Abstract
Calocybe indica mushroom was exposed under natural and artificial UVB light to enhance vitamin D2 contents in the fruit bodies. When Kinetic model was designed to examine the rate of conversion of ergosterol into vitamin D2 at different time intervals (0, 15, 45, 60 and 90 min), it was found that the conversion was linear with time. The maximum content of vitamin D2, that is 78.33 µg/g in sunlight and 140.58 µg/g in UVB radiated fruit bodies, was recorded in the samples exposed for 60 min. Interestingly, UVB radiations triggered the synthesis of β-glucan from their actual content (22.42–44.36 g/100 g) and improved the contents of phenols (12.46–47.38 mg GAE/g) and flavonoids (0.85–2.15 mg Quercetin/g). The estimated antioxidant activities, viz., free radical DPPH scavenging activity and ferric reducing antioxidant power was also found to significantly (p < 0.05) increase after 60 min of UVB exposure. For DPPH and FRAP, lowest IC50 values obtained was 1.90 and 4.60 respectively, which are suggestive of high antioxidant capacity. Additionally, the paper also describes how UVB rays chemically altered the scores for all seventeen amino acids that were analyzed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ergosterol (C28H44O) found in the cell wall of fungi is the precursor of ergocalciferol i.e. vitamin D2, which gets converted under UV exposure. The conversion phenomenon is similar to that of conversion of cholecalciferol (vitamin D3) into vitamin D in human skin under sunlight exposure. It is imperative to maintain the vitamin D levels in the body as it reportedly protects the body from conditions like osteoporosis (Weaver et al. 2016), diabetes (Al-Daghri et al. 2014), cardiovascular risks (Schnatz and Manson 2014), neurogenerative diseases (DeLuca 2004) and also a range of cancers (Garland et al. 2006). The dietary recommendation of vitamin D for an adult male or female is 600 IU or 15 µg/day (Institute of Medicines 2010). However, the unfortunate fact is that 70 per cent of the worldwide population has vitamin D deficiency. This percentile is particularly alarming in India where vitamin D levels of 97% of the adults are below < 75 nmol (Palacios and Gonzalez 2014). Limited availability of food sources that contain adequate levels of vitamin D (i.e. only cholecalciferol containing fish, egg and meat) might be one of the reasons for this deficiency. Increasingly modern lifestyle that limits people’s exposure to sunlight might be another reason.
This tenacious problem requires a reliable solution that can battle against the increasing vitamin D deficiency rate. Edible fungi emerge as a significant part of the solution. As a demanding food source, they can play an essential role in meeting the increasing demands of this particular vitamin. Mushrooms can not only provide vitamin D but are also the carriers of therapeutically active compounds including beta-glucan (β-glucan), terpenoids, steroids, essential amino acids and antioxidants (Rathore et al. 2017). These are again reportedly useful in achieving cure as well as prevention of various life threatening diseases (Prasad et al. 2015).
However, it has been noticed that the vitamin D2 content varies with the season, latitude, weather conditions, various mushroom species and the condition in which they are grown. A huge disparity in the data for vitamin D2 content of some commonly consumed mushroom was seen from literature (Table 1). For example, vitamin D2 contents of widely consumed species Agaricus bisporus is presented differently by authors: 0.05, 3.9 and 12.48 μg/g (dw) by Simon et al. (2011), Urbain and Jakobsen (2015) and Mau et al. (1998) respectively. Likewise, for medicinal mushroom Lentinus edodes the content was found to be varying widely: 26.20 μg/g by Sławinska et al. (2016), 2.8 μg/g by Ko et al. (2008) and 53.9 μg/g by Jashinge and Perera (2006) on a dry weight basis.
On the other hand, a complete lack of documentation about the content in vitamin D was found in Calocybe indica (C.indica) mushroom species, an Indian origin mushroom. C. indica (P&C) was first cultivated by Prukashthya and Chandra in 1974 (Prukashthya and Chandra 1974) in India. C. indica is reportedly rich in protein (2.75–3.22 g/100 g), dietary fibre (1.11–1.63 g/100 g), ash (1.28–2.30 g/100 g) and antioxidant properties (Rathore et al. 2018b; Subbiah and Balan 2015). The fruit body extracts of C. indica are known to exhibit therapeutic properties against diabetes (Rajeswari and Krishnakumari 2013), oxidative stress (Babu and Rao 2013), anti-lipid peroxidation effects (Subbiah and Balan 2015) and a range of cancers (Selvi et al. 2011; Ghosh 2015; Ganapathy and Renitta 2014). The species also contains a number of secondary metabolites such as calocyban (β-glucan), triterpenpoids, phenols, flavonoids etc. (Mandal et al. 2010; Subbiah and Balan 2015). This mushroom species is now becoming popular functional food in other countries, including China, Singapore, Malaysia, and Bangladesh. Hence for this reason, the exploration of vitamin D2 contents in C. indica could prove to be a natural vitamin D supplement to assuage the increasing vitamin D deficiencies amongst the population.
Thus, the present study was designed with the aim of accounting for the vitamin D2 contents of C. indica along with its enhancement by exposing under natural sunlight as well as artificial UVB light. Furthermore, the study also attempts to establish the effects of these radiations on the associated nutraceutical properties, including β-glucan, antioxidants, and amino acids.
Materials and methods
UVB exposure of C. indica
Fresh harvested mushrooms (Calocybe indica P&C) were obtained from the Haryana Agro Research & Development Centre (HAIC) in Murthal, India and were treated on the same day. Whole mushroom fruit bodies were cleaned to remove dirt using a damp cloth, chopped lengthwise (3.0 ± 0.2 cm), placed on shelves and exposed to the UV-B (ultra-violet-B) radiation on both sides in the same conditions, at 5.3 w/m2 intensity under a UV-B lamp (Philips UV-B narrowband TL-20 W) in a chamber for the time period of 15, 30, 45, 60 and 90 min. The experiments pertaining to the natural sunlight irradiation were carried out during the month of June at Indian Institute of Technology, Delhi, India. The samples were always exposed to direct sunlight from 10:00 a.m. to 04:00 p.m. on sunny days without clouds. The irradiated samples were separately lyophilized, sealed and stored at – 20 °C for further analysis.
Ergosterol assay
Mushrooms were analyzed according to the method given by Urbain and Jackobsen (2015), albeit with slight modifications. Freeze dried mushroom sample powders (0.5 g) were accurately weighed and mixed with 4 ml of sodium ascorbate (Sigma chemicals) solution (17.5 g of sodium ascorbate in 100 ml of 1 M sodium hydroxide), 50 ml of ethanol (95% pure), 10 ml of 50% potassium hydroxide (85% pure, Merck Chemicals). The mixture was saponified under reflux at 80 °C for 1 h, and immediately cooled to room temperature beforeit was transferred into a separating funnel. The mixture was first extracted with 15 ml de-ionized water, followed by 15 ml ethanol, and then with three-stages of hexane of volumes 50, 50 and 20 ml, respectively. The pooled organic layers were washed three times with 50 ml of 3% KOH in 5% ethanol and then finally with de-ionized water until neutralized. The organic layer was transferred into a round bottom flask, rotary evaporated (Buchi R 300, India) to dryness at 40 °C, and immediately re-dissolved in 5 ml ethanol.
Chromatographic conditions
The volume of 20 μl filtered sample (0.45 μm, Millipore, Billerica, MA, USA) was injected into a high-performance liquid chromatography (HPLC, PerkinElmer series 2000, USA) equipped with a 2487 dual absorbance detector (PerkinElmer Corp., USA) and eluted through a reverse phase C18 column (Symmetry 4.6 × 250 mm, PerkinElmer Corp. USA). The mobile phase used was methanol/acetonitrile, 25:75, at flow rate of 1 ml/min, and UV detection was at 264 nm. Vitamin D2 was taken as standard (Sigma chemicals, Germany) obtained, and the quantification was carried out using a calibration curve.
Determination of nutraceutical components
β-Glucan contents of irradiated fruiting bodies
Quantitative estimation of β-glucan was estimated by enzymatic procedure using enzymatic kit (Megazyme, Ireland) adopting the AACC method 32–22 and standard method given by McCleary and Glennie-Holmes (1985). The method involved the conversion of (1 → 3)(1 → 4)-β-d-glucan into glucose, which was subsequently, measured using the glucose oxidase/peroxidase procedure with visible spectrophotometer.
Preparation of methanolic extract for antioxidant analysis
The dried mushroom samples (5 g) were extracted by stirring ground sample in 200 ml of methanol at room temperature for 48 h and then filtered through Whatman no. 1 filter paper (Sigma Aldrich, USA). The residue was re-extracted with additional 100 ml portion of methanol. This was followed by the dried of the combined methanolic extracts in a rotary evaporator at 40 °C. These were then re-dissolved in methanol.
Total phenol content
Total phenolic content (TPC) was measured according to the method given by Rathore et al. (2018b) with slight modifications. Methanolic extracts of fruit bodies (10 µl) was mixed with 50 µl of 50% Folin-Ciocalteu reagent. To this mixture 2% sodium carbonate was added and the tubes were vortex. The absorbance was measured at 750 nm after an incubation period of 30 min. The results were expressed as mg of Gallic Acid Equivalents (GAE) per gram of mushroom extract.
Total flavonoid content
Colorimeteric method was followed to determine the total flavonoid contents (TFC) with a slight modification as described by Upadhyay et al. (2015). Briefly, 1 ml of methanolic extract was mixed with an equal volume of 2% AlCl3.6H2O and shaken vigorously. The mixture was then incubated at room temperature for 10 min and absorbance was measured at 367 nm spectrophotometrically. Quercetin was taken as the standard.
Free radical scavenging activity
Free radical 2,2-dipheynl-1-picrylhydrazyl (DPPH) scavenging activity was measured according to the protocol described by Rathore et al. (2018a). To the 50 µl of different concentrations of methanolic extract (0.65 to 20 mg/ml), 200 µl of DDPH solution (0.2 mM) was mixed. Tubes were vortex mixed and incubated in dark for 30 min. The absorbance was measured with the help of a spectrophotometer (PrkinElmer, USA) at 517 nm. Methanol was kept as blank. The percent of reduction of DPPH was calculated according to the following Eq. 1, where Abs control is the absorbance of DPPH solution without extracts.
The results are expressed as IC50 which signifies concentration of the extracts that cause 50% inhibition.
Ferric reducing antioxidant power (FRAP)
FRAP assay was done according to the method given by Rathore et al. (2018a). The oxidant in the FRAP assay was prepared by mixing 2,4,6-tri[2-pyridyl]-s-triazine, TPTZ (10 mM in 40 mMHCl, 2.5 ml), acetate buffer (0.3 M pH 3.6, 25 ml), and 2.5 ml of FeCl3.6H2O (20 mM). The combination prepared by mixing all these reagents is known as “FRAP reagent”. To the amount of 1.8 ml freshly prepared FRAP reagent, 100 µl of sample and 100 µl of distilled water was added. The tubes were then incubated at 37 °C for 30 min and absorbance was taken at 595 nm using a spectrophotometer.
The results are expressed as IC50 which signifies the concentration of the extracts that cause 50% inhibition.
Determination of amino acid
Amino acid analysis was carried out using a method given by Rathore et al. (2018b) but with slight modifications. Five grams of lyophilized mushroom sample was digested using 100 ml of 6 N, HCL for 3 h at 100 °C. The suspension was collected and diluted using distilled water and then filtered. The samples obtained were derivatized using phenylisothiocyanate and converted to phenylthiocarbamoyl amino acids. The derivatized samples (20 µl) were injected into the high-performance liquid chromatography (HPLC, Agilent 1260 Infinity, USA) and read using a florescent detector.
Statistical analysis
All the treatments were carried out in replicates, indicating means and standard deviations. The data was analyzed statistically by analysis of variance (ANOVA) and significant differences among mean values at 95% level of confidence by Duncan’s test using SPSS version 17.0 statistics software. Pearson correlation coefficient and Principal Component Analysis (PCA) was carried out using Origin Pro version 9.1 statistic software.
Results and discussion
Vitamin D2 concentration of C. indica exposed under natural sunlight and UVB light
The vitamin D2 content in the C. indica was evaluated as 34.55 ± 0.88 µg/g DW. The fruit bodies exposed under natural sunlight and UVB light radiations produced significant (p < 0.05) amount of vitamin D2 in C. indica (Table 2). The vitamin D2 content elevated to the fourfold from 34.55 ± 0.88 µg/g DW to 140.58 ± 0.38 µg/g DW after 60 min of UVB light exposure (Supplementary Fig. 1). This is because of the particular UV band region of 280–315 nm in which opening of the B-ring 5,7 diene of pro-vitamin D leads to the production of pre-vitamin D (Simon et al. 2011). We postulate that the slow conversion of vitamin D2 in sunlight exposed samples might be due to the differences in UV intensities of sunlight such as UVA (365 nm), UVC (254 nm) and UVB (315 nm) at different time leaps during the sampling. However, the continuous exposure of UVB rays at set conditions of laboratory could be one of the reasons for converting vitamin D2. The above statement is further justified with a study reported by Jasinghe and Perera (2006) describing that UV-B is the most effectual intensity for converting ergosterol to vitamin D2 as compared to UVC and UVA. High levels of vitamin D2 in UVB exposed samples have also been reported by Urbain et al. (2016). Further, this group of researchers concluded that even providing the similar conditions as with the UVB radiated mushrooms, the sunlight exposed oyster samples did not reach to the marked levels of vitamin D2 estimated in UVB irradiated samples. As represented in Table 1, it is noteworthy to mention that the content of vitamin D2 in C. indica fruit bodies estimated for the first time was found to be higher as reported for the prominent edible mushroom species namely Agaricus bisporus, Lentinus edodes and Pleurotus ostreatus (Huang et al. 2015; Slawinska et al. 2016; Urbain and Jakobsen 2015). Simon et al. (2011) reported the vitamin D2 content of only 3.8 µg/g DW in A. bisporus mushroom, exposed under sunlight, which is exceedingly lesser as compared to the contents measured in the current report. In contrast a recently published study represented high contents of vitamin D2 i.e. 44 and 406 µg/g DW in control and UVB irradiated button mushrooms respectively (Nolle et al. 2017). Now, the question arises whether the UVB exposed mushrooms are safe to consume or not? As per the literature they are safe and beneficial too. A clinical study conducted on rat models, by Calvo et al. (2013) revealed that the consumption of UV exposed mushrooms is not only safe but also support the bone growth and mineralization without any toxicity.
Kinetics model parameters of vitamin D2 in C. indica
Kinetic studies are helpful in predicting the effect of processing on quality parameter(s). In the present study, the kinetics of vitamin D2 formation under the exposure sunlight and UVB is monitored. It was observed that vitamin D2 formation in C. indica is a function of exposure time and follows zero order kinetics with rate of vitamin D2 formation as:
where, C is the vitamin D2 content (µg/g) and t is the exposure time (min).
It is observed that the rate of vitamin D2 formation is higher when fruiting bodies are exposed to UVB in comparison to sunlight (Table 2). This marks the potential of UVB radiations in stimulating and accelerated the photochemical reactions in mushroom fruit bodies faster in comparison to the natural sunlight. This approach to quantify the conversion of ergosterol to vitamin D2 via kinetic model was also demonstrated by Jashinge et al. (2007). Their group of researchers concluded that although the mushroom followed the zero order kinetic reaction in conversion of ergosterol, the conversion rate varied with the type of mushroom species in the order of oyster > shitake > albone > button.
For the present study the formation of vitamin D2 by C. indica fruit bodies on exposure to sunlight and UVB as function of time can be calculated using the following equations:
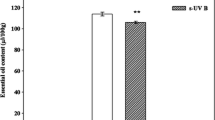
Effect of UVB and sunlight exposure on the β-glucan contents of C. indica
Mushroom fruiting bodies exposed under UVB light at different time interval observed to have significantly (p < 0.05) high β-glucan contents as compared to both the control and sunlight exposed C. indica fruit bodies. As depicted in Fig. 1 the initial content of β-glucan (22.45 g/100 g) calculated for the unexposed samples enhanced to 44.37 g/100 g after 60 min of UVB exposure. The UV rays of sunlight also helped to spike the content to some extent and the highest value obtained with natural light recorded was 33.57 g/100 g at 60 min. However, no increment in the content was observed in both experimental setups after 60 min. Pearson correlation coefficient (R value) analysis further confirmed that the contents of β-glucan content found in close proximity with the vitamin D2 contents measured the R2 value of 0.97 (Table 4). We also found scarcity of data in the β-glucan contents of irradiated mushroom fruit bodies. However, a quite related study by Huang et al. (2015) says that the effect of UV exposure on the total polysaccharide content vary species to species, the UVB irradiated fruit bodies of white and pink oyster were found to have increased β-glucan contents after exposure. Moreover, no changes were detected in the in the β-glucan contents of similarly treated golden oyster mushroom.
Effect of sunlight and UVB radiations on the content of total phenol, flavonoid, DPPH and FRAP activitiesof C. indica fruit bodies
Mushrooms are known to contain polyphenols, widely known for their marvelous antioxidant activities. In the present study, methanolic extracts prepared from the freeze dried samples were evaluated for their total phenol and flavonoid contents using standard procedure. Results pertaining to TPC indicated a substantial linear increase up to 60 min of exposure under UVB light with the highest content of 47.38 ± 0.40 mg/g. The content was significantly (p < 0.05) higher compared to that of the control sample (Table 3). The correlation coefficient data (Table 4) also indicated a positive correlation (R2 = 0.85) between the phenol content and exceeding vitamin D2 values of the C. indica fruit bodies. On the other hand, sunlight could only enhance the total phenol content upto 28.37 mg/g which was again observed to be in linearity with the mentioned exposure time (Table 3). A few studies available on the polyphenol contents of C. indica have reported much lesser content of phenols (18.53 ± 0.02 and 19.80 ± 0.02 mg/g in cap and stipe respectively) as recorded in the current findings (Babu and Rao 2013; Mishra et al. 2014).
Flavonoids known as the potent antioxidant compounds were also recorded. Similar to phenols, their contents were found to be significantly (p < 0.05) influenced by the UVB radiations. The contents were in the range of 0.88–1.75 mg/g and 0.88–2.15 mg/g in sunlight and UVB exposed C. indica fruiting bodies respectively. Results are consistent with the findings of Jiang et al. (2010) in which UVC treated L. edodes mushrooms showed increased contents of total phenols and flavonoids. In contrast, Huang et al. (2015) stated that continuous UV exposure lasting up to 2 h did not change the values for total phenols and flavonoids in eleven species of mushroom fruit bodies. We envisaged that UV radiations elicited the stress conditions in the fungus, which might have turned the biosynthesis pathways for the production of secondary metabolites. Furthermore, it has been reported that stress created by UV stimulate the enzyme systems by playing an important role in scavenging reactive oxygen species and inducing chalcone synthase, the first committed enzyme in flavonoid biosynthesis (Springob et al. 2003; Jiang et al. 2010).
The IC50 values calculated for the antioxidant properties DPPH and FRAP were also found to augment from the existing values (Table 3). With respect to the content calculated for DPPH, the highest IC50 (1.90) was in UVB exposed and 2.26 was found in sunlight exposed samples obtained after 60 min of exposure. Nevertheless, the scavenging effects of DPPH reduced to 50% after 60 min. A similar pattern as free radical DPPH scavenging activity was seen with the ferric ions. The highest value recorded was 4.60 obtained after 60 min of UVB exposure, followed by 90 > 45 > 30 > 15 > control (0 min). The enhanced levels of phenols and flavonoids might be the rationale for high scavenging effects and ferric reducing capacity. The correlation matrix depicted in Table 4 also indicated strong positive correlations amongst the phenol and DPPH and FRAP IC50 values (low IC50 value indicates high antioxidant activity). The statement is further confirmed by Huang et al. (2012) suggesting that the presence of bioactive compound such as phenolics, flavonoids and anthocyanidin contributed to the momentous antioxidant activities in berry fruits. Current findings are in agreement with the literature available on the edible mushrooms (Huang et al. 2015), spices such as clove (Patwardhan and Bhatt 2015) and blueberry fruit (Nguyen et al. 2014) all of these reporting increased levels of antioxidants due to the UVB exposure.
Effect of sunlight and UVB radiations on the amino acid profiling of the C. indica fruit bodies
The current study is mapping the amino acid profiling of C. indica fruiting bodies for the first time. It was found that UVB irradiation of the mushroom fruit bodies significantly (p < 0.05) improved their amino acids content. The most increased amino acid calculated was glutamic acid in the UVB treated C. indica with chemical score of 4.47 g/100 g (Table 5). However, UVB was not found to influence the contents of aliphatic amino acids such as alanine (1.22 g/100 g) and threonine (0.51 g/100 g) and methionine. But the most affected amino acids recorded was aspartic acid which reduced from 1.65 g/100 g to 1.52 g/100 g. The rest of the amino acids were observed to be enhanced positively from their respective contents. Noaman et al. (2016) also showed increased alanine and glutamic acid contents in UVB exposed algae samples. This could be due to the triggered aminotransferase reaction and influencedglutamate synthase pathways responsible for their productions. There was a marked increment noticed in the aromatic and secondary amino acid contents as well. It was hypothesized that UVB radiations could have penetrated inside the nucleus and the protein chromatophores of the mushroom fruit bodies. This in turn, lead to the changes in their chemical pathways responsible for the protein synthesis and other enzyme activities involved in their synthesis. In contrast, Simon et al. (2011) did not find any significant changes in the amino acid contents of the UV radiated button mushrooms.
Correlation effect of UVB exposure on vitamin D2, β-glucan, phenol contents and antioxidant activity of C. indica fruit bodies
Principal component analysis (PCA) was also performed to see the pattern of correlation with in the set of observed variables and how these different variables varied in different treatments. PCA biplot (Fig. 2) of all the variables and treatments (observations) revealed 95.64% of the total variance and the contribution of the principal component 1 and component 2 represented 86.33% and 9.31% of total variation in the observed variables.It was clear that the phenol, β-glucans and vitamin D2 content were grouped together on the right lower side of the biplot, suggesting their positive correlation, with the time period of the UVB exposure. On the other hand, the DPPH and FRAP IC50 were seen on the opposite side, i.e. left side of the PCA plot suggesting their strong positive correlation with enhancing vitamin D2, phenol and β-glucans content.
Conclusion
This is the first scientific report to establish the contents of vitamin D2 in the indigenous edible mushroom C. indica. The rate of conversion of ergosterol was found linear with time and both the UVB exposed as well as sunlight exposed mushrooms were found to contain excellent contents of vitamin D, sufficient to meet the recommended daily dietary intakes of adults. The study also signified the role of UVB radiations in the enhancement of the contents of bioactive compounds, including β-glucans, phenols and flavonoids. The UVB irradiated fruit bodies were also found to attain high levels of antioxidant properties (DPPH and FRAP) along with the enhanced essential amino acid contents. We believe that introduction of such kind of economical post-harvest treatment as well as technology for the mushrooms could prove to be a beneficial approach for combating the prevailing vitamin D deficiencies, without compromising the nutritional and nutraceutical quality parameters. However, a detailed in vivo study involving vitamin D deficient subjects at clinical levels is warranted.
References
AlDaghri NM, AlAttas OS, Alkharfy KM, Khan N, Mohammed AK, Vinodson B, Ansari MG, Alenad A, Alokail MS (2014) Association of VDR-gene variants with factors related to the metabolic syndrome, type 2 diabetes and vitamin D deficiency. Gene 542:129–133
Babu DR, Rao GN (2013) Antioxidant properties and electrochemical behaviour of cultivated commercial Indian edible mushrooms. Food Sci Technol 50(2):301–308
Calvo MS, Babu US, Garthoff LH, Woods TO, Dreher M, Hill G, Nagaraja S (2013) Vitamin D2 from light-exposed edible mushrooms is safe, bioavailable and effectively supports bone growth in rats. Osteoporos Int 24(1):197–207
DeLuca HF (1696S) Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr 80(6):1689S–1696S
Ganapathy J, Renitta E (2014) Evaluation of anti-cancer activity and anti-oxidant status of Calocybe indica (milky mushroom) on Dalton’s Lymphoma ascites induced mice. Aust J Basic Appl Sci 8(10):466–475
Garland CF, Garland FC, Gorham ED, Lipkin M, Newmark H, Mohr SB, Holick MF (2006) The role of vitamin D in cancer prevention. Am J Public Health 96(2):252–261
Ghosh SK (2015) Study of anticancer effect of Calocybe indica mushroom on breast cancer cell line and human Ewings sarcoma cancer cell lines. N Y Sci J 8(5):10–15
Huang W, Zhang H, Liu W, Li C (2012) Survey of antioxidant capacity and phenolic composition of blueberry, blackberry, and strawberry in Nanjing. J Zhejiang Univ Sci 13(2):94–102
Huang SJ, Lin CP, Tsai SY (2015) Vitamin D2 content and antioxidant properties of fruit body and mycelia of edible mushrooms by UVB-irradiation. J Food Compos Anal 42:38–45
Institute of Medicine, Food and Nutrition Board (2010) Dietary reference intakes for calcium and vitamin D. National Academy Press, Washington, DC
Jashinge J, Perera CO (2006) Distribution of ergosterol in different tissues of mushrooms and its effect on the conversion of ergosterol to vitamin D2 by UV irradiations. Food Chem 92:541–546
Jashinge J, Perera CO, Sablani SS (2007) Kinetics of the conversion of ergosterol in edible mushrooms. J Food Eng 79:864–869
Jiang T, Jahangir MM, Jiang Z, Lu X, Ying T (2010) Influence of UV-C treatment on antioxidant capacity, antioxidant enzyme activity and texture of postharvest shiitake (Lentinus edodes) mushrooms during storage. Postharvest Biol Tec 56(3):209–215
Keflie TS, Nolle N, Lambert C, Nohr D, Biesalski HK (2018) Impact of the natural resource of UVB on the content of vitamin D2 in oyster mushroom (Pleurotus ostreatus) under subtropical setting. Saudi J Biol Sci. https://doi.org/10.1016/j.sjbs.2018.07.014
Ko JA, Lee BH, Lee JS, Park HJ (2008) Effect of UV-B exposure on the concentration of vitamin D2 in sliced shiitake mushroom (Lentinus edodes) and white button mushroom (Agaricus bisporus). J Agric Food Chem 56:3671–3674
Koyyalamudi SR, Jeong SC, Pang G, Teal A, Biggs T (2011) Concentration of vitamin D2 in white button mushrooms (Agaricus bisporus) exposed to pulsed UV light. J Food Compos Anal 24:976–979
Mandal S, Maitya KK, Bhunia SK, Dey B, Patra S, Sikdar SR, Islam SS (2010) Chemical analysis of new water-soluble (1→6)-, (1→4)-α, β-glucan and water-insoluble (1→3)-, (1→4)-β-glucan (Calocyban) from alkaline extract of an edible mushroom, Calocybe indica (Dudh Chattu). Carbohydr Res 345(18):2657–2663
Mau JL, Chen PR, Yang JH (1998) Ultraviolet irradiation increased vitamin D2 content in edible mushrooms. J Agric Food Chem 46:5269–5272
McCleary BV, GeneryHolmes M (1985) Enzymatic quantification of (1–3) (1–4)-β-d-glucan in barley and malt. J I Brew 91:285–295
Mishra KK, Pal RS, Arunkumar R (2014) Antioxidant activities and bioactive compound determination from caps and stipes of specialty medicinal mushrooms Calocybe indica and Pleurotus sajor-caju (higher basidiomycetes) from India. Int J Med Mushrooms 16(6):555–567
Nguyen CTT, Kim J, Yoo KS, Lim S, Lee JL (2014) Effect of pre-storage UV-A, -B, and -C radiation on fruit quality and anthocyanin of ‘Duke’ blueberries during cold storage. J Agric Food Chem 62(50):12144–12151
Noaman NH, Faiza MA, Shafik MA, Kareem MSMAK, Manesi MW (2016) Effect of ultraviolet-b irradiation on fatty acids, amino acids, protein contents, enzyme activities and ultra structure of some algae. Adv J Biol Earth Sci 1(1):126–152
Nolle N, Argyropoulos D, Ambacher S, Muller J, Biesalski HK (2017) Vitamin D2 enrichment in mushrooms by natural or artificial UV-light during drying. Food Sci Technol 85:400–404
Palacios C, Gonzalez L (2014) Is vitamin D deficiency a major global public health problem? Steroid Biochem Mol Biol 144:138–145
Patwardhan J, Bhatt P (2015) Ultraviolet-B protective effect of flavonoids from Eugenia caryophylata on human dermal fibroblast cells. Pharmacogn Mag 11:397–406
Phillips KM, Rasor AS (2013) A nutritionally meaningful increase in vitamin D in retail mushrooms is attainable by exposure to sunlight prior to consumption. J Nutr Food Sci 3:1
Prasad S, Rathore H, Sharma S, Yadav AS (2015) Medicinal mushrooms as a source of novel functional food. Int J Food Sci Nutr Diet 04(5):221–225
Purkayastha RP, Chandra A (1974) New species of edible mushroom from India. Trans Br Mycol So 62(2):415–418
Rajeswari P, Krishnakumari S (2013) Potent anti-hyperglycaemic activity of Calocybe indica in streptozotocin induced diabetic rats. Int J Pharm Sci 5:512–515
Rathore H, Prasad S, Sharma S (2017) Mushroom nutraceuticals for improved nutrition and better human health. A review. PharmaNutrition 5:35–46
Rathore H, Sharma A, Prasad S, Sharma S (2018a) Selenium bioaccumulation and associated nutraceutical properties in Calocybe indica mushroom cultivated on Se-enriched wheat straw. J Biosci Bioeng 126(4):482–487
Rathore H, Sharma A, Prasad S, Kumar A, Sharma S, Singh A (2018b) Yield, nutritional composition and antioxidant properties of Calocybe indica cultivated on wheat straw basal substrate supplemented with nitrogenous tree leaves. Waste Biomass Valori. https://doi.org/10.1007/s12649-018-0416-5
Schnatz PF, Manson JE (2014) Vitamin D and cardiovascular disease: an appraisal of the evidence. Clin Chem 60(4):600–609
Selvi S, Umadevi P, Murugan S, GiftsonSenapathy J (2011) Anticancer potential evoked by Pleurotus florida and Calocybe indica using T24 urinary bladder cancer cell line. Afr J Biotechnol 10(37):7279–7285
Simon RR, Phillips KM, Horst RL, Munro IC (2011) Vitamin D mushrooms: comparison of the composition of button mushrooms (Agaricus bisporus) treated postharvest with UVB light or sunlight. J Agric Food Chem 59(16):8724–8732
Slawinska A, Fornal E, Radzki W, Skrzypczak K, Zalewska-Korona M, Michalak-Majewska M, Parfieniuk E, Stachniuk A (2016) Study on vitamin D2 stability in dried mushrooms during drying and storage. Food Chem 199:203–209
Springob J, Nakajima JI, Yamazaki M, Saito K (2003) Recent advances in the biosynthesis and accumulation of anthocyanins. Nat Prod Rep 20:288–303
Subbiah KA, Balan V (2015) A comprehensive review of tropical milky white mushroom (Calocybe indica P&C). Mycobiology 43(3):184–194
Upadhyay R, Sehwag S, Singh SP (2015) Antioxidant activity and polyphenol content of Brassica oleracea varieties. Int J Veg Sci 22(4):353–363
Urbain P, Jakobsen J (2015) Dose–response effect of sunlight on vitamin D2 production in Agaricus bisporus mushrooms. J Agric Food Chem 63:8156–8161
Urbain P, Valverde J, Jakobsen J (2016) Impact on vitamin D2, vitamin D4 and Agaritine in Agaricus bisporus mushrooms after artificial and natural solar UV light exposure. Plant Foods Hum Nutr 71(3):314–321
Weaver CM, Alexander DD, Boushey CJ, Dawson-Hughes B, Lappe JM, LeBoff MS, Liu S, Looker AC, Wallace TC, Wang DD (2016) Calcium plus vitamin D supplementation and risk of fractures: an updated meta-analysis from the National Osteoporosis Foundation. Osteoporos Int 27:367–376
Funding
The authors grateful acknowledge the financial support provided by the University Grant Commission (UGC) (Grant no. 2201011-NETJRF-10299-69) and National Horticulture Board (NHB) for carrying out the research work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Rathore, H., Prasad, S., Sehwag, S. et al. Vitamin D2 fortification of Calocybe indica mushroom by natural and artificial UVB radiations and their potential effects on nutraceutical properties. 3 Biotech 10, 41 (2020). https://doi.org/10.1007/s13205-019-2024-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-019-2024-x