, making cellulose fibers more accessible to the cellulase enzymes. Similar surface structure changes in rice straw pretreated with electron beam irradiation (Bak et al. 2009) and aqueous ammonia (Ko et al. 2009) due to the removal of external fibers were reported.
FESEM image analysis of scb. a Raw biomass, b alkali pretreated scb by 1% (w/v) NaOH at 50 °C for 2 h, c only organosolv pretreated scb using 85%, v/v phosphoric acid at 50 °C for 1 h followed by chilled acetone, and d sequentially pretreated (1%, w/v NaOH for 2 h + organosolv) scb. The dried scb samples were placed over a carbon tape and a double coated by gold film for 90 s using Polaron Sputter Coater, SC7620 “Mini” (Quorum technologies, England) for the analysis
FT-IR analysis of raw and pretreated scb
The FT-IR spectroscopic analyses of raw scb, the scb pretreated by NaOH for 2 h, only organosolv, and the best sequentially pretreated scb (NaOH pretreated for 2 h + organosolv) were carried out, and the results are shown in Fig. 2. The raw scb biomass showed the existence of the peaks at wavenumber 1265 cm−1 and 1749 cm−1 which are associated with the lignin and its aromatic ring, respectively (Fig. 2). The presence of lignin and aromatic lignin ring in Arabidopsis (Xu et al. 2013) and scb biomass (Bi et al. 2016) at 1215 cm−1 and 1770 cm−1, respectively, was also reported earlier. However, only NaOH pretreated scb displayed reduced peaks at 1265 cm−1 and 1749 cm−1 (Fig. 2) showing the effective removal of lignin from scb. The FT-IR spectrum of sequentially pretreated scb showed a prominent peak at 1062 cm−1 for crystalline cellulose and relatively sharp peak at 1171 cm−1 for hemicellulose (Fig. 2), while in only organosolv and NaOH pretreatments, these peaks were not prominent (Fig. 2). Similarly, the corresponding peaks 1053 cm−1 associated with crystalline cellulose and 1160 cm−1 with hemicellulose in the pretreated wheat straw biomass were earlier reported (Bekiaris et al. 2015). These results displayed that cellulose and hemicellulose are more exposed after the sequential pretreatment of scb than the other two pretreatments.
FT-IR spectra of scb. a Raw biomass, b alkali pretreated by 1% (w/v) NaOH at 50 °C for 2 h, c only organosolv pretreated by 85%, v/v phosphoric acid at 50 °C for 1 h followed by chilled acetone, and d sequentially pretreated (1%, w/v NaOH for 2 h + organosolv) scb. The scb samples were ground with potassium bromide (KBr) in the ratio 1:100 (w/w) and were made into a pellet by hydraulic press. The pellet samples were scanned between wavenumber, 400 and 4000 cm−1
A small but very prominent and sharp peak at 890 cm−1 characteristic of amorphous cellulose appeared in the sequentially pretreated scb, which was absent in the other all pretreated and raw scb. This result further confirmed that the sequentially pretreated scb gave enhanced cellulose exposure as compared with all other two pretreatments of scb. Similarly, a sharp peak at 898 cm−1 in the alkali-treated seaweed (Gelidiella aceroso) powder associated with β-glycosidic linkages between the anhydroglucose rings in the cellulose was reported earlier (Singh et al. 2017).
Relatively, a broad peak was observed at 1632 cm−1 in the sequentially pretreated scb (Fig. 2). This peak is principally associated with absorbed water, due to strong affinity of hemicellulose for water, suggesting that the pretreated sample has disordered structure. In vetiver grass, the corresponding peak at 1633 cm−1 was observed in the pretreated sample which was suggested to be associated with the water absorption spectrum of the hemicellulose (Chaikumpollert et al. 2004). A wide peak near 3420 cm−1 was observed in the sequentially pretreated scb sample (Fig. 2). This wide peak is associated with OH vibrations due to intermolecular and intramolecular hydrogen bonds in cellulose. The corresponding peak at 3426 cm−1 for the cellulose from pretreated kans grass was described earlier (Gupta et al. 2017). A higher CH2 peak stretching near 2913 cm−1 in the sequentially pretreated scb was observed (Fig. 2), which is a distinguishing feature of cellulose. Similarly, the peak at 2900 cm−1 associated with cellulose from the pretreated biomass Lantana camara was described earlier (Borah et al. 2016). The FT-IR spectral analysis of raw and sequentially pretreated scb clearly showed that the cellulose fibers are exposed and hemicellulose and lignin contents are significantly removed in the pretreated scb. The best sequentially pretreated scb was saccharified by a cocktail of cellulase enzymes, and the hydrolysate obtained was used for separate hydrolysis and fermentation for small-scale bioethanol production.
Small-scale fermentation of enzymatic hydrolysate
The SHF was employed for 34 mL hydrolysate (containing 2.78 ± 0.33 g/L of glucose) obtained after enzymatic saccharification of best sequentially pretreated (NaOH for 2 h + organosolv) scb by cellulase cocktail of chimera and cellobiohydrolase for preliminary investigation of bioethanol production at a small scale. The fermentation of hydrolysate was carried out by taking it as a carbon source with supplementation of other nutrients as mentioned in methods. The fermentation profile depicting glucose and ethanol concentration along with the cell OD at 600 nm during the fermentation process are shown in Fig. 3. The ethanol concentration reached a maximum of 1.18 ± 0.18 g/L at 2 h of fermentation with concomitant decrease in glucose concentration (Fig. 3) giving ethanol yield 0.42 g/g of glucose resulting in 82.4% of ethanol conversion efficiency (using Eqs. 1 and 2). However, this is a preliminary investigation for bioethanol production at a small scale from the current optimized saccharification and pretreatment strategies. Therefore, the process could be a possible approach for a large-scale production upon further optimization. Moreover, in earlier reports, as summarized in Table 4, the ethanol conversion efficiency obtained from dilute alkali pretreated scb was 23% after 72 h of fermentation (Asgher et al. 2013) and 40.84% after 24 h of fermentation (Liu et al. 2015), while with dilute ammonia pretreatment of scb, it was 42% after 72 h of fermentation (Cao and Aita 2013). Thus, above ethanol conversion efficiencies also indicated that the production of ethanol depends on the pretreatment and hydrolysis (Cao and Aita 2013). Nevertheless, the pretreatment should result in maximum lignin removal and higher cellulose exposure for efficient ethanol conversion efficiency (Liu et al. 2015; Qiu et al. 2012). In a previous study, 3% (w/v) NaOH pretreatment of sugarcane bagasse which gave delignification of 70% (w/w) these resulting in efficient cellulose-to-glucose conversion that led to the maximum ethanol conversion efficiency of 67.5% (Wang et al. 2019).
Separate hydrolysis and fermentation (SHF) profile of the hydrolysate obtained after enzymatically saccharification of the cellulosic fraction recovered from optimized sequential pretreatment by 1% (w/v) NaOH at 50 °C for 2 h followed by organosolv (85%, v/v phosphoric acid at 50 °C for 1 h + chilled acetone) of scb. The small-scale fermentation was performed by Saccharomyces cerevisiae at 30 °C and 150 rpm for 24 h. The profile is showing the cell growth (the cell optical density at 600 nm), glucose utilization (g/L), and ethanol concentration (g/L) with time. 500 µL sample from fermentation broth was withdrawn, and glucose and ethanol were determined simultaneously by HPLC using Metacarb 67C analyt column
Overall mass balance
The mass balance based on 100 g raw scb for optimized sequentially pretreated (NaOH for 2 h + organosolv) scb and its subsequent enzymatic hydrolysis and fermentation process steps were carried out (Fig. 4). The best sequentially pretreated scb gave 46.1 g of solid residue containing 30.4 g cellulose, 3.7 g hemicellulose, and 3.7 g lignin. The pretreated hydrolysate contained 12.1 g cellulose, 20.7 g hemicellulose, and 18.3 g lignin. The enzymatic saccharification of the best sequentially pretreated scb by chimera and CtCBH5A in 2:3 ratio under optimized conditions for 96 h released TRS, 10.6 g, and glucose, 6.3 g (Fig. 4). The preliminary investigation for bioethanol production at a small scale by SHF of glucose obtained from enzymatic saccharification of the best sequentially pretreated scb produced 2.7 g of ethanol (Fig. 4). This showed that the production of ethanol was 82.4% of the theoretical yield.
The overall mass balance of 100 g raw scb after, the best sequential pretreatment by 1% (w/v) NaOH at 50 °C for 2 h followed organosolv (85%, v/v phosphoric acid at 50 °C for 1 h + chilled acetone), the enzymatic saccharification of sequentially pretreated solid scb residue by cellulase cocktail (chimera and cellobiohydrolase) at 30 °C for 96 h, and the preliminary investigation of bioethanol production of enzymatic hydrolysate at small scale using SHF at 30 °C and 150 rpm for 24 h
Conclusion
The sequential pretreatment by 1% (w/v) NaOH at 50 °C for 2 h followed by phosphoric acid–acetone pretreatment was found to be an effective pretreatment strategy for sugarcane bagasse. It showed 83.2% (w/w) delignification. resulting in higher cellulose recovery of 66.1% (w/w), demonstrating that this pretreatment is effective for the exposure of cellulose content. The enzymatic hydrolysis using cellulase cocktail comprising chimera and cellobiohydrolase of above sequentially pretreated scb resulted in maximum TRS yield of 230 mg/g and glucose yield of 137 mg/g of pretreated scb. This resulted in 37% higher cellulose-to-glucose conversion than with only organosolv pretreated scb. This sequential pretreated scb gave maximum CrI of 45% indicating higher removal of hemicelluloses and lignin than only organosolv pretreated (38%) and raw scb (25%). The surface morphological changes of the sequentially pretreated scb analyzed by FESEM displayed the smooth surface with fibrous structures suggesting the exposure of cellulose fibers. The FT-IR analysis of sequentially pretreated scb showed the enhanced cellulosic peaks as compared with only organosolv pretreated and raw scb. The preliminary investigation for bioethanol production at a small scale from enzymatic hydrolysate obtained from the sequentially pretreated scb upon separate hydrolysis and fermentation by S. cerevisiae gave ethanol yield of 0.42 g/g of glucose, which resulted in the ethanol conversion efficiency of 82.4%. These optimized pretreatment strategies could be used as a possible approach for higher cellulosic conversion to glucose for bioethanol production on a large scale using further optimization.
References
Asadi N, Zilouei H (2017) Optimization of organosolv pretreatment of rice straw for enhanced biohydrogen production using Enterobacter aerogenes. Bioresour Technol 227:335–344
Ascencio JJ, Chandel AK, Philippini RR, da Silva SS (2019) Comparative study of cellulosic sugars production from sugarcane bagasse after dilute nitric acid, dilute sodium hydroxide and sequential nitric acid-sodium hydroxide pretreatment. Biomass Convers Biorefin 10:813–822
Asgher M, Ahmad Z, Iqbal HMN (2013) Alkali and enzymatic delignification of sugarcane bagasse to expose cellulose polymers for saccharification and bio-ethanol production. Ind Crop Prod 44:488–495
Bak JS, Ko JK, Han YH, Lee BC, Choi IG, Kim KH (2009) Improved enzymatic hydrolysis yield of rice straw using electron beam irradiation pretreatment. Bioresour Technol 100:1285–1290
Bekiaris G, Lindedam J, Peltre C, Decker SR, Turner GB, Magid J, Bruun S (2015) Rapid estimation of sugar release from winter wheat straw during bioethanol production using FTIR-photoacoustic spectroscopy. Biotechnol Biofuels 8:85
Bharadwaja STP, Singh S, Moholkar VS (2015) Design and optimization of a sono-hybrid process for bioethanol production from Parthenium hysterophorus. J Taiwan Inst Chem Eng 51:71–78
Bi S, Peng L, Chen K, Zhu Z (2016) Enhanced enzymatic saccharification of sugarcane bagasse pretreated by combining O2 and NaOH. Bioresour Technol 214:692–699
Binod P, Satyanagalakshmi K, Sindhu R, Janu KU, Sukumaran RK, Pandey A (2012) Short duration microwave assisted pretreatment enhances the enzymatic saccharification and fermentable sugar yield from sugarcane bagasse. Renew Energy 37:109–116
Borah AJ, Singh S, Goyal A, Moholkar VS (2016) An assessment of the potential of invasive weeds as multiple feedstocks for biofuel production. RSC Adv 6:47151–47163
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Cao S, Aita GM (2013) Enzymatic hydrolysis and ethanol yields of combined surfactant and dilute ammonia treated sugarcane bagasse. Bioresour Technol 131:357–364
Chaikumpollert O, Methacanon P, Suchiva K (2004) Structural elucidation of hemicelluloses from Vetiver grass. Carbohydr Polym 57:191–196
Gupta A, Rajulapati V, Das D, Goyal A (2017) Comparative analysis of bioethanol production involving saccharification by mixed recombinant clostridial enzymes using sugarcane leaves and kans grass as sustainable feed stocks from North-east India. Indian J Biotechnol 16:199–210
Gupta R, Aswal VK, Saini JK (2020) Sequential dilute acid and alkali deconstruction of sugarcane bagasse for improved hydrolysis: insight from small angle neutron scattering (SANS). Renew Energy 147:2091–2101
Hatzis C, Riley C, Philippidis G (1996) Detailed material balance and ethanol yield calculations for the biomass-to-ethanol conversion process. In: Seventeenth symposium on biotechnology for fuels and chemicals, Humana Press, Totowa, NJ, pp 443–459
Kim JS, Lee YY, Kim TH (2016) A review on alkaline pretreatment technology for bioconversion of lignocellulosic biomass. Bioresour Technol 199:42–48
Ko JK, Bak JS, Jung MW, Lee HJ, Choi IG, Kim TH, Kim KH (2009) Ethanol production from rice straw using optimized aqueous-ammonia soaking pretreatment and simultaneous saccharification and fermentation processes. Bioresour Technol 100:4374–4380
Lee HL, Chang CK, Teng KH, Liang PH (2011) Construction and characterization of different fusion proteins between cellulases and β-glucosidase to improve glucose production and thermostability. Bioresour Technol 102:3973–3976
Liu Y, Zhang Y, Xu J, Sun Y, Yuan Z, Xie J (2015) Consolidated bioprocess for bioethanol production with alkali-pretreated sugarcane bagasse. Appl Energy 157:517–522
Liu CG, Xiao Y, Xia XX, Zhao XQ, Peng L, Srinophakun P, Bai FW (2019) Cellulosic ethanol production: progress, challenges and strategies for solutions. Biotechnol Adv 37:133–158
Marques NP, de Cassia PJ, Gomes E, da Silva R, Araujo AR, Ferreira H, Bocchini DA (2018) Cellulases and xylanases production by endophytic fungi by solid state fermentation using lignocellulosic substrates and enzymatic saccharification of pretreated sugarcane bagasse. Ind Crops Prod 122:66–75
McKee LS, Pena MJ, Rogowski A, Jackson A, Lewis RJ, York WS, Marles-Wright J (2012) Introducing endo-xylanase activity into an exo-acting arabinofuranosidase that targets side chains. Proc Natl Acad Sci USA 109:6537–6542
Mesa L, Gonzalez E, Cara C, Gonzalez M, Castro E, Mussatto SI (2011) The effect of organosolv pretreatment variables on enzymatic hydrolysis of sugarcane bagasse. Chem Eng J 168:1157–1162
Moura HO, Campos LM, da Silva VL, de Andrade JC, de Assumpçao SM, Pontes LA, de Carvalho LS (2018) Investigating acid/peroxide-alkali pretreatment of sugarcane bagasse to isolate high accessibility cellulose applied in acetylation reactions. Cellulose 25:5669–5685
Nath P, Dhillon A, Kumar K, Sharma K, Jamaldheen SB, Moholkar VS, Goyal A (2019) Development of bi-functional chimeric enzyme (CtGH1-L1-CtGH5-F194A) from endoglucanase (CtGH5) mutant F194A and β-1, 4-glucosidase (CtGH1) from Clostridium thermocellum with enhanced activity and structural integrity. Bioresour Technol 282:494–501
Nedumaran M, Singh S, Jamaldheen SB, Nath P, Moholkar VS, Goyal A (2020) Assessment of combination of pretreatment of Sorghum durra stalk and production of chimeric enzyme (β-glucosidase and endo β-1, 4 glucanase, CtGH1-L1-CtGH5-F194A) and cellobiohydrolase (CtCBH5A) for saccharification to produce bioethanol. Prep Biochem Biotechnol 50:883–896
Nelson N (1944) A photometric adaptation of the Somogyi method for the determination of glucose. Int J Biol Chem 153:375–380
Panagiotopoulos IA, Chandra RP, Saddler JN (2013) A two-stage pretreatment approach to maximise sugar yield and enhance reactive lignin recovery from poplar wood chips. Bioresour Technol 130:570–577
Pandey A, Soccol CR, Nigam P, Soccol VT (2000) Biotechnological potential of agro-industrial residues. I: sugarcane bagasse. Bioresour Technol 74:69–80
Pathak KK, Das S (2020) Impact of bioenergy on environmental sustainability. Biomass valorization to bioenergy. Springer, Singapore, pp 133–158
Qiu Z, Aita GM, Walker MS (2012) Effect of ionic liquid pretreatment on the chemical composition, structure and enzymatic hydrolysis of energy cane bagasse. Bioresour Technol 117:251–256
Raabo BE, Terkildsen TC (1960) On the enzymatic determination of blood glucose. Scand J Clin Lab Investig 12:402–407
Rabelo SC, Fonseca NA, Andrade RR, Maciel Filho R, Costa AC (2011) Ethanol production from enzymatic hydrolysis of sugarcane bagasse pretreated with lime and alkaline hydrogen peroxide. Biomass Bioenerg 35:2600–2607
Rezende CA, de Lima MA, Maziero P, deAzevedo ER, Garcia W, Polikarpov I (2011) Chemical and morphological characterization of sugarcane bagasse submitted to a delignification process for enhanced enzymatic digestibility. Biotechnol Biofuels 4:54
Rocha GJM, Andrade LP, Martin C, Araujo GT, Mouchrek Filho VE, Curvelo AAS (2020) Simultaneous obtaining of oxidized lignin and cellulosic pulp from steam-exploded sugarcane bagasse. Ind Crops Prod 147:112227–112232
Sant’Anna C, Souza W (2012) Microscopy as a tool to follow deconstruction of lignocellulosic biomass. In: Mendez-Vilas A (ed) Current microscopy contributions to advances in science and technology. Formatex Research Center, Badajoz, pp 639–645
Satari B, Karimi K, Kumar R (2019) Cellulose solvent-based pretreatment for enhanced second-generation biofuel production: a review. Sustain Energy Fuels 3:11–62
Segal LGJMA, Creely JJ, Martin AE Jr, Conrad CM (1959) An empirical method for estimating the degree of crystallinity of native cellulose using the X-ray diffractometer. Text Res J 29:786–794
Sindhu R, Binod P, Janu KU, Sukumaran RK, Pandey A (2012) Organosolvent pretreatment and enzymatic hydrolysis of rice straw for the production of bioethanol. World J Microbiol Biotechnol 28:473–483
Singh S, Gaikwad KK, Park SI, Lee YS (2017) Microwave-assisted step reduced extraction of seaweed (Gelidiella aceroso) cellulose nanocrystals. Int J Biol Macromol 99:506–510
Somogyi M (1945) A new reagent for the determination of sugars. Int J Biol Chem 160:61–68
Sritrakul N, Nitisinprasert S, Keawsompong S (2017) Evaluation of dilute acid pretreatment for bioethanol fermentation from sugarcane bagasse pith. Agric Nat Resour 51:512–519
Talha Z, Ding W, Mehryar E, Hassan M, Bi J (2016) Alkaline pretreatment of sugarcane bagasse and filter mud codigested to improve biomethane production. Biomed Res Int 2016:1–10
TAPPI (1992) Technical Association of Pulp and Paper Industry Atlanta Georgia, USA
Wang Q, Wang W, Tan X, Chen X, Guo Y, Yu Q, Zhuang X (2019) Low-temperature sodium hydroxide pretreatment for ethanol production from sugarcane bagasse without washing process. Bioresour Technol 291:121844–121850
Xu F, Yu J, Tesso T, Dowell F, Wang D (2013) Qualitative and quantitative analysis of lignocellulosic biomass using infrared techniques: a mini-review. Appl Energy 104:801–809
Yan X, Cheng JR, Wang YT, Zhu MJ (2020) Enhanced lignin removal and enzymolysis efficiency of grass waste by hydrogen peroxide synergized dilute alkali pretreatment. Bioresour Technol 301:122756–1222764
Zhang YHP, Cui J, Lynd LR, Kuang LR (2006) A transition from cellulose swelling to cellulose dissolution by o-phosphoric acid: evidence from enzymatic hydrolysis and supramolecular structure. Biomacromol 7:644–648
Zhang K, Pei Z, Wang D (2016) Organic solvent pretreatment of lignocellulosic biomass for biofuels and biochemicals: a review. Bioresour Technol 199:21–33
Zhang H, Zhang J, Xie J, Qin Y (2020) Effects of NaOH-catalyzed organosolv pretreatment and surfactant on the sugar production from sugarcane bagasse. Bioresour Technol 312:123601–123607
Zhao X, Cheng K, Liu D (2009) Organosolv pretreatment of lignocellulosic biomass for enzymatic hydrolysis. Appl Microbiol 82:815
Acknowledgements
Authors acknowledge the financial support provided by DBT-Pan-IIT Grant (BT/EB/PAN-IIT 2012), Centre for Bioenergy from Department of Biotechnology, Ministry of Science and Technology, New Delhi, India to Prof. Arun Goyal. The fellowship to Priyanka Nath was supported by funding from DST Inspire fellowship from Department of Science and Technology, New Delhi, India.
Author information
Authors and Affiliations
Contributions
AG conceived the idea and designed the objectives. PN, MPD, and SS performed the experiments. VR performed FT-IR analysis. AG, PN, MPD, and SS wrote the paper.
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest to declare.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nath, P., Maibam, P.D., Singh, S. et al. Sequential pretreatment of sugarcane bagasse by alkali and organosolv for improved delignification and cellulose saccharification by chimera and cellobiohydrolase for bioethanol production. 3 Biotech 11, 59 (2021). https://doi.org/10.1007/s13205-020-02600-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-020-02600-y